SHANGHAI ARCHIVES OF PSYCHIATRY INSTRUCTIONS TO AUTHORS
SHANGHAI ARCHIVES OF PSYCHIATRY INSTRUCTIONS TO AUTHORS
(February 2012)
The Shanghai Archives of Psychiatry is a general psychiatry journal published bimonthly by the Shanghai Mental Health Center of Shanghai Jiao Tong University Medical Center. Starting publication in 1959, it was China’s first specialty psychiatry journal and is currently one of the core psychiatry journals published in China. Starting with the first issue of 2012 all the content in the Shanghai Archives of Psychiatry will be published in English, though there will continue to be Chinese-language translations of the abstracts of published research papers. The Shanghai Archives of Psychiatry considers manuscripts on the full range of topics relevant to mental health in China and elsewhere, including research in the basic neurosciences, clinical practice, epidemiology, and health services. We welcome original papers on new research and secondary analyses that report on new aspects of high-quality studies that have been published previously. We also consider meta-analyses, papers on biostatistical and methodological issues relevant to psychiatry, commentaries and letters about previously published research, and forum pieces that discuss different viewpoints on controversial issues of interest to mental health professionals. The Shanghai Archives of Psychiatry is an open-access journal, doi versions of all articles that are accepted are immediately placed on our website and can be downloaded free of charge from our website (www.saponline.org). If authors have questions about a potential submission that are not covered in the following instructions, they should contact the editorial staffat shtougao3296@yahoo.com.cn.
1.TYPES OF MANUSCRIPTS
1.1 Research articles
As described below in section 3, the structure and content of research articles varies somewhat based on the topic. Generally speaking the main text (including introduction, methods, results and discussion) should be under 4 500 words, and there should be no more than 5 tables or figures and less than 30 references.
1.2 Reviews
These are comprehensive reports on the current state of knowledge about a topic of current theoretical, clinical or public health significance. We are particularly interested in reviews that summarize and interpret the research or clinical practices in China and put it in its international context. Transparency about the material included in reviews is essential, so all reviews should include brief sections entitled ‘Search strategy and selection criteria’ stating the sources of the material covered and the criteria used to include or exclude studies. Reviews should be under 5 000 words and have at least 50 references. Authors interested in preparing reviews should contact the editors BEFORE writing the review to ensure that the topic will be of interest to our readership.
1.3 Commentaries
These are detailed discussions about research articles published in the Shanghai Archives of Psychiatry by Chinese and international experts who were not involved in the original research. They can consider the methodological issues raised by the original article, the implications of the report, or provide the details of other on-going research projects related to the original report. Commentaries should be under 1 500 words in length, include no more than 2 tables or figures and have under 15 references.
1.4 Forum
These papers present a particular point of view on a controversial topic in mental health. They can involve scientific, clinical or policy issues. The manuscripts should be under 1 500 words in length and have less than 20 references. In each issue two or more papers with varying viewpoints on a topic of interest by Chinese and international experts will be placed in the ‘Forum’ section of the journal. Authors interested in preparing a manuscript for the Forum section should contact the editors BEFORE they prepare the paper; if we think it of sufficient interest we will recruit other experts to write a papers with alternative viewpoints about the topic.
1.5 Correspondence
Readers are encouraged to write letters of less than 700 words with no more than 5 references and no more than one table or figure. Letters will usually discuss some aspect of the research, commentaries, forums or other content previously published in the journal, but they can also briefly present data from studies or raise other areas of interest to readers.
1.6 Case reports
These are single cases or case series that highlight an interesting or important clinical or theoretical issue. We are particularly interested in case reports that highlight specific characteristics of patients in China or specific aspects of the Chinese mental health care system. Case reports should be less than 1000 words in length, include no more than one table or figure and have less than 10 references.
2.GENERAL INFORMATION FOR PREPARATION OF ALL TYPES OF MANUSCRIPTS
Writing in all manuscripts should be clear and concise with no unnecessary use of technical terms or abbreviations, making the manuscripts accessible to all mental health professionals not only to those who work in a particular field. This section lists general issues relevant for all manuscripts; the next section provides more detailed information about preparing research articles.
2.1 Language of submission
Our preference is to receive manuscripts in English but manuscripts can be submitted in both English and Chinese.All Chinese-language manuscripts will be translated and edited by the Journal office. Research articles will also include a Chinese-language abstract so once the final English version of research articles are approved the editorial office will prepare a Chinese abstract. Starting in 2013 all manuscripts must be submitted in English.
2.2 Formatting of the manuscript
Manuscripts should be submitted in Word format (i.e., ‘manuscript.doc’ or ‘manuscript.docx’). Single spacing should be used in the text but main sections and subsections within the text should be divided by empty lines to facilitate reading. The main text should use 12-point Calibri typeface, but smaller gauge Calibri typeface can be used in tables if necessary. In Chinese-language manuscripts standard simplified Chinese characters in number 5 ‘songti’ ( ’宋體’) typeface should be used. The typeface used to distinguish primary, secondary and tertiary levels within the manuscript should be different and used consistently throughout the text. The heading of a main section or a subsection (level 1 and level 2) should be in bold and written on a separate line; the heading for a sub-sub heading(i.e., level 3 heading) should be in italic, not using bold, and on a separate line. The title page, abstract, main text,references, and each figure and table should begin on a separate page in the manuscript. Whenever possible figures and tables should be placed at the end of the main document (after the references) rather than being submitted as separate documents. All pages of the manuscript should be sequentially number starting 1,2,3 and so forth.All scientific terms should follow the terms specified by the terminology committee of the National Committee of Scientific Terminology. (For authors submitting English-language manuscripts who may not have access to this reference the editorial office will edit the manuscript to conform to these specifications.)
