Compound Formula Rehmannia alleviates levodopainduced dyskinesia in Parkinson’s disease
Long Teng, Fang Hong, Chenguang Zhang, Jiancheng He, Haiying Wang
Compound Formula Rehmannia alleviates levodopainduced dyskinesia in Parkinson’s disease
Long Teng, Fang Hong, Chenguang Zhang, Jiancheng He, Haiying Wang
Shanghai University of Traditional Chinese Medicine, Shanghai, China
Long Teng and Fang Hong contributed equally to this work.
Compound Formula Rehmannia has been shown to be clinically effective in treating Parkinson’s disease and levodopa-induced dyskinesia; however, the mechanisms remain unclear. In this study, we established a model of Parkinson’s disease dyskinesia in rats, and treated these animals with Compound Formula Rehmannia. Compound Formula Rehmannia inhibited the increase in mRNA expression of N-methyl-D-aspartate receptor subunits 1 and 2 and excitatory amino acid neurotransmitter genes, and it inhibited the reduction in expression of γ-aminobutyric acid receptor B1, an inhibitory amino acid neurotransmitter gene, in the corpus striatum. In addition, Compound Formula Rehmannia alleviated dyskinesia symptoms in the Parkinson’s disease rats. These experimental findings indicate that Compound Formula Rehmannia alleviates levodopa-induced dyskinesia in Parkinson’s disease by modulating neurotransmitter signaling in the corpus striatum.
nerve regeneration; traditional Chinese medicine; Parkinson’s disease; dyskinesia; excitatory amino acid; inhibitory amino acid; neurobehavior; Compound Formula Rehmannia; NSFC grant; neural regeneration
Funding: This study was supported by the National Natural Science Foundation of China, No. 30672684, 30973722; Science and Technology Support Traditional Chinese Drug Research and Development Project of Shanghai, No. F50102; Traditional Chinese Medicine Research Fund of Shanghai Municipal Health Bureau, No. 2012J009A; Annual Research Budget of Shanghai University of Traditional Chinese Medicine in 2013, No. 2013JW25.
Teng L, Hong F, Zhang CG, He JC, Wang HY. Compound Formula Rehmannia alleviates levodopa-induced dyskinesia in Parkinson’s disease. Neural Regen Res. 2014;9(4):407-412.
Introduction
Parkinson’s disease is a degenerative disorder of the central nervous system in the elderly, and is associated with the loss of dopamine in the substantia nigra pars compacta in the midbrain. It is the most common chronic progressive motor disorder, and is characterized by tremor, rigidity, bradykinesia and postural instability. Levodopa is the preferred treatment for Parkinson’s disease in the clinic. However, long-term use of levodopa may lead to various motor complications, among which levodopa-induced dyskinesia is the most common, severely affecting patients’ quality of life[1-2]. The positive clinical outcomes and substantial ef fi cacy of traditional Chinese medicines have brought hope for Parkinson’s disease treatment. Accumulating evidence indicates that traditional Chinese medicines not only alleviate Parkinsonian symptoms, but also reduce levodopa-induced dyskinesia and other complications[3]. Current studies suggest that most Parkinsonian symptoms and levodopa-induced dyskinesia are the result of yin deficiency stirring wind pattern[4]. Compound Formula Rehmannia was developed based on clinical experiences (National Invention Patent No. ZL200810043734.2). This formulation has satisfactory clinical ef fi cacy in the treatment of Parkinson’s disease and levodopa-induced dyskinesia[5-6].
Studies have shown that amino acid neurotransmitters are closely linked with the pathogenesis of Parkinson’s disease and levodopa-induced dyskinesia[7-11]. Previous studies by our research group indicate that excitatory amino acids have certain links with levodopa-induced dyskinesia in Parkinson’s disease. We showed that levels of excitatory amino acids (aspartate and glutamate) in the striatum are increased, while rotational behavior and stereotyped movement worsened in a rat model of levodopa-induced dyskinesia[12]. Excitatory amino acid levels can be an indicator of the severity of disease. Intervention with Compound Formula Rehmannia greatly reduced aspartate and glutamate content, demonstrating the therapeutic potential of Compound Formula Rehmannia in the treatment of levodopa-induced dyskinesia. The present study aimed to examine the effects of Compound Formula Rehmannia on the expression of amino acid neurotransmitter-related genes in the corpus striatum of Parkinsonian rats with dyskinesia.
