One Step Bioleaching of Sulphide Ore with Low Concentration of Arsenic by Aspergillus niger and Taguchi Orthogonal Array Optimization*
Sadia Ilyas, CHI Ru’an (池汝安)**, Jae Chun Lee and Haq Nawaz Bhatti Key Laboratory for Green Chemical Process of Ministry of Education, School of Chemical Engineering & Pharmacy, Wuhan Institute of Technology, Wuhan 430073, China
2 Mineral Resoures Research Division, Korea Institute of Geoscience and Mineral Resources (KIGAM), 124 Gwahang-no,Yuseong-gu, Daejeon 305-350, Korea
3 Department of Chemistry and Biochemistry, University of Agriculture, Faisalabad 38040, Pakistan
1 INTRODUCTION
In Pakistan various geological regional surveys confirm the presence of ores of arsenic, aluminum, copper, gold, iron, lead, silver, uranium and zinc but most of these minerals occur as low grade ores and are not fit for extraction of metals by conventional metallurgical techniques. Large amount of these low grade ores,which do not contain commercially attractive amounts of precious metals, will be exposed to the atmosphere.At the surface of the rock piles, wind, rainfall and other weather factors (i.e., temperature) will produce exhaustive leaching of metals (Zn, Cu, Cd, Ni,etc.) [1].
Various groups of native microorganisms such as bacteria and fungi can accelerate the production of acid and enhance the leach ability of precious metals to a concentration more commercially attractive, giving value to depleted ores [2, 3]. Although the microbial leaching of metals is successful by using chemolithoautotrophic bacteria to solubilize metals from materials containing sulphide and/or ferrous compounds [4,5], the use of fungi may be an interesting alternative[6]. Bioleaching with fungal microorganisms is based on the following mechanisms: acidolysis (with solubilization of matrix), complexolysis (with complexation of metals by excreted organic acids or amino acids),redoxolysis (reduction of ferric iron mediated by oxalic acid) and bioaccumulation of metals by the mycelium of organism. Although the process of leaching using fungi looks promising, few studies have been performed in this field. The investigation includes the evaluation of the leaching of carbonaceous low-grade ores and nickeliferous laterites [7-10].
The present study is to investigate and optimize a process for the fungal leaching of arsenic containing sulphide ore, to identify and evaluate the parameters that affect the bioleaching process and to determine if the orthogonal array optimization can improve the process. This may be helpful for the commercial implementation of process.
Taguchi method has been shown to be one of effective means for the improvement of productivity in research and development. It has found many applications because of its universal applicability to all engineering fields [11]. The Taguchi experimental array design is therefore used to determine the optimum leaching conditions. Accordingly, the effects of experimental parameters such as initial pH, particle size,pulp density, initial inoculums size and residence time are investigated using an L25 (55) orthogonal array.
2 MATERIALS AND METHOD
2.1 Collection of fungal sample
Samples for isolation of fungal culture were collected from Kalabagh iron ore deposits. A 10 m×10 m area was sampled and 100 g of samples, which contain almost 70% of sulphide ore, 20% of clay, and 10% of silt, were taken from 0 to 10 cm depth after removing 3 cm surface residues, and then homogenized by mixing, sieved and placed in a sterile tightly closed polyethylene bag. The samples were stored at 4 °C and processed within 48 h. For isolation of microorganisms, 10 g samples were added to 100 ml sterilized water and mixed on the magnetic blender for 20 min to separate microorganisms from the sample completely.The serially diluted sample solution was plated on potato dextrose agar (PDA) growth media [12] at 30 °C for 3 days. The mature conidia were washed off from the surface of the PDA medium with sterilized deionized water. The number of spores was counted using a Petroff-Hausser counting chamber and concentration was adjusted to about 3×107spores per ml.
For growth in liquid medium, the culture medium was composed of 100 g·L-1sucrose, 1.5 g·L-1NaNO3,0.5 g·L-1KH2PO4, 0.025 g·L-1MgSO4·7H2O, 0.025 g·L-1KCl and 1.6 g·L-1yeast extract. One ml of fungal spores (approximately 3×107spores) were added to 100 ml of media and incubated at 30 °C at 120 r·min-1for 15 days. Morphological characteristics of the isolates were determined according to the methods described by Weiet al[12]. Physiological and biochemical characteristics were performed as per usual by plate assays. The 18S rRNA genes of the isolate was amplified and sequenced according to the methods described by Whiteet al[13]. The sequences of 18S rRNA genes were first analyzed using the BLAST searching program at the National Center for Biotechnology Information (NCBI) website: http://www.ncbi.nlm.nih.gov/BLAST/. Related sequences were preliminarily aligned with the default setting of Clustal X (2.0)[14]. Phylogenetic and molecular evolutionary analyses were conducted using MEGA version 4.0 [15].
2.2 Source and analysis of ore
A sample of sulphide ore was obtained from PCSIR Laboratories, Lahore, Pakistan and large pieces were crushed in a jaw-crusher separately. Then all pieces were ground to relatively smaller particles using disc-grinding machine. The final grinding of the ore was carried out using ring grinder (FRITSCH Pulverisette, Germany). In order to separate the particles according to their sizes, ASTM sieves were used.
Finely powdered sample (1.0 g) of ore was refluxed with 100 ml of aqua regia in round bottom flask for 1 h. The solution was allowed to cool at room temperature and was filtered through Whatman No. 42 filter paper. The concentration of metals was determined by atomic absorption spectrophotometer (VarianAA-400)and the percentage of these metals in the ore sample was calculated.
2.3 Orthogonal experimental array
An orthogonal experimental array design was constructed for this study that consisted of L25(55)orthogonal array [16]. The degree of influence of individual parameter and the best co-optimization combination of these parameters for bioleaching of metals from sulphide ore sample was observed with culture of KBS4.
Experimental parameters and their levels were determined in the light of preliminary tests and temperature was kept at 30 °C. Preliminary tests were performed and significant factors affecting the metal dissolution were identified as pulp density, initial pH,particle size, initial inoculums size and residence time.