2.3 Title
Many electronic searches are based on titles so the title should clearly describe the main content of the paper.Avoid abbreviations and empty works (e.g., “research study”, “discussion of”) in the title.
2.4 Authors
Only persons who make substantial contribution to the work should be listed. This should generally be six or fewer. Do not list a ‘research group’ as the author, though a few authors could be the representatives of a ‘research group’, the members of which are listed in the acknowledgment section. The order of the authors and the person assigned as the corresponding author need to be determined at the time of submission, they cannot be changed later. The journal can occasionally accept two ‘co-first authors’ or two ‘co-corresponding authors’ but the reason for this needs to be explained in the cover letter for the manuscript to the editor. We will not accept more than two first authors or more than two corresponding authors.
2.5 Institutional affiliations
The institution where the first author worked at the time of completing the work reported in the manuscript should be the primary institutional affiliation reported in the manuscript. If the first author currently works at a different institution or if the first author was a trainee from another institution at the time of completing the reported work the current institution or the home institution can also be noted as secondary institutional affiliations of the first author, but should not be identified as the primary institution for the first author of the manuscript.
2.6 Abstracts and summaries
Research articles should have structured abstracts of less than 350 words (see Section 3.2 below). Reviews should have unstructured summaries of less than 150 words that highlight the main points of the manuscript. Case reports should have an unstructured summary of less than 100 words. Commentaries and Forums will not have abstracts or summaries.
2.7 References
Only references directly relevant to the content of the manuscript that the authors have read in FULL should be listed; copying references from other papers or referring to articles only seen in abstract is not acceptable. Authors must carefully check the information in the reference against the original to ensure accuracy. Numbered references should appear sequentially in the text. In the text the number or numbers of the reference(s) cited should appear as superscripts in square brackets (for example;[2-3,6]) . If a reference only appears in a table or a figure the sequence number for the reference should be the number that would be appropriate for text that appears at the point the table or figure is first mentioned in the text. Unpublished materials should not appear in the reference list unless there is a doi number already specified that readers can use to identify the material. Chinese-language references and those in other non-English languages should be translated into English and ‘(in Chinese)’ or ‘(in other language)’should be placed at the end of the reference. If the original article in the foreign language includes an English title this should be employed in the reference rather than a re-translation of the original title.
The Shanghai Archives of Psychiatry employs the standard method for referencing recommended by International Committee of Medical Journal Editors (ICMJE) Uniform Requirements for Manuscripts Submitted to Biomedical Journals (http://www.nlm.nih.gov/bsd/uniform_requirements.html). For English-language references, authors’names are written with the family name followed by one or more capitalized initials for the first name(s); different authors’ names are separated by commas. In Chinese manuscripts Chinese references are also written in this general format but the full Chinese name of authors is provided and the names of Chinese journals are written out in full. For example:
Loo CK, Mitchell PB. A review of the efficacy of transcranial magnetic stimulation (TMS) treatment for depression,and current and future strategies to optimize efficacy. J Affect Disord, 2005, 88(3): 255-267.
王繼軍, 江開達, 徐一峰. 經顱磁刺激治療抑郁癥的機制和臨床應用現(xiàn)狀. 上海精神醫(yī)學, 2008, 20(1): 49-52.
If a reference has more than six authors, the first six are listed followed by ‘et al’ in English manuscripts and in English references in Chinese-language manuscripts, and by ‘等’in Chinese-language references in Chinese-language manuscripts. Authors should not simply copy references from other publications, because these often employ different formats. Authors should be particularly careful in writing the abbreviated titles of English-language journals;the correct abbreviation for journal titles are provided at the following website: http://www.nlm.nih.gov/tsd/serials/terms_cond.html. Authors should check the website (http://www.nlm.nih.gov/bsd/uniform_requirements.html) for examples of how to compose references in different types of situations. If in doubt about how to compose a reference authors could also check reference lists from articles in recent issues of the Shanghai Archives of Psychiatry.
2.8 Figures
Figures should be self-explanatory and abbreviations used should be explained in footnotes. If at all possible figures should be inserted in the Word document with the main manuscript rather than being supplied as a separate document in a different format. Figures are numbered sequentially as they appear in the text, if there is only one figure it is labeled ‘Figure 1’. The title for a figure should appear above the figure. All figures will be edited prior to publication so, if the manuscript is accepted, authors will subsequently be expected to submit versions of their figures that can be edited.
2.9 Tables
Tables should be composed using the ‘Tables’ function in WORD rather than importing it from Excel, SPSS or some other format. The title and footnotes should be part of the table, not separate from the table; they are the top and bottom lines of the WORD table that have merged all cells across the width of the table, allowing the title and footnote content to be moved as the table is moved or re-formatted. If a table has many columns it can be presented on a horizontally oriented page, but it should never be wider than the maximum 150 characters (including spaces).All tables should be numbered sequentially as they appear in the text; if there is only one table, it is labeled ‘Table 1.’Tables should be self explanatory so that readers can understand the content of the table without reference to the text. All abbreviations used in tables should be explained in footnotes. All tables should indicate the actual numbers of cases on which the results are based (i.e., do not present percents or means without specifying the denominator on which they are based in a parenthesis beside the statistic, in the column title, or in a footnote). It is generally better to present specific p-values in separate columns of a table rather than categorizing p-values a “p<0.05, p<0.01,p<0.001”. P-values smaller than 0.001 should be written as ‘<0.001’ NOT as ‘0.000’. The number of significant digits for the figures within a column should be the same (that is, write ‘0.030, 0.300, 0.311’ NOT ‘0.03, 0.3, and 0.311’). For Tables that are reprints or adapted versions of previously published tables, the original publication of the table should be cited in the footnote (and in the reference list).