Results
Quantitative analysis of experimental animals
A total of 200 healthy adult Sprague-Dawley rats were included in this study. Sixteen animals were selected as normal controls, and another 16 rats were injected with salinecontaining 0.2% ascorbic acid into the left substantia nigra to serve as the sham surgery group. The remaining 168 rats were used for the Parkinson’s disease models and received injection of 6-hydroxydopamine into the left substantia nigra. Among these, 50 rats successfully displayed signs of Parkinsonism, while the remaining 118 rats failed to show Parkinsonian symptoms. Seven days later, the 50 Parkinson’s disease model rats were intraperitoneally injected with levodopa and benserazide to produce a model of levodopa-induced dyskinesia. Among these, 32 rats successfully displayed signs of dyskinesia and were equally and randomly divided into control and Compound Formula Rehmannia groups. At the same time, as levodopa + benserazide injection, the Compound Formula Rehmannia group was given Compound Formula Rehmannia, while the control group was given intraperitoneal injection of an equal amount of water containing 0.05% ethanol and 0.1% ascorbic acid. A total of 64 rats were included in the fi nal analyses, with 16 animals in each of the control, sham surgery, model and Compound Formula Rehmannia groups.
Compound Formula Rehmannia alleviated levodopainduced dyskinesia in Parkinson’s disease rats
Abnormal involuntary movement (AIM) scores
Typical AIMs were found in the rat model of levodopa-induced dyskinesia, including involuntary flapping of the contralateral forelimb (forelimb AIM), involuntary bending to the opposite side and twisting of the body and neck (axial AIM) and involuntary licking towards the opposite side (orofacial AIM). The AIM scores gradually increased over time in the rats with levodopa-induced dyskinesia (P < 0.01 or P < 0.05). The AIM scores decreased after treatment with Compound Formula Rehmannia (P < 0.01). Over the treatment period, the AIM scores gradually decreased (P < 0.05; Table 1).
Dynamic changes in the number of peak rotations to the contralateral side in Parkinson’s disease rats with levodopainduced dyskinesia