The most significant factors were chosen for optimization, using a five-level orthogonal array with L25(55) matrix, which denotes five parameters, each with five levels, since it is the most suitable for the conditions being investigated and each experiment was repeated twice under the same conditions to monitor the effects of noise sources of the laboratory medium in the bioleaching process. The exact levels were selected using the levels obtained from the previous results. In the proposed method, possible interactions between variables were not in the matrix, and the focus was placed on the main effects of the five most important factors with levels as given in Table 1.
The order of experiments was obtained by inserting parameters into columns of orthogonal arrays, L25(55),chosen as the experimental plan given in Table 2, but the order of experiments was made randomly in order to avoid noise sources during an experiment, which affect results in a negative way. Table 2 is an L25orthogonal array, in which column elements represent the range of levels of the column factors. Each row of orthogonal array represents a run, which is a specific set of factor levels to be tested [17].
2.4 Bioleaching experiments
Experiments on bioleaching of metals from ore were conducted in 250 ml Erlenmeyer flasks containing100 ml of bioleaching medium in each flask at different levels of various factors (initial pH, particle size,pulp density, initial inoculums and residence time).After sterilization by autoclaving at 121 °C and 103.4 kPa pressure for 15 min, 25 batch bioleaching tests were carried out with different combinations of factors and levels for a period of 20 days. One ml of KBS4 spores suspension was incubated with ore sample,with 15% pulp density, in one step bioleaching system.All flasks were weighed before sampling, and then two milliliters of samples were taken by disposable sterilized pipettes at regular intervals. Subsequently, the volume was made up by sterile deionizer water. Samples were centrifuged at 10000 r·min-1for 20 min and filtered through 0.45 μm membrane filters before analyses of pH, concentrations of organic acids and metal ions in solution. All flasks were sealed with four layers pledget and incubated at 30 °C and 120 r·min-1.Each flask experiment was carried out in duplicate.
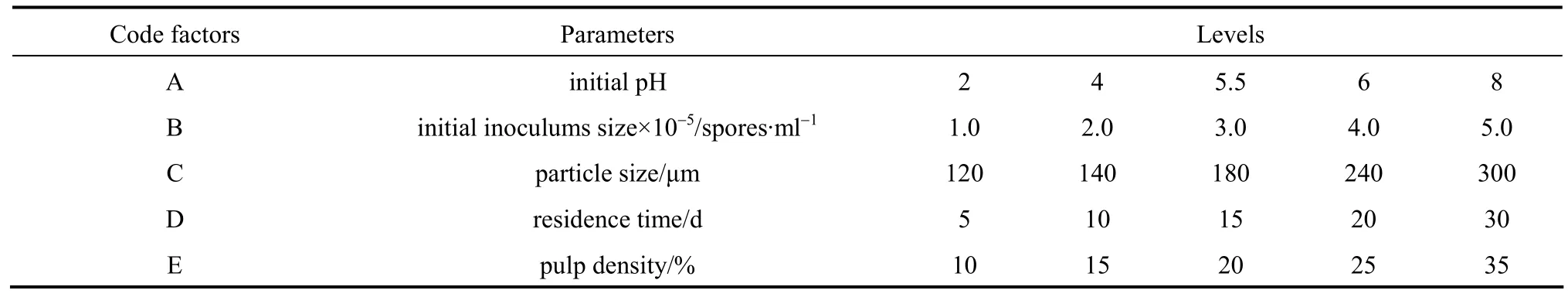
Table 1 Coded values of experimental parameters and their levels
2.5 Chemical leaching experiments
To evaluate the effect of pH changes on the extraction of metals and the effectiveness of different organic acids in metal dissolution, the chemical leaching tests of 1 g·L-1ore sample were conducted in 250 ml Erlenmeyer flasks with citric, oxalic, and gluconic acids at concentrations of 50 mmol·L-1as well as a mixture of commercial citric, oxalic, and gluconic acids at concentrations equal to the acids produced in the bioleaching process. Experiments were performed in duplicates and under the same conditions as bioleaching.
2.6 Adsorption study
Three strategies were adopted for studying the fungal biosorption behavior in bioleaching process: (1)10 g·L-1of ore and 100 ml (62.2 mmol) mixed organic acid was agitated at 120 r·min-1and 30 °C for 24 h.The leaching liquor was centrifuged at 10000 r·min-1for 20 min and filtered through 0.45 μm membrane filter; (2) (1.00±0.03) g wet biomass and 10 g·L-1ore were added in 100 ml (62.2 mmol) mixed organic acid solution and agitated at 120 r·min-1and 30 °C for 24 h;(3) (1.00±0.03) g wet biomass was added to the filtered leachate and agitated at 120 r·min-1and 30 °C for 24 h. The concentration of metal ions in the filtrates was analyzed by atomic absorption spectrophotometry.
2.7 Analytical methodology
The metal concentration was analyzed using an atomic absorption spectrophotometer (Varian AA-400).The solid residues were dried at RT and samples were taken for chemical analysis and X-ray diffraction (XRD).Spores were counted by direct counting in a counting chamber with an optical microscope (×1000). The pH of the bioleaching medium was monitored at room temperature with a pH meter calibrated with a low pH buffer. For the analysis of organic acids, 10 μl of filtrate was injected to high performance liquid chromatography (HPLC; Agilent 1100), using C18 columns(Thermo electron corporation). The mobile phase consisted of a phosphate buffer (50 mmol·L-1KH2PO4,pH 2.0) and acetonitrile (2.0%, by volume). The organic acids were detected at 214 nm with a flow rate of 1.0 ml·min-1for 20 min. The signal was monitored by a UV detector.
3 RESULTS AND DISCUSSION
3.1 Identification of the isolate
Morphological, physiological and biochemical characteristics of isolate-KBS4 are summarized in Table 3. Accession number of 18S rDNA sequence for isolate was obtained by depositing sequence with Gen-Bank of National Center for biotechnology Information (www.ncbi.nlm.nih.gov). The comparison for 18S rRNA gene sequences revealed that KBS4 had 99%similarity withAspergillus nigerstrain JC (EF068267).Based on their 18S rRNA gene sequences and phylogenetic positions, the isolate was designated asAspergillus niger(Fig. 1).