2.10 Units of measurement
The written versions of different units of measurement used in manuscripts should be based on Use of Standard Units of Measurement in Medicine published in Chinese in 1991 by the Chinese Medical Association. (For authors submitting English-language manuscripts who may not have access to this reference, the editorial office will edit the manuscript to conform to these specifications.) When preceded by a number, units of time use the following symbols:‘d’ (days), ‘h’ (hours), ‘min’ (minutes), ‘s’ (seconds). There is no plural form for the symbols representing units of measurement; for example, write ‘23 min’ NOT ’23 mins’. A unit of measurement may combine physical and nonphysical entities; for example, ‘times/min’, ‘persons/year’, and so forth. If there are more than one dividing entity in a unit of measurement it should be written using units to the power of -1; for example, ‘ng·kg-1·min-1’ NOT ‘ng/kg/min’.The units for standard deviations (SD) of a parameter do not need to be repeated if the standard deviation is reported with the mean in the mean (SD) format; for example, ‘4.5 (1.2) times/d’ NOT ‘4.5 times/d (1.2 times/d)’.
2.11 Numerals
The method of expressing numbers is based on the 1995 publication (in Chinese) Rules for the Use of Numbers in Publications. (For authors submitting English-language manuscripts who may not have access to this reference the editorial office will edit the manuscript to conform to these specifications.) Dates should always be based on the Gregorian calendar. All numbers are written as Arabic numerals. For numbers with many digits, every three digits before and after the decimal is separated by a space equal to half the width of a typed letter; for example‘5 678 430.015 43’. Decimal numbers must always have a ‘0’ preceding the decimal; for example, ‘0.143’ NOT ‘.143’.When using the percent symbol in a sequence or range the symbol should be repeated; for example, ‘10%~20%’ NOT’10~20%’. But when using the percent for a mean and standard deviation when written together in the mean (SD)format the % should follow the parenthesis; for example ‘54.2 (12.4)%’ NOT ’54.2% (12.4%)’. When multiplying units of length, the unit of measurement should be repeated with each number; for example, ‘4 cm × 3 cm × 5 cm’ NOT‘4×3×5 cm3’.
2.12 Statistics
The symbols for the t-test (t), F-test (F), Chi Square test (χ2), correlation coefficient (r), degrees of freedom (df),probability (p) and other statistical tests or measures should all be written in italics. Do NOT use the ± symbol to identify standard deviations as it implies symmetry around the mean that may not be the case; the standard deviation of a mean should usually be expressed as a number in parenthesis following a mean value (for example, ‘75.2 (13.5)’)or in a separate column in a table beside a column with the corresponding means. Do not report p-values without indicating the value for the corresponding statistical test and (in most cases) the degrees of freedom. Degrees of freedom can be written in three ways: ‘t=1.98, df=32’, ‘t(30)=1.98’ or ‘t30=1.98’. P-values should be preceded by a ‘0’and written to three significant digits; for example, ‘p=0.120’, NOT ‘p=.120’, and NOT ‘p=0.12’. If the p-value is less than 0.001 it should be written ‘p<0.001’, NOT as ‘p=0.000’. For results that meet predetermined criteria of statistical significance, the statement in the text should be ‘…the result was statistically significant..’, NOT ‘..the result was significant’, and NOT ‘…the result was very significant’.
2.13 Abbreviations
Abbreviations should not appear in the title and should be used sparingly in the text. Authors should limit the use of abbreviations, particularly in the abstract; in cases where a term only occurs once or twice in the abstract or in the main text there is no need to include the abbreviation. In Chinese-language text the first appearance of any abbreviations must give the full term in Chinese followed by a parentheses with the FULL term in English followed by a comma and then the abbreviation that will be used subsequently; for example, ‘簡明精神病評定量表 (Brief Psychiatric Rating Scale, BPRS)’. In English-language text in the first instance the full term is written out followed by a parenthesis with the abbreviation that will be used subsequently; for example, ‘Hamilton Depression Rating Scale(HAMD)’. Some readers will not be familiar with abbreviations commonly used in psychiatric publications such as‘DSM-IV’, ‘WHO’, ‘ICD-10’, ‘BPRS’ and so forth; for this reason ALL abbreviations need to be written out in their first appearance in the text. If an abbreviation appears in the abstract and in the main text it needs to be written out fully the first time it appears BOTH in the abstract and in the main text; thus if it only appears once or twice in the abstract it would be better to use the full term (without the abbreviation) in the abstract and reserve the use of the abbreviation for the main text. Abbreviations that appear in tables or figures must be explained in the footnote of the table or figure, even if the full name is provided in the main text.
2.14 Drug names
The Chinese names of drugs should be based on the most recent official Chinese pharmacopeia or the drug list of the Chinese Ministry of Health pharmacopeia committee. In English only generic drug names should be used.