The number of peak rotations to the contralateral side within 5 minutes, triggered by apomorphine, increased over time in rats with levodopa-induced dyskinesia, although this increase over time was not statistically signi fi cant (P >0.05). The differences between the model and Compound Formula Rehmannia groups at 4 and 6 weeks after modeling were statistically significant (P < 0.05 or P < 0.01). In the Compound Formula Rehmannia group, the number of peak rotations signi fi cantly decreased after treatment (P < 0.05 or P < 0.01), although this decrease over time was statistically insigni fi cant (P > 0.05; Table 2).
Dynamic changes in time to commencement of rotational behavior in rats with levodopa-induced dyskinesia
In the model group, the time to commencement of apomorphine-elicited rotational behavior tended to decrease over time, although there were no signi fi cant differences across the different time points (P > 0.05). In the Compound Formula Rehmannia group, the time to commencement of rotational behavior increased over the duration of treatment (P < 0.05). The differences between the model and Compound Formula Rehmannia groups at 6 weeks were statistically signi fi cant (P < 0.01; Table 3).
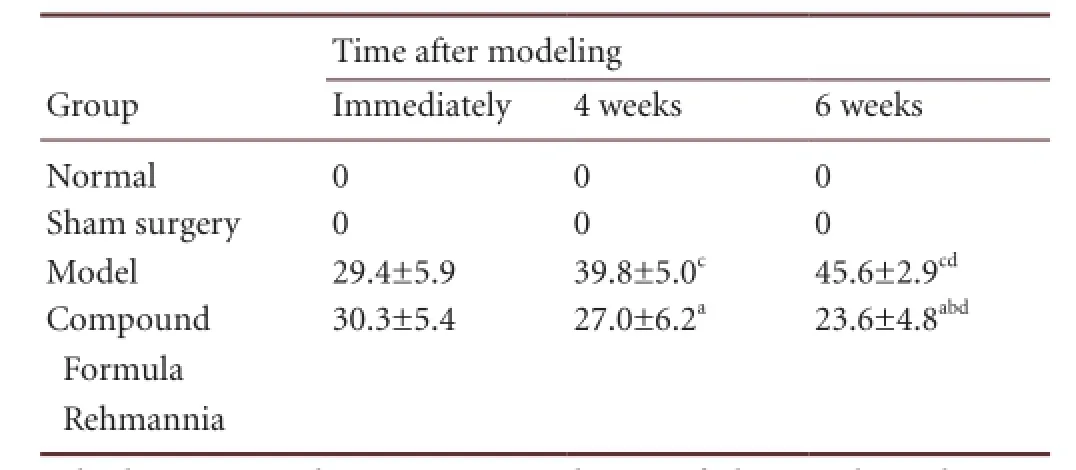
Table 1 Effect of Compound Formula Rehmannia on abnormal involuntary movement scores in Parkinson’s disease rats with levodopa-induced dyskinesia
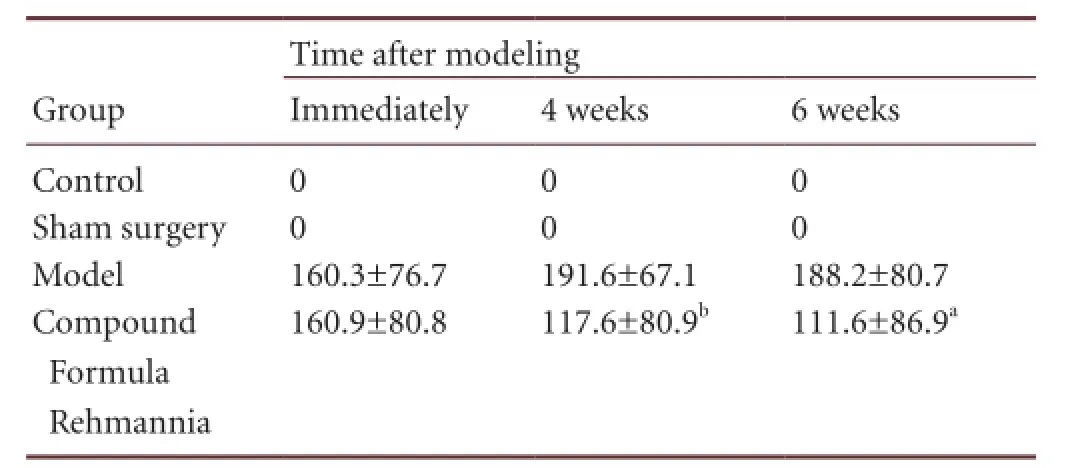
Table 2 Effect of Compound Formula Rehmannia on the number of peak rotations to the contralateral side (rotations/5 minutes) in Parkinson’s disease rats with levodopa-induced dyskinesia

Table 3 Effect of Compound Formula Rehmannia on time (seconds) to commencement of rotational behavior in rats with levodopainduced dyskinesia
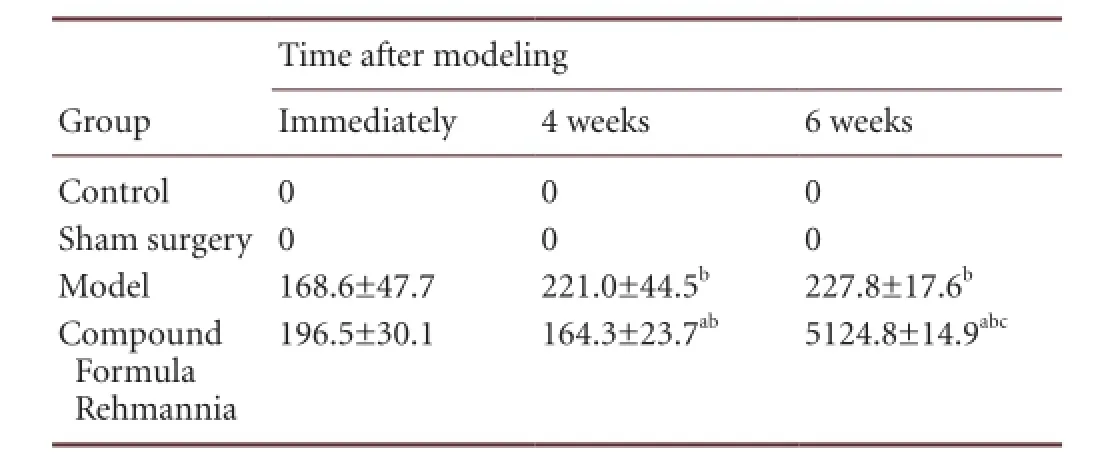
Table 4 Effect of Compound Formula Rehmannia on the duration (minutes) of rotational behavior in rats with levodopa-induced dyskinesia
Dynamic changes in the duration of rotational behavior in rats with levodopa-induced dyskinesia
In the model group, the duration of rotational behavior triggered by apomorphine gradually increased over time (P < 0.01). In the Compound Formula Rehmannia group, the duration signi fi cantly decreased after treatment (P < 0.01), and this reduction continued over time (P < 0.05 or P < 0.001; Table 4).