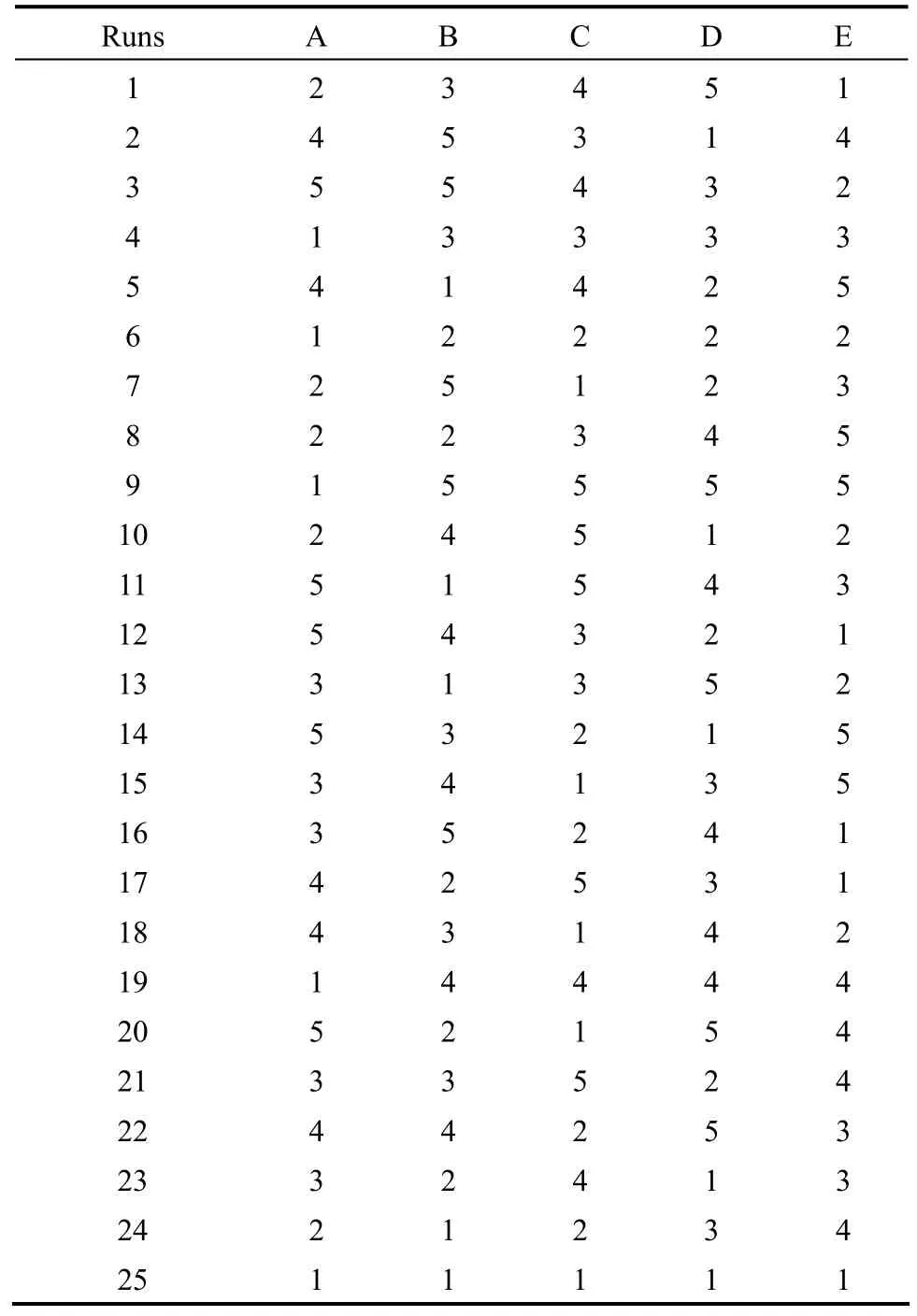
Table 2 L25 (55) experimental work plan
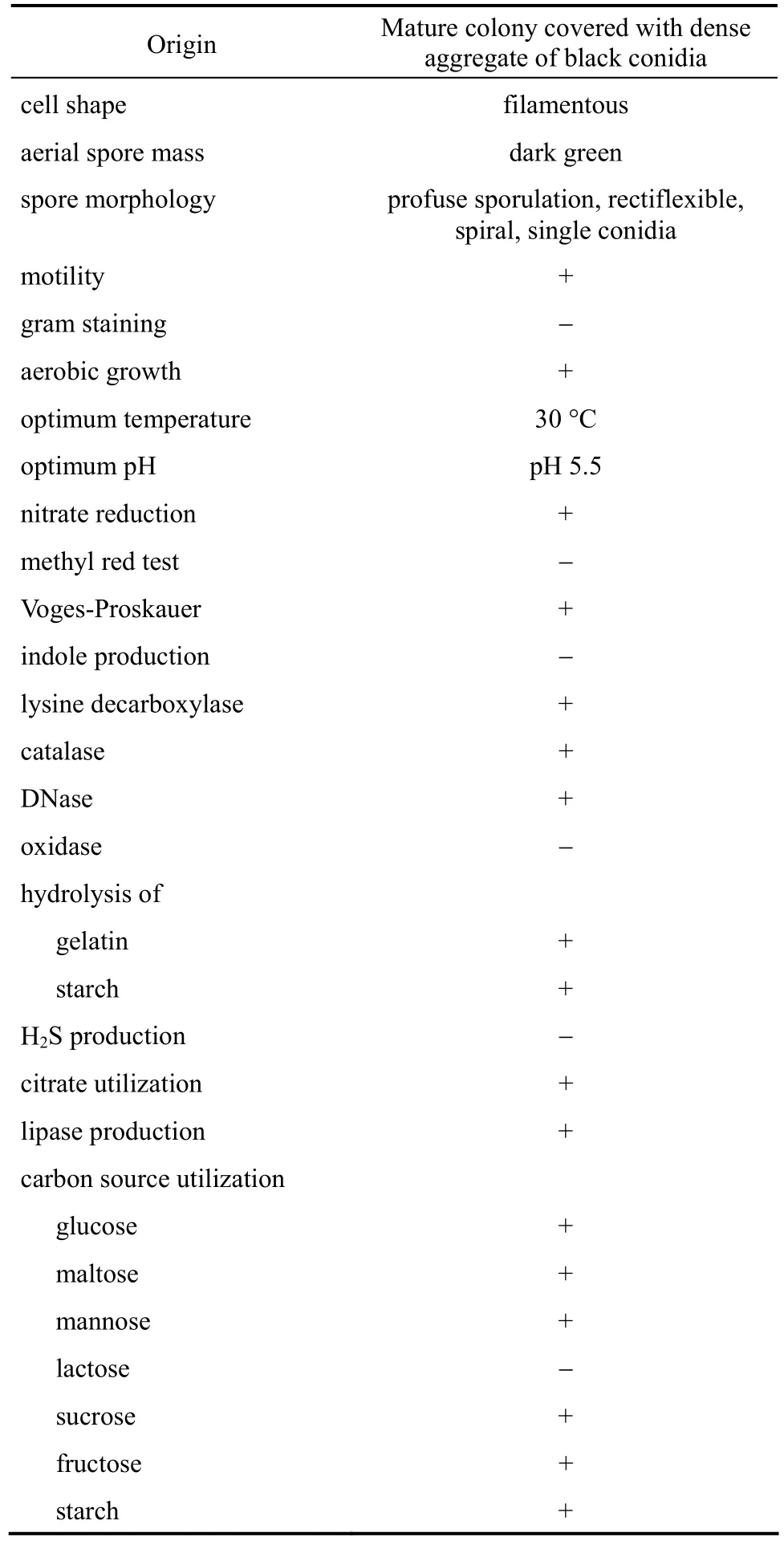
Table 3 Morphological, physiological, and biochemical characteristics of A. niger strain KBS4
3.2 Chemical analysis of ore
Chemical analysis of sulphide ore sample used in the study was carried out to determine the concentration of various metals present. The major metals in the ore sample were found to be Fe, Zn, As, Cu and Al as shown in Table 4.