3.SPECIFIC INFORMATION ABOUT PREPARATION OF RESEARCH ARTICLES
All of the issues raised in the previous section are relevant for the preparation of manuscripts reporting research results. The following points provide additional information specific to the preparation of research articles.
3.1 Information that should be included in research articles
The information readers need to know about the conduct of a research study in order to evaluate the internal and external validity of the study (and, thus, decide whether or not the results are important) varies depending on the type of study. There are, therefore, different internationally accepted guidelines and checklists describing the information that should be included when reporting different types of studies. Researchers should consider these guidelines when designing their studies (to ensure that all the necessary information is collected) and when preparing their research reports. Reviewers for Shanghai Archives of Psychiatry will consider these guidelines when assessing a manuscript, so authors are strongly advised to be familiar with the guidelines.
· randomized controlled trials: CONSORT guidelines (http://www.consort-statement.org)
· observational studies (cohort, case-control or cross-sectional designs): STROBE (http://www.strobe-statement.org)
· genetic association studies: STREGA guidelines (PLoS Med 6(2): e1000022. doi:10.1371/journal.pmed.1000022)
· studies of diagnostic accuracy: STARD guidelines (http://www.consortstatement.-org/stardstatement.htm)
· systematic reviews and meta-analyses: PRISMA guidelines (http://www.prisma-statement.org)
· meta-analyses of observational studies in epidemiology: MOOSE guidelines (http://www.consortstatementorg/Initiatives/MOOSE/moose.pdf)
3.2 Overall structure of manuscripts for research articles
All authors of biomedical research reports should be familiar with the widely accepted requirements of reporting research. The most authoritative and detailed description of these requirements is the Uniform Requirements for Manuscripts Submitted to Biomedical Journals (www.ICMJE.org). Our journal abides by these requirements.Manuscripts for Research Articles submitted to the Shanghai Archives of Psychiatry should organized as follows as shown to the left.
All manuscripts need to have these sections unless there are no acknowledgments, figures, tables or appendices,in which case the corresponding section is omitted. The main text (Introduction, Methods, Results and Discussion) can be further divided into additional numbered level-two subsections (e.g., 2.1, 2.2, 2.3..) or level-three sub-subsections(such as 4.2.1, 4.2.2, 4.2.3…) if needed, but the subsections and sub-subsections in each major section (Introduction,Methods, Results and Discussion) should be limited to 5 or 6 at most.
Title Page (preferably limited to one page)
(starting on a new page)
1.Introduction (starting on a new page)
2.Methods
2.1 Biostatistical methods
3.Results
4.Discussion
4.1 Main Findings
4.2 Limitations
4.3 Implications
Acknowledgments
Conflict of interest statement
Funding
starting on a new page)
Figures (each figure on a new page)
Tables (each table starting on a new page)
Appendices (each appendix starting on a new page)
3.3 Title page (If possible limit it to a single page)
Includes title, authors, institutional affiliations of the authors, and the name and contact information for the corresponding author. Wherever possible titles should include the design employed in the study (e.g., Randomized Controlled Study; Case-control Study; Cohort Study, Cross-sectional Study; Meta-analysis, etc.). In Chinese-language manuscript the formal English-language name of the participating institutions must be provided to prevent inaccurate translation of these Chinese location names. Only persons who make a substantial intellectual contribution to the study should be named authors; individuals who organized funding or run the center where the study is conducted but did not directly participate in the study should NOT be named authors (though they can be mentioned in the acknowledgements). The corresponding author is not, as is commonly the case in China, an honorary position for the primary authors’ supervisor or superior but, rather, the individual who will be responsible for corresponding with the Journal in the process of revising the manuscript; in most cases this is the first author himself or herself.
3.4 Abstract (on a separate page)
We use structured abstracts for Research Articles that include the following sections:
Background (current state of knowledge about subject and/or why the current study is important);
Hypothesis/Aim (specific question that will be resolved by the study);
Method (sample selection and procedures);
Results (primary and secondary outcomes);
Interpretation (implication of the findings);
Trial Registration Number (if available for randomized controlled trials);
(3-6 words selected from MeSH headings).
Abstracts should be written in the third person and be under 350 words in length. Most readers only scan the abstracts and many electronic search systems only provide abstracts so the abstract needs to prove sufficient information for readers to determine what the problem was, what was done, what was found and the likely validity and generalizability of the results. All RCTs and case-control studies need to have a specific main hypothesis stated;other types of studies may have a hypothesis or an aim. Any statistical results provided in the abstract should include the test statistic, the degrees of freedom, and the p-value with three significant digits; do not present a p-value without the corresponding test statistic. Important values that are main findings should be presented with 95%confidence intervals. Do not, however, overburden the results section of the abstract with numbers and statistics;present the main results with supporting statistics but limit the use of statistics when describing secondary results,preferably describing them with text.
Following international standards we strongly recommend that researchers who plan to conduct randomized controlled clinical intervention trials register the studies on an international registry prior to starting patient enrollment for the study. The registration number for the study provided by the trial registry should then be put in the abstract following the discussion section. One such registry is http://prsinfo.clinicaltrials.gov/, but any registry that participates in the WHO Clinical Trail Registry Platform (http://www.who.int/ictrp/data_set/en/index1.htlm) is acceptable. Most important international medical journals require registration of RCTs as a condition of acceptance for publication. Starting in January 2013, all manuscripts about clinical RCTs submitted to the Shanghai Archives of Psychiatry will need to have registry numbers.