Effect of Compound Formula Rehmannia on the expression of excitatory and inhibitory amino acids in the corpus striatum of rats with levodopa-induced dyskinesia
Compared with the control group, NMDA-R1 and NMDA-R2 mRNA expression in the corpus striatum of levodopa-induced dyskinetic rats was significantly increased (P < 0.01), and continued to increase over time (P < 0.01). In contrast, expression of GABA-RB1 mRNA in levodopa-induced dyskinetic rats was signi fi cantly decreased (P < 0.01). After treatment with Compound Formula Rehmannia, NMDA-R1 and NMDA-R2 mRNA levels significantly decreased (P < 0.01), although they remained higher than in the control group (P < 0.01). Expression gradually increased over time (P <0.05). After treatment with Compound Formula Rehmannia, expression of GABA-RB1 mRNA in levodopa-induced dyskinetic rats signi fi cantly increased (P < 0.01), but was still lower than in the control group (P < 0.01; Table 5).
Discussion
In the present study, we injected 6-hydroxydopamine into the brain of normal rats to establish a Parkinson’s disease model. A sham surgery group was included in the analyses to verify that there was no direct correlation between brain injury and Parkinson’s disease. After 6-hydroxydopamine was injected to lesion the substantia nigra, rats began to exhibit Parkinsonian signs and symptoms. We then administered levodopa for 2 weeks via lumbar injection to prepare the levodopa-induced dyskinesia model. In previous studies, symptomology was assessed by observing physical signs, neurological behavior, tongue pathology, and other objective indicators (e.g., levels of oxidative stress, cAMP, cGMP, 6-Keto-PGF1α, TXB2 and ET, and electron microscopy). Previous studies also examined the effects of various drugs (Gastrodia and Uncaria Beverage, Phlegm-Flushing Decoction, Peach Kernel and Carthamus Four Agents Decoction)[13-14]. Gastrodia and Uncaria Beverage have been shown to be the most effective[14-16], demonstrating that the rat model results from yin de fi ciency stirring wind pattern, according to traditional Chinese medicine.
Based on the literature and personal clinical experience, we created Compound Formula Rehmannia. The compound is composed of rehmannia, root of herbaceous peony, uncaria, nacre, salvia miltiorrhiza, grass-leaved sweet fl ag, scorpion and green tea leaves.
A reduction in nigrostriatal dopaminergic neurons leads to a decrease in activity of the direct pathway and an increase in activity of the indirect pathway, thereby leading to increased inhibition of the thalamus and cortex, resulting in hypokinesia[17]. The dyskinesia of Parkinson’s disease patients using levodopa is marked by acrokinesia, with stereotyped or dancing activities, which may involve the limbs, tongue, neck, body and abdomen, and symptoms such as sustained muscle contraction (dystonia) and myoclonia[18]. Thus, the pathology of levodopa-induced dyskinesia is distinct from that of Parkinson’s disease.
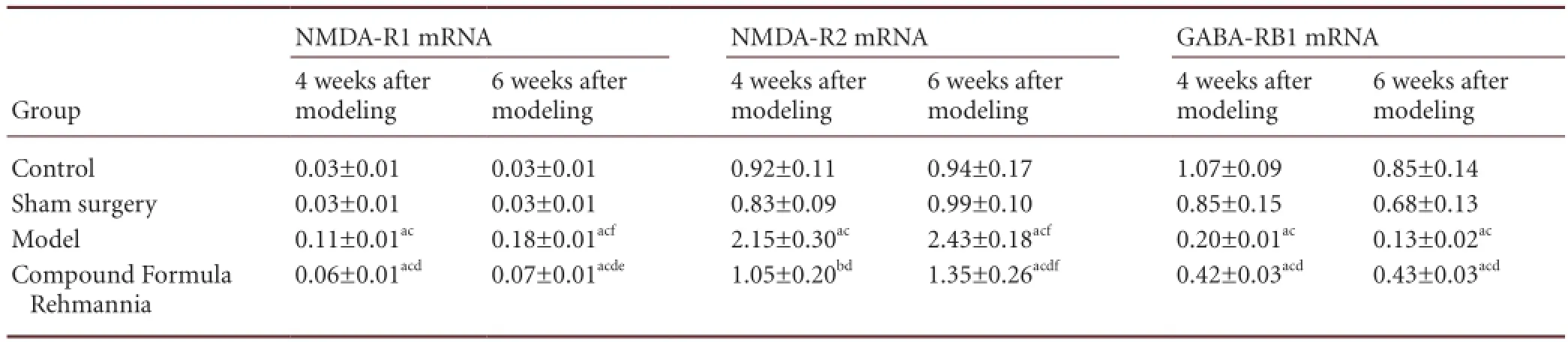
Table 5 Effect of Compound Formula Rehmannia on the expression of excitatory (NMDA-R1, NMDA-R2) and inhibitory (GABA-RB1) amino acids mRNAs in the corpus striatum of levodopa-induced dyskinetic rats
Neurological behavior is an important indicator of levodopa-induced dyskinesia in rats with Parkinson’s disease, and is used to judge the success of the model and the severity of the disease. AIMs are an important indicator for neurobehavioral evaluation. In the present study, typical AIMs were observed in the rat model of levodopa-induced dyskinesia, including involuntary flapping of the forelimb (forelimb AIM), involuntary bending to the opposite side and twisting of the body and neck (axial AIM) and involuntary licking towards the opposite side (orofacial AIM). The AIM scale scores increased over time and were reduced by treatment of Compound Formula Rehmannia.