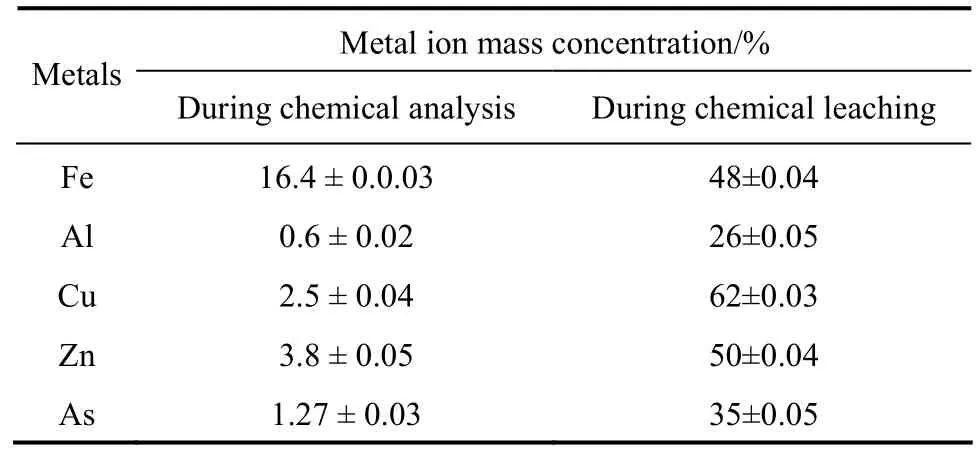
Table 4 Chemical analysis of sulphide ore sample for metal content and chemical leaching using mixture of acids (62.2 mmol·L-1)
3.3 Bioleaching study
Preliminary leaching experiments were conducted at pH 5, temperature 30 °C, particle size 50-150 μm,agitation of 120 r·min-1, initial inoculums size 2.2×107spores per ml, pulp density 10% and residence time of 30 days. The recovery of metal ions was very small(data not shown), so an orthogonal experimental array was constructed for optimization of bioleaching process parameters prior to bioleaching while agitation and temperature was kept at 120 r·min-1and 30 °C. Corresponding leaching efficiencies with two replications obtained under the candidate conditions are displayed in Table 5. The collected data were then analyzed by Origin pro 7.5 software to evaluate the effect of each parameter on the optimization criteria. The maximum amount of metal ion dissolution was defined as the optimization criterion. The mean response calculation was performed to achieve the aim. For better realizing of the experimental conditions related to each response,the corresponding conditions were brought in each row of that table. Taguchi recommends analyzing the mean response for each run and uses graphs of the marginal means of each factor, as shown in Fig. 2, but these graphs are only used to show the trend of each factor and it is incorrect to use these graphs to predict other values that are not experimented. The usual approach is to examine the graphs and select the maximum value. Fig. 2 displays the effects of controllable factors on mean responses for metals of tailing sample. The optimum conditions for enhancing metal ion dissolution are found to be pH 5.5, particle size 180 μm, initial inoculums size 3×107spores per ml, pulp density 15% and residence time of 20 days.