The abstract is followed by “Key Words”—a list of 3-5 words and phrases (separated by semicolons) in which the first letter of the word or phrase is capitalized. This list should be placed on the same page as the abstract just below the abstract. These words and phrases will be used for the electronic searches of the manuscript so they should reflect the core content of the manuscript. These terms are NOT just any words the authors think appropriate,authors should first select the relevant English-language terms from the most recent version of MeSH terms of the Index Medicus published by the National Library of Medicine in the United States (http://www.nlm.nih.gov/mesh/),and then provide Chinese-language translations of the terms in the Key Words section of the Chinese abstract.
A Chinese-language abstract will be prepared by the editorial office after the final version of the English-language manuscript is approved and then placed at the end of the printed version of the English-language paper.
3.5 Introduction
The introduction must provide a clear overview of the current state of knowledge or research both in China and internationally. This should not be an exhaustive list of previous work but, rather, a summary of previous work emphasizing the theoretical, methodological, clinical or other problems that remain to be clarified or resolved. If there are existing systematic reviews or meta-analyses about the subject, these need to be cited. The authors must then indicate how the current research project will add to the sum of knowledge about the subject. For all RCTs and case-control studies the presumed theoretical relationship of the variables considered in the current project need to be specified and, based on this theoretical model, a main hypothesis should be presented that clearly specifies the main outcome variable and the methods that will be used to assess the outcome variable. Up to three secondary hypotheses can also be specified.
3.6 Methods
Methods need to be described in sufficient detail such that a person knowledgeable in the field could replicate the study and such that readers can assess the reliability of the findings (i.e., internal validity) and the generalizability of the results (i.e., external validity). Research reports in China rarely provide this level of detail, so the methods section of submitted manuscripts will generally need to be longer than is typical for Chinese journals. The reporting guidelines mentioned above (in section 3.1) indicate the types of information needed for each type of study. Authors need to provide details about the source population for the study; about the procedures for selecting (sampling)individuals from the source population; about the randomization procedure (if there is one); about the diagnostic,evaluative and therapeutic procedures undertaken; and about the method used to assess the outcomes or other variables of interest in the study. In addition, the method section should include the following information:
· A brief statement about the sample size needed to address the primary hypothesis should be included. (In most cases a detail computation of sample size is not needed).
· The numbers of individuals involved at each step of the project—selection, follow-up and outcome—should be specified in a ‘subject flowchart’ (see ‘Figures’ below) .
· The basic characteristics of the final sample should be described. (In some cases this information can be put in the beginning of the results section.) If a substantial portion (10% or more) of potential subjects do not enter the study, the basic characteristics of those who are and are not included in the study should be compared. Similarly, if a substantial proportion of subjects enrolled in the study do not complete the study the characteristics of those who do and do not complete the study should be compared.
· The provenance (i.e., original source), source of the translated version, reliability, and validity of any questionnaires or other evaluative instruments used in the study need to be specified. If the study was done in China but the reliability and validity parameters of the employed scales are not available for China (a common problem) this should be stated and the current report should provide values (based on data collected in the study) that help readers assess these parameters (e.g., alpha values for the total score and subscale scores of scales that are used to assess outcomes).
· The origin of numerical values employed in the analysis and the theoretical range of such variables need to be specified. For example, if the total score of a scale is employed as a primary outcome measure the text could read: “The total score was the primary outcome measure employed; this was the sum of the 17 items in the scale (which were each scored 1-3) so the potential range of values of the total score was 17~51.”This information is needed so readers can understand the numerical values presented in the results and the relative importance of a reported change in mean scores.
· The methods of training persons who conduct the evaluations and their inter-rater reliability should be reported.
· The details of any novel laboratory procedures should be specified.
· The process of obtaining informed consent from subjects should be described and institutional review board (or committee) that provided ethical approval for the study should be identified.
For all research papers a figure presenting the flowchart for the enrollment and follow-up of subjects in the study needs to be prepared and placed after the references and before the tables in the manuscript. The figure should show the sampling frame from which the subjects were selected, reasons for non-inclusions and the numbers and reasons subjects drop out during the course of the study. Two examples are as follows:

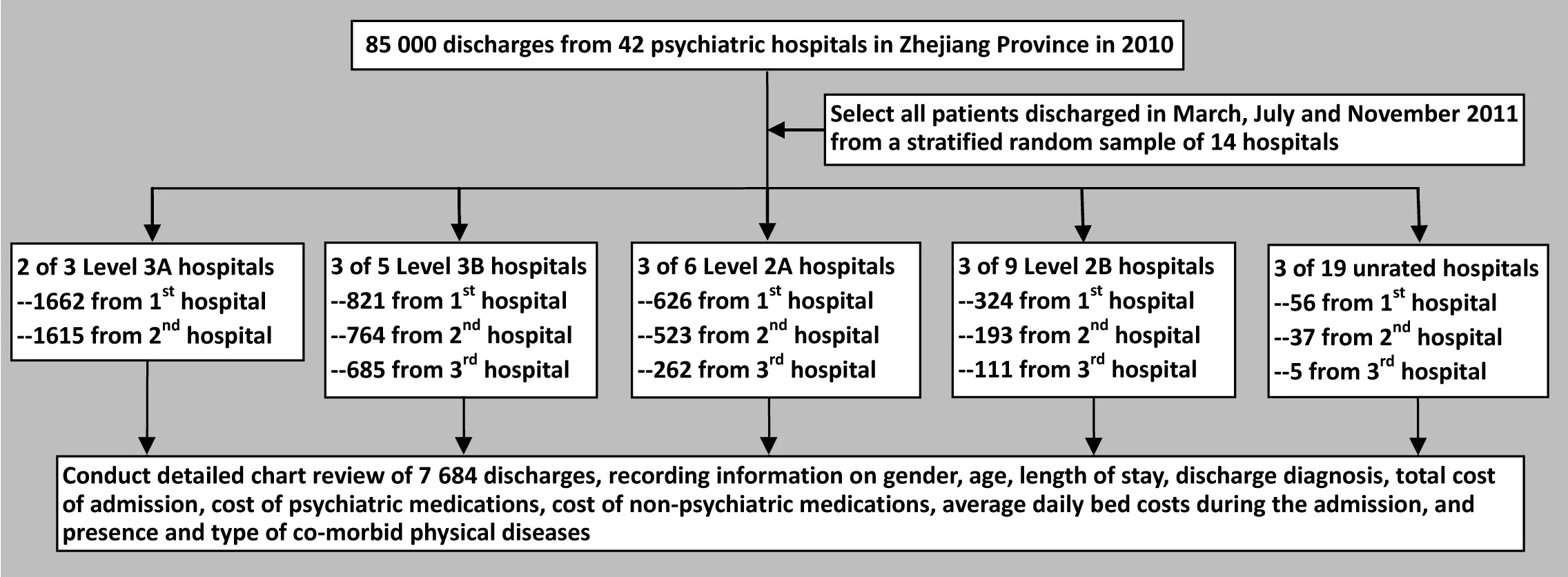
3.6.1 Statistical methods
All the statistical tests employed in the primary and the secondary analyses should be specified and the software employed to conduct the analyses should also be specified. Authors need to ensure that the statistical tests employed are appropriate to the design and to the type of data available; avoid using chi square tests for ranked data, unpaired tests when paired tests are appropriate, and so forth. If the sample is weighted in the analysis the method of weighting the sample needs to be described. If any novel or uncommon statistical procedures are employed these need to be described in detail and references that describe the use of these methods should be provided. In general for intervention studies intention-to-treat analyses (i.e., including data on ALL subjects who entered the study) are preferred to analyses that are limited to the subjects who complete the study. For intervention studies the main outcome measure should usually be the absolute difference between groups with a 95% confidence interval for the difference. When comparing multiple groups within a sample (e.g., mean total score of an outcome measure for four different treatment groups) multiple-comparison tests need to be used; it is not appropriate to simply report the overall statistic and assume all the groups are different from each other or to compute multiple bivariate tests on each pair of results.
The methods for categorizing outcome variables and other variables used in the analyses should be specified.For example, if a scale is used to assess ‘clinical improvement’ the cut-off score (or % improvement from baseline)for determining ‘improvement’ should be specified. Similarly, if a continuous variable is dichotomized or divided into multiple categories in the analysis, the method for doing so should be specified; for example, “…in the logistic regression analysis family income level was divided into ‘high’ and ‘low’ using the median value for the total sample”.
3.7 Results
The results of the primary analysis and any secondary analyses of interest should be presented, but secondary analyses should be clearly distinguished from those that are based on a priori hypotheses. Simple statistical results with only a few data points can be presented in the text but detailed data is often best presented in tables or—if a figure provides a clearer presentation of the data—in a figure. Data provided in the tables or figures can be summarized in the text but they should NOT be duplicated in the text. Main outcomes (e.g., prevalence, rate of improvement, odds ratios, etc.) should be presented with 95% confidence intervals. All p-values presented should be accompanied by the value for the corresponding statistical test and, in most cases, the degrees of freedom (e.g.,χ2=0.95, df=2, p=0.301; t24=3.21, p=0.003). P-values should be presented with three significant figures (e.g., p=0.172);if the p-value is less than 0.001 it should be written as “p<0.001” not as “p=0.000”. The denominators for all percents and means presented should be specified unless it is clear from the text. Do not present new statistical methods in the results section, these should be described in the statistical section of the methods.
3.8 Discussion
The discussion section for ALL research papers should be subdivided into the following three subsections: Main findings, Limitations, and Implications.
3.8.1 Main findings
There should be a summary of the main findings as it relates to the original hypotheses. This is NOT a simple repetition of the results and does not usually need to repeat the numerical findings reported in the results. It is not appropriate to introduce new results that have not been reported in the results section. Avoid arriving at conclusions that are not justified by the results, such as assuming that a correlation identified in a cross-sectional study is a causal relationship. The relationship between existing evidence (prior studies) and the new findings of the current study should be clarified; but this should NOT be an exhaustive listing of all prior research, only prior work DIRECTLY relevant to the current results should be discussed.
3.8.2 Limitations
All factors that affect the accuracy of the findings should be mentioned and their potential affect on the study results discussed. Similarly factors that affect the representativeness of the findings and the generalizability of the results should be described and discussed.
3.8.3 Implications
The potential impact of the results on theoretical understanding of the condition, on clinical practice, and on the organization of health services should be described and future research that needs to be conducted to confirm or extend the results should be outlined. Do not claim to have ‘discovered’ something if the data is only preliminary or if researchers in other locations have been conducting similar studies. Be conservative in making recommendations for changes in policy or practice, particularly if this study is the first one with this specific result or if the result is based on retrospective or cross-sectional data. Avoid overstating the importance of the results by making unfounded inferences and avoid making non-specific (i.e., empty) recommendations based on the results like “clinicians should be aware of the identified risk factors” or “It is important to develop preventive programs for this problem”.