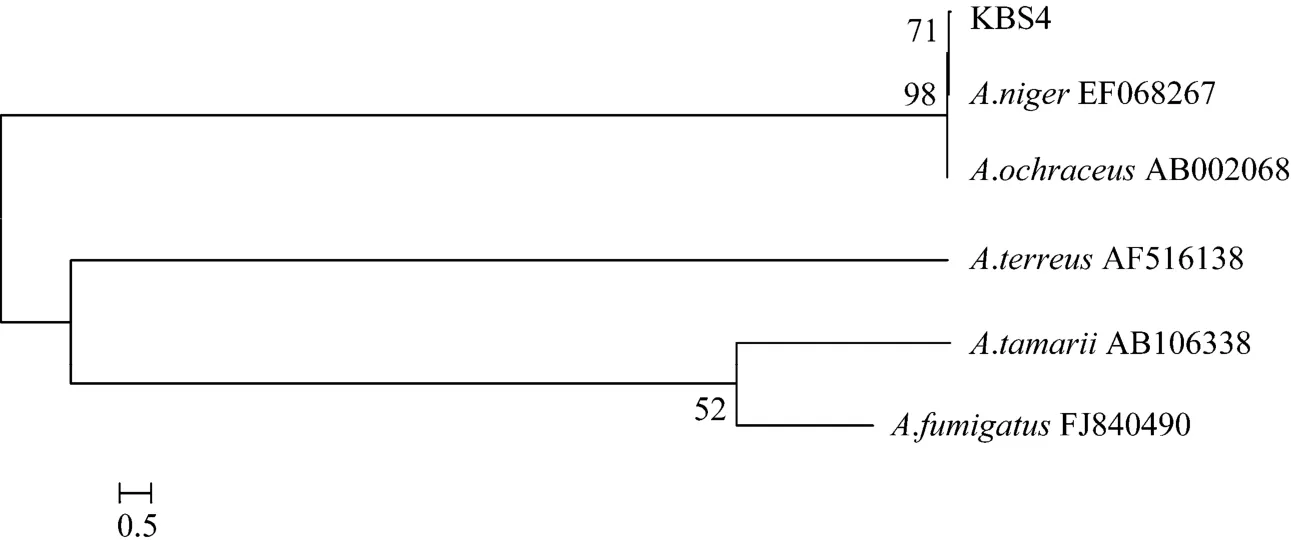
Figure 1 18S rRNA-based phylogenetic relationship between the isolates and representatives of other related tax (Numbers at nodes—levels of bootstrap support based on data for 1000 replicates; values—greater than 50% presented; scale bar—0.5 substitution per nucleotide position)
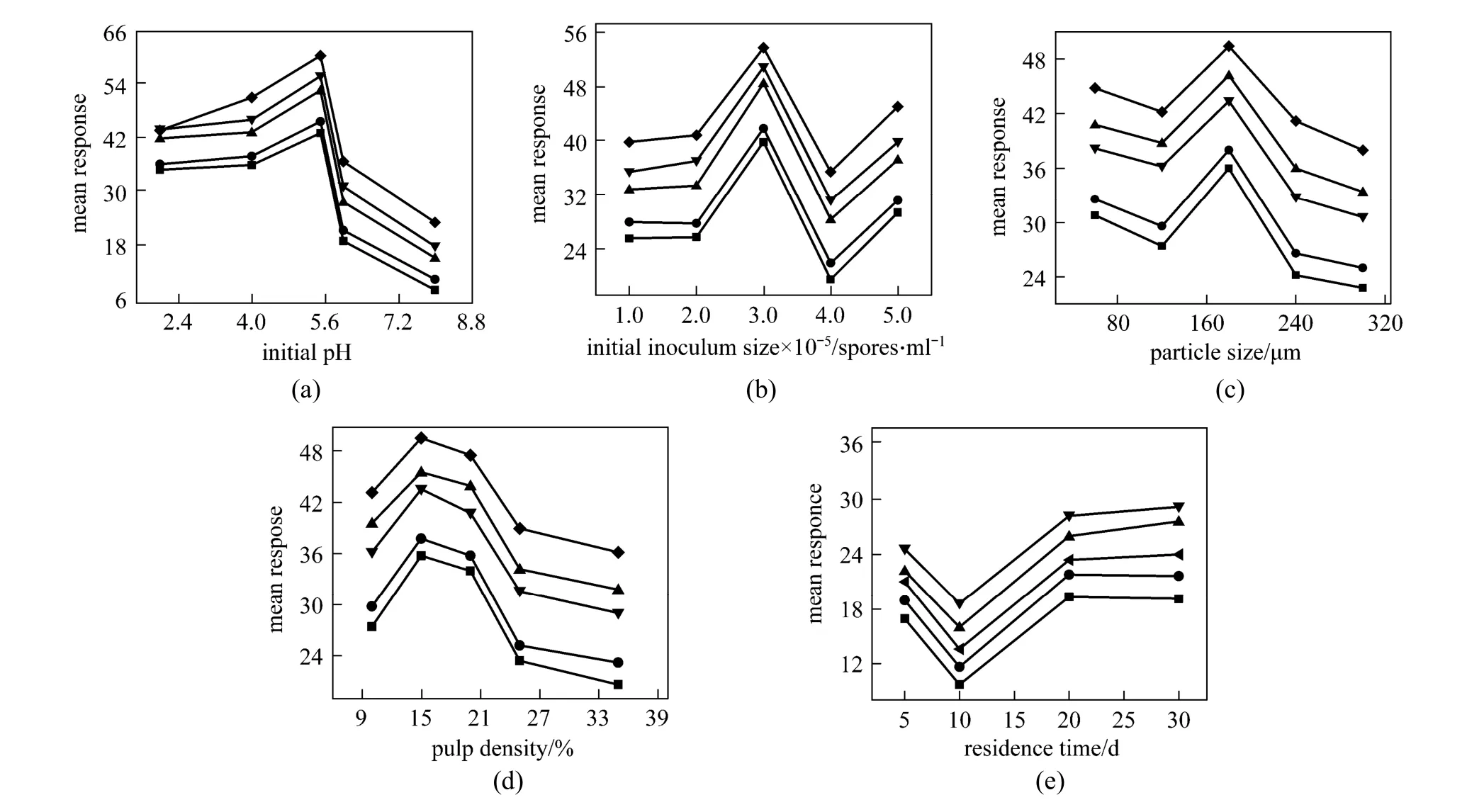
Figure 2 Effects of controllable factors on mean responses for dissolution percent of metal ions■ Fe; ● Zn; ▲ As; ▼ Cu; ◆ Al
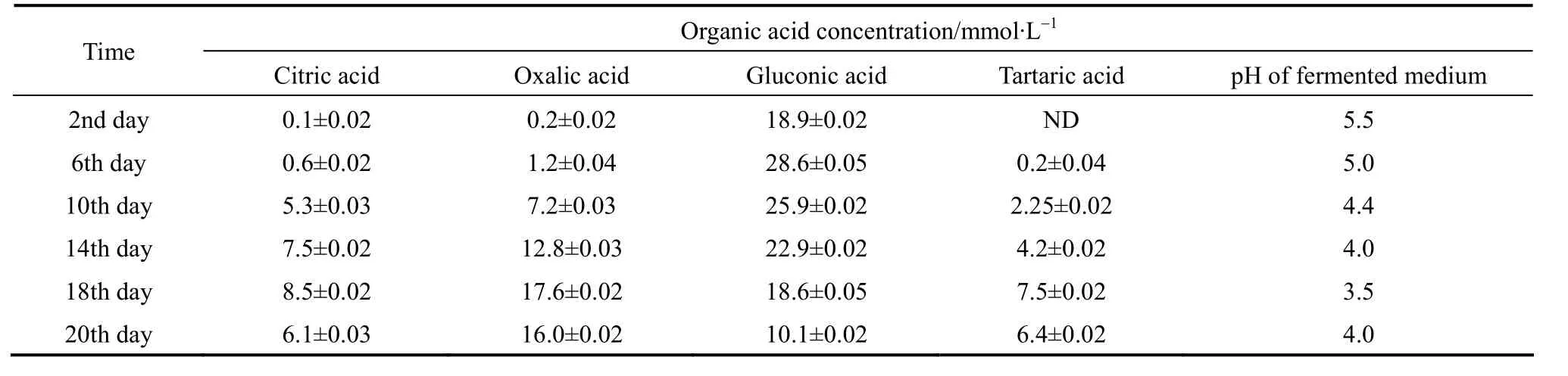
Table 5 Organic acid concentration at different time intervals during 20 days of bioleaching experiment
3.4 pH variation
During bioleaching process,A.nigermetabolized organic acids (citric, oxalic, gluconic acid,etc.) by aerobic fermentation using bioleaching medium,resulting in a decrease of pH (Table 5). This result is in accordance with that of Bosshardet al[9]. The increase in biomass concentration and the decrease in pH at the 4th day also indicated thatA.nigerwas in the active growth phase. The initial pH was 5.5 and it decreased to 5.2 and then rapidly decreased from 5.2 to 4.5 within 216 h and reached 3.5 within 432 h, as shown in Table 6, and then pH increased from 3.3 to 4.0 within 480 h, at the termination of bioleaching period. The pH decrease represents that the bio-produced organic acids exceeds the demand for converting metals into soluble salts, while the pH increase is a representative of the consumption of protons.