3.9 Acknowledgements
Individuals who provided practical or strategic support for the study and/or in the preparation of the manuscript but do not meet the requirement of authorship should be mentioned. Their specific contribution can be described(simply) (e.g., ‘assisted in the data collection’, ‘assisted in the analysis’, ‘provided valuable comments on a prior draft’, etc.). The lead author (or the corresponding author) must obtain the written consent of persons who are acknowledged (the editorial office may request this documentation during the review process). Institutions that supported the conduct of the study can also be mentioned, but the funding institution does not need to be mentioned because it is mentioned in a subsequent section.
3.10 Confict of interest statement
The presence of any financial or other conflict of interest by any of the named authors should be stated (and explained in the cover letter to the editors). If there is no conflict of interest of any of the authors this should be stated (e.g., ‘The authors report no conflict of interest related to this manuscript.’)
3.11 Funding
If the study was supported by one or more research funds, the title and number of the grant(s) and the name of the institution(s) that provide grants or financial support to the authors to conduct, analyze or write-up the study should be specified. The role of the funder in the design, implementation, analysis and write-up of the study needs to be indicated. If there is no specific funding agency for the study this section should still be included in the manuscript and the statement “This study received no external funding.” should be placed under the section heading.
3.12 References (starting on a separate page)
See section 2.7. As stated in that section, the Shanghai Archives of Psychiatry employs the standard method for referencing recommended by International Committee of Medical Journal Editors (ICMJE) Uniform Requirements for Manuscripts Submitted to Biomedical Journals available at (http://www.nlm.nih.gov/bsd/uniform_requirements.html). For examples of how to compose references in different types of situations look at this website. If in doubt about how to compose a reference authors could also check reference lists from articles in recent issues of the Shanghai Archives of Psychiatry.
3.13 Figures (each figure is on a separate page, not inserted in the text of the results section)
See section 2.8. As described in section 3.6, all research articles must have a figure that shows enrollment and follow-up of subjects during the study. This is usually ‘Figure 1’. Other sequentially numbered figures are placed on separate pages in the manuscript after this study flowchart (before the tables). Diagrams and figures must be clearly labeled and provided in a form that will allow the editorial staff to re-format them for publication in the Journal.
3.14 Tables (each table is on a separate page, not inserted in the text of the results section)
See section 2.9. Tables are numbered sequentially as they appear in the text. If there is only one table it is still labeled as ‘Table 1’. Authors should check recent issues of the Shanghai Archives of Psychiatry for examples of how to format a present tabular data.
3.15 Appendices (each appendix is put on a separate page)
In some circumstances appendices will be included in the print version of an article. Articles that describe the reliability and validity of a questionnaire or scale should usually have a copy of the final version of the scale in an appendix for the article. Details about laboratory methods or complex sampling strategies can also be placed in an appendix rather in the methods section.
4.SUBMISSION OF MANUSCRIPTS
Manuscripts can be submitted on our website (www.saponline.org) or by e-mail directly to the editorial office(shtougao3296@yahoo.com.cn). Starting in January 2013 we will only accept English-language manuscripts on our website. The following documents should be included as part of each submission:
·COVER LETTER TO THE EDITORS. This brief letter (in WORD format) to the editors provides a list of the documents submitted, the reason this manuscript is considered appropriate for the Shanghai Archives of Psychiatry, full contact information for the corresponding author, the first author and, if different from the corresponding author, the individual you has access to the data set used to write the paper and who conducted the statistical analyses described in the paper. The cover letter can also include any additional information the authors wish to communicate about the submission. This letter should be signed by the first author and the corresponding author (if different from the first author).
·MANUSCRIPT. The full manuscript in WORD (.doc or .docx) format. We strongly prefer to have all tables and figures inserted into a single document, not as separate documents.
·INSTITUTIONAL APPROVAL. [Only for Research Articles]A signed document from the first author’s institution needs to be submitted with manuscripts indicating that the manuscript has not been submitted elsewhere,that there is no conflict about the content of the manuscript among the authors, that the manuscript does not contain confidential data, and that the content is not plagiarized, fabricated or falsified. Chinese and English versions of this document can be downloaded from the www.saponline.org website or obtained from the editorial office (shtougao3296@yahoo.com.cn). Preferably the signed document is scanned and submitted as a pdf file along with the manuscript, but it can be separately submitted to the editorial office by fax (86(0)21-64685661).
·AUTHORS’ STATEMENTS. Signed ‘Authorship Statement, Copyright Transfer, Financial Disclosure, and Acknowledgment Permission’ forms from EACH author (one form for each author) should be submitted with the other documents. All authors must certify that the material in the manuscript has not been published elsewhere, that it is not currently being considered by another journal, and that it will not be submitted for consideration at another journal while under consideration by the Shanghai Archives of Psychiatry. All authors must also report on the form any financial or other potential conflicts of interest and indicate their level of participation in the preparation of the manuscript. Chinese and English versions of this document can be downloaded from the www.saponline.org website or obtained from the editorial office (shtougao3296@yahoo.com.cn). Preferably the signed documents are scanned and submitted together as pdf files along with the manuscript, but if this is difficult to do because of the wide dispersal of authors or some other reasons,these forms can also be submitted separately to the editorial office by e-mail or by fax (86(0)21-64685661).
·IRB APPROVAL. [Only for Research Articles]A copy of the document certifying Institutional Review Board approval (or its equivalent) for the study reported in the manuscript is needed. This approval needs to indicate that the committee responsible agreed that the study met internationally accepted standards for the protection of the rights and safety of human subjects. Preferably the approval document is scanned and submitted as a pdf file along with the manuscript, but it can also be separately submitted to the editorial office by fax (86(0)21-64685661). If no IRB approval was obtained for the reported study this needs to be explained in the cover letter to the editor.
·COPIES OF RELATED MANUSCRIPTS. If the authors (or other authors) have published or submitted a manuscript elsewhere on a similar topic using the same data set as the current manuscript, PDF or WORD files of these published papers and manuscripts need to be included with the submission so the editors can determine the amount of overlap between the submitted manuscript and previous publications.
·SUPPLEMENTAL INFORMATION. Any supplemental information the authors would like the editors and reviewers to consider in the assessment of the manuscript.
5.THE REVIEW PROCESS
5.1 Based on the agreement signed by all authors at the time of submission, authors are not permitted to submit the manuscript to other journals while it is under consideration by the Shanghai Archives of Psychiatry. This is a legal agreement that all medical journals take seriously; authors who submit articles to multiple journals and their institutions are identified and entered on lists of delinquent authors and institutions. If, however,the corresponding author receives formal notification from the editorial office that the manuscript has been rejected, the authors are then permitted to submit it elsewhere.
5.2 Manuscripts are initially reviewed by editorial staff before being sent out for review. Within 14 working days of receipt of the manuscript authors will be informed about whether or not the manuscript will be considered for the journal (this is NOT an acceptance of the paper).
5.3 Authors of papers that will be considered will be informed of the methodological and/or statistical revisions needed before the manuscript will be sent out to our content reviewers. If authors have difficulty making the revisions recommended the editorial office will do its best to assist the authors in making the necessary revisions. Authors who have not provided a revised version of the manuscript or corresponded with the editorial office about the manuscript for one month will be notified by e-mail and telephone that the manuscript will be dropped from consideration by the journal if a revision is not received within the next 10 days.
5.4 When a revised manuscript that satisfactorily addresses the issues raised by the editorial review is received it will be sent to two content reviewers for a blinded evaluation.
5.5 If both reviewers recommend rejection the manuscript will be rejected. If both reviewers recommend acceptance, the manuscript will be reviewed by the editorial management group. If there is disagreement from the two reviewers the manuscript will be sent to a third reviewer for an independent assessment; if the third reviewer recommends rejection it will be rejected, if the third reviewer recommend acceptance it will be sent to the editorial management group.
5.6 The editorial management group (including members of the editorial board) review manuscripts for which two reviewers recommend acceptance and make the final decision on rejection or conditional acceptance. In most cases this review process is completed within three months of receipt of the revised manuscript (following the initial review by the editorial office). If the review process takes longer than three months the editorial office will contact the corresponding author and tell him/her about the current status of the manuscript.
5.7 Authors of papers that are rejected will be sent the detailed reviews from the content experts.
5.8 Authors of conditionally accepted manuscripts will be asked to make additional revisions or additions to their manuscripts based on the comments of the reviewers and the editors. If necessary, editorial staff will assist authors make the requested revisions, which often require new, more sophisticated analyses of the data. Authors who have not provided a revised version or corresponded with the editorial office about the manuscript for six weeks will be notified by e-mail and telephone that the manuscript will be dropped from consideration by the journal if a revision is not received within the next 10 days.
5.9 Revised manuscripts submitted in Chinese will be sent for translation into English.
5.10 The revised English-language manuscripts will then be line-edited by a native English-speaking editor. This detailed process often results in further revisions to the text and identifies additional problems with the manuscript. Multiple interactions (either by e-mail or phone) with the author and, if different, the individual responsible for the analysis, are usually needed to resolve these issues and arrive at a final version of the manuscript.
5.11 Once the final revision to the manuscript is prepared, the article will be formally accepted and the corresponding author will be notified of the acceptance.
5.12 The accepted version of the manuscript will be typeset and a proof in pdf format will be sent to the corresponding author for checking and approval.
5.13 Once the typeset proof has been corrected and approved by the corresponding author it will be converted into a doi version and placed on the website for the journal so that it can be made freely available prior to publication in the paper version of the Journal. The typeset version of the manuscript will also be placed in the cue to be published in the paper version of the journal.
6.FAST-TRACK SUBMISSION
We will also prove a fast-track option for authors who can justify the need for rapid publication of their results.Authors who wish to have their manuscripts considered for fast-track publication should indicate this in the cover letter to the editor that accompanies the manuscript. If we agree to fast-track a publication and the authors are able to provide rapid revisions based on reviewers comments we will endeavor to place a doi version of the manuscript on our website within one month of receipt of the manuscript.
- 上海精神醫(yī)學的其它文章
- Heterogeneity of treatment effects
- Predictors of re-hospitalization over a two-year follow-up period among patients with schizophrenia enrolled in a community management program in Chengdu, China
- Comparison of family functioning and social support between families with a member who has obsessivecompulsive disorder and control families in Shanghai
- Efficacy of contingency management in improving retention and compliance to methadone maintenance treatment:a random controlled study
- Brainnetome of schizophrenia: focus on impaired cognitive function
- Where is the path to recovery when psychiatric hospitalization becomes too difficult?