3.5 Concentration variation of organic acids and metals
The bio-produced organic acids byA.nigerresult in the environmental mobility of metal ions [17-19].Some of these acids act as complexing agents with 2 or more electron donors for metal ions [20].
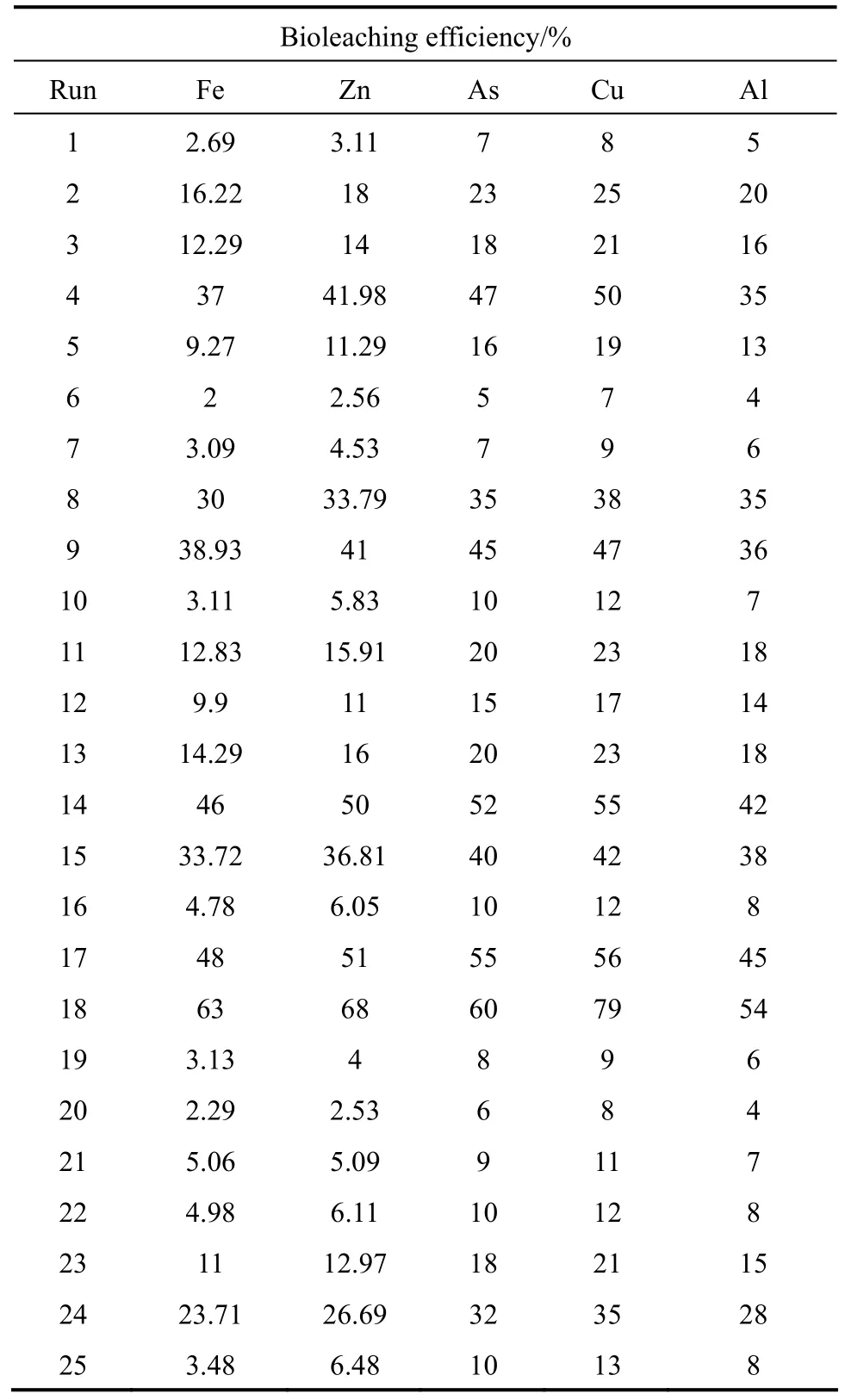
Table 6 Bioleaching efficiency of metals in orthogonal array design
To investigate the effectiveness and utilization of organic acids in metal solubilization during leaching process, organic acids produced were analyzed by HPLC. It is obvious from the HPLC data that major acid produced is gluconic acid followed by oxalic, citric and tartaric acids as shown in Table 6. It had been reported that the variety of organic acids metabolized byA.nigeris significantly related with the pH and metals in the culture medium [19, 21]. Tables 5 and 6 describe the concentrations of organic acids during one-step bioleaching with 15% pulp density of ore sample.
The results indicate that the gluconic acid concentration increases significantly at the beginning of bioleaching. It is noteworthy that the concentration of gluconic acid decreases and that of tartaric acid increases after 144 h, which is dissimilar to the results of Wu and Ting [22] where the concentration of gluconic acid increased until the end of bioleaching and that of Yanget al. [23] where the concentration of gluconic acid decreased and that of oxalic acid increased after 48 h. It is presumably due to the difference ofA.niger. More study about this phenomenon is in progress.
As shown in Table 5, the concentrations of Fe, Zn,As, Cu and Al (metal ions) increase significantly and achieve the maximum values at 18th day (63% Fe,68% Zn, 60% As, 79% Cu and 54% Al) and then decrease. To determine the role of the bio-produced organic acids in the bioleaching process, a comparison between chemical leaching and bioleaching was made by using a mixture of commercial citric, oxalic, and gluconic acids at the same concentration of the organic acids produced in the one-step bioleaching process of ore sample with 15% pulp density. The mixture contained 8.5 mmol·L-1citric acid, 28.6 mmol·L-1gluconic acid, 17.6 mmol·L-1oxalic acid and 7.5 mmol·L-1tartaric acid. It was observed that bioleaching gave a relatively higher extraction yield for Fe, Zn, As, Cu and Al than mix-organic acids leaching (48% Fe, 50%Zn, 3 % As, 62% Cu and 26% Al). Castroet al. [24]reported that chemical leaching with citric acid was less effective in the extraction of nickel from garnierite compared to bioleaching byA.niger. Aung and Ting [25] also showed that in bioleaching of spent fluid catalytic cracking catalyst, bioleaching had 2.7%-20% higher metal extraction efficiency than chemical leaching with commercial organic acids at the same concentration. These results affirm that secondary metabolite should be included in the bioleaching process [26]. However, the differences in metal extraction yield between bioleaching and mix-organic acids leaching show that acidolysis of bio-produced organic acids exhibits the most important mechanism in the bioleaching process. Furthermore, according to the profiles of gluconic acid and extracted metals, it can be concluded that the gluconic acid offers the main contribution to the metal extraction. In the case of control medium (without fungal spores), pH decreased to 5.1 from 5.5 at the end of the leaching period and small amount of metal ions (3.5% Fe, 4.2%Zn, 3.2% As, 5.2% Cu and 3.2% Al) was recovered.
3.6 Variation in biosorption behavior
Table 7 depicts the results of biosorption of metal ions during bioleaching. It is noteworthy that less biosorption occurs when ore and biomass are present in mixed organic acid, strategy 2. Results of Al and Fe are in accordance to Yanget al. [27] but those of Zn,Cu and As are different. In the case of strategy 1, biosorption is more then strategy 2 but less then strategy 3.The order for bio-sorption of metal ions in all strategies is Al>Fe>Cu=Zn>As. Less biosorption of metal ions by fungus in the presence of ore may be due to the competition of ore particles for available binding sites.Tang and Valix [28] reported that biosorption of nickel and cobalt by gangue minerals showed a potential inhibition effect on the metal dissolution from laterite minerals.It is assumed that similar phenomenon occurs in our study. A more detailed study will be carried out in future.
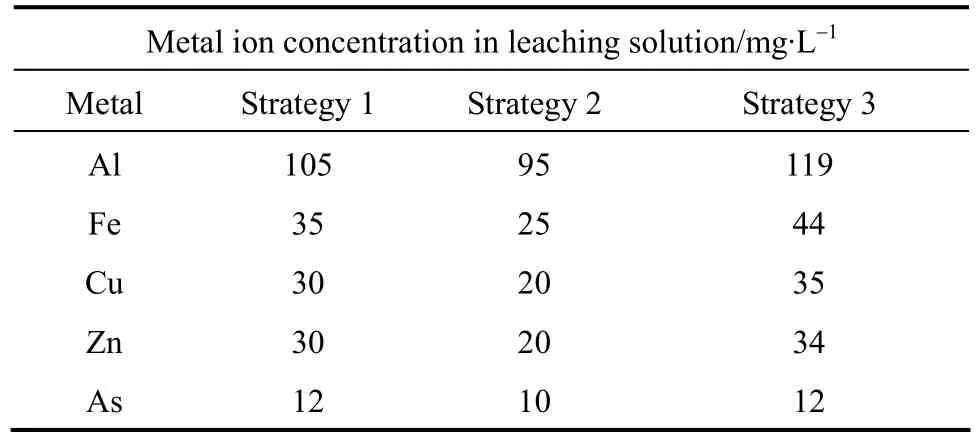
Table 7 Variation in biosorption behavior under different conditions
4 CONCLUSIONS
The optimization criterion is defined as the maximum dissolution percent of metals. pH 5.5, particle size 180 μm, pulp density 15%, initial inoculums size of 3×107spores per ml and residence time of 20 days are recommended for optimal bioleaching conditions.Under the optimum condition, 63% Fe, 68% Zn, 60%As, 79% Cu and 54% Al were leached out withA.nigerstrain KBS4. The gluconic acid offers the main contribution to the metal extraction. Bioleaching gives a relatively higher extraction yield for Fe, Zn, As, Cu and Al than mix-organic acids leaching (48% Fe, 50%Zn, 35% As, 68% Cu and 2% Al). Thus the secondary metabolites should be included in the bioleaching process. Biosorption of metal ions by fungal biomass may occur during the bioleaching process but the bio-sorption does not hinder the removal of metal ions.
1 Mulligan, C.N., Galvez-Cloutier, R., “Bioleaching of copper mining residues byAspergillus niger”,Water Sci.Technol., 41, 255-262(2000).
2 Valix, M., Usai, F., Malik, R., “Fungal bioleaching of low-grade laterite ores”,Min.Eng., 14, 197-203 (2001).
3 Murr, L.E., Berry, V.K., “Direct observations of selective attachment of bacteria on low grade sulfide ores and other mineral surfaces”,HydrometallurgyJ., 2, 11-24 (1976).
4 Sukla, L.B., Panchanadikar, V.V., Kar, R.N., “Microbial leaching of lateritic nickel ore”,World J.of Microbiol.and Biotech., 9, 255-257(1993).
5 Krebs, W., Brombacher, C., Bosshard, P.P., Bachofen, R., Brandl, H.,“Microbial recovery of metals from solids”,FEMS Microbiol.Rev.,20, 605-617 (1997).
6 Bosecker, K., “Bioleaching: metal solubilization by microorganisms”,FEMS Microb.Rev., 20, 591-604 (1997).
7 Wenberg, G.M., Erbisch, F.H., Volin, M.E., “Leaching of copper by fungi”,Mining Eng., 23, 207-212 (1971).
8 Tzeferis, P.G., Agatzini, S., Nerantzis, E.T., “Mineral leaching of non-sulphide nickel ores using heterotrophic micro-organisms”,Lett.Appl.Microbiol., 18, 209-213 (1994).
9 Bosshard, P.B., Bachofen, R., Brandl, H., “Metal leaching of fly ash from municipal waste incineration byAspergillus niger”,Environ.Sci.Technol., 30, 3066-3070 (1996).
10 Tasa, A., Vourinen, A., Garcia, O., Tuovinen, O., “Biologically enhanced dissolution of pyrite rich black shale concentrate”,J.Environ.Sci.Health, 32, 2683-2695 (1997).
11 ?opur, M., ?zmetin, C., ?zmetin, E., Kocakerim, M.M., “Optimization study of the leaching of roasted zinc sulphide concentrate with sulphuric acid solutions”,Chem.Eng.Process., 43, 1007-1014(2003).
12 Wei, J.C., Handbook of Fungal Identification, Shanghai Scientific and Technical Publisher, Shanghai (1979).
13 White, T. J., Bruns, T., Lee, S., Talor, J., “Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics”, PCR Protocols: A Guide to Methods and Applications, In Innis, M.A.,Gelfand, D.H., Sninsky, J.J., White T.J., Eds., Academic, San Diego,315-322 (1990).
14 Thompson, J. D., Gibson, T. J., Plewniak, F., Jeanmougin, F., Higgins, D. G., “The Clustal X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools”,Nucleic Acids Research, 24, 4876-4882 (1997).
15 Tamura, K., Dudley, J., Nei, M., Kumar, S., “Molecular evolutionary genetics analysis (MEGA) software version 4.0”,Molecular Biology and Evolution, 24, 1596-1599 (2007).
16 Safarzadeh, M.S., Moradkhani, D., Ilkhchi, M.O., Golshan, N.H.,“Determination of the optimum conditions for the leaching of Cd-Ni residues from electrolytic zinc plant using statistical design of experiments”,Sep.Purif.Technol., 58, 367-376 (2008).
17 Xu, T.J., Ting, Y.P., “Fungal bioleaching of incineration fly ash:metal extraction and modeling growth kinetics”,Enzyme Microb.Technol., 44, 323-328 (2009).
18 Sayer, J.A., Gadd, G.M., “Binding of cobalt and zinc by organic acids and culture filtrates ofAspergillus nigergrown in the absence or presence of insoluble cobalt or zinc phosphate”,Mycol.Res., 105,1261-1267 (2001).
19 Mulligan, C.N., Kamali, M., Gibbs, B.F., “Bioleaching of heavy metals from low-grade mining ore usingAspergillus niger”,J.Hazard.Mater., 110, 77-84 (2004).
20 Elliott, H.A., Shastri, N.L., “Extractive decontamination of metal-polluted soils using oxalate”,Water,Air,Soil Pollut., 110,335-346 (1999).
21 Karaffa, L., Kubicek, C.P., “Aspergillus nigercitric acid accumulation: do we understand this well working black box?”,Appl.Microbiol.Biotechnol., 61, 189 (2003).
22 Wu, H.Y., Ting, Y.P., “Metal extraction from municipal solid waste(MSW) incinerator fly ash-chemical leaching and fungal bioleaching”,Enzyme Microb.Technol., 38, 839 (2006).
23 Yang, J., Wang, Q.H., Wang, Q., Wu, T.J., “Comparisons of one-step and two-step bioleaching for heavy metals removed from municipal solid waste incineration fly ash”,Environ.Eng.Sci., 5, 783-789(2008).
24 Castro, I.M., Fietto, J.L.R., Vieira, R.X., Trópia, M.J.M., Campos,L.M.M., Paniago, E.B., Branda, O, R.L., “Bioleaching of zinc and nickel from silicates usingAspergillus nigercultures”,Hydrometallurgy, 57, 39-49 (2000).
25 Aung, K.M.M., Ting, Y.P., “Bioleaching of spent fluid catalytic cracking catalyst usingAspergillus niger”,J.Biotechnol., 116,159-170 (2005).
26 Schinner, F., Burgstaller, W., “Extraction of zinc from industrial waste by aPenicilliumsp.”,Appl.Environ.Microbiol., 55, 1153-1156(1989).
27 Yang, J., Wang, Q., Luo, Q., Wang, Q., Wu, T., “Biosorption behavior of heavy metals in bioleaching process of MSWI fly ash byAspergillus niger”,Biochem.Eng.J., 46, 294-299 (2009).
28 Tang, J., Valix, M., “Leaching of low-grade nickel ores by fungi metabolic acids”, In: ECI Conference on Separations Technology VI:New Perspectives on Very Large-Scale Operations, Kingfisher Resort, Fraser Island, Queensland, Australia (2006).
 Chinese Journal of Chemical Engineering2012年5期
Chinese Journal of Chemical Engineering2012年5期
- Chinese Journal of Chemical Engineering的其它文章
- Adsorption and Desorption of Praseodymium (III) from Aqueous Solution Using D72 Resin*
- Reactive Distillation for Producing n-Butyl Acetate: Experiment and Simulation
- Adsorption of Chlortetracycline from Water by Rectories*
- Optimization of Fermentation Media for Enhancing Nitrite-oxidizing Activity by Artificial Neural Network Coupling Genetic Algorithm*
- Effect of Propanoic Acid on Ethanol Fermentation by Saccharomyces cerevisiae in an Ethanol-Methane Coupled Fermentation Process*
- Numerical Study on Laminar Burning Velocity and Flame Stability of Premixed Methane/Ethylene/Air Flames*