It has been reported that levodopa-induced dyskinesia is associated with an imbalance in brain amino acid neurotransmitters[7-11]. Dopamine receptor activation leads to enhanced cortical striatal glutamatergic synaptic transmission[19]. As the most important excitatory neurotransmitter in the central nervous system, glutamate functions through ionotropic receptors and metabotropic receptors. The NMDA ionotropic receptor mediates the glutamatergic excitation of striatal and subthalamic nucleus neurons. The NMDA receptor is mainly composed of NMDA-R1 and NMDA-R2 subunits, where NMDA-R1 serves as the ion channel and NMDA-R2 plays a regulatory role[20].
Previous experiments showed that glutamic acid content in the striatum is signi fi cantly increased in rats with levodopa-induced dyskinesia[12]. In the present study, we found that the expression of NMDA-R1 and NMDA-R2 in the striatum was significantly increased, and that expression levels increased with time. This indicates that levodopa-induced dyskinesia is related to an increase in NMDA receptor function in striatal neurons[21]. This increase in glutamate receptor expression increases channel activity in response to corticostriatal glutamatergic input, thereby leading to a change in direct and indirect pathway activation and resulting in motor complications. Compound Formula Rehmannia down-regulated the expression of NMDA-R1 and NMDA-R2.
As the most important inhibitory system, the GABAergic system plays a crucial role in the regulation of excitatory glutamatergic neurons. Indeed, the blockade of GABA inhibition can signi fi cantly increase neuronal excitability[22]. GABA in the striatum plays an important role in the regulation of the excitability of the basal ganglia-thalamo-cortical loop[23]. In this study, the expression of GABA-RB1 in the corpus striatum of Parkinsonian rats with levodopa-induced dyskinesia was signi fi cantly decreased compared with the control and sham surgery groups. Furthermore, striatal GABA-RB1 expression declined in these dyskinetic rats at 6 weeks compared with 4 weeks for the model group. This indicates that the reduction in GABA receptor expression is related to the onset of levodopa-induced dyskinesia. Compound Formula Rehmannia up-regulated the expression of GABA-RB1.
After treatment with Compound Formula Rehmannia, the expression of NMDA-R1 and NMDA-R2 declined signi fi cantly, while GABA-RB1 expression signi fi cantly increased. These changes in gene expression became more striking over the course of treatment. Previous studies have shown that Compound Formula Rehmannia can lower levels of excitatory amino acids and raise those of GABA[12], indicating that the medicine can modulate NMDA and GABA content, thereby regulating the balance between excitation and inhibition in the striatum and relieving levodopa-induced dyskinesia.
Materials and Methods
Design
A randomized, controlled animal experiment.
Time and setting
The experiment was performed at the Shanghai University of Traditional Chinese Medicine and Shanghai Hongyuan Biological Technology Co., Ltd., China from March 2009 to February 2011.
Materials
Animals
A total of 200 healthy male Sprague-Dawley rats, of speci fi c pathogen free grade, weighing 180–220 g, aged 6–8 weeks, were provided by the Animal Experimental Center of Shanghai University of Traditional Chinese Medicine, China (license No. SYXK (Hu) 2004-2005). Rats were housed in wire cages under constant temperature (23 ± 2°C) and humidity (60–65%), and a 12-hour dark-light cycle (light: 7:00–19:00; dark: 19:00–7:00), allowing free access to water and food. Procedures using experimental animals were in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, issued by the Ministry of Science and Technology of China[24].
Drug
Compound Formula Rehmannia has been granted the National Invention Patent (ZL200810043734.2), and is composed of 15 g of rehmannia, 30 g of root of herbaceous peony, 15 g of ramuli umcariae cum uncis, 15 g of nacre, 20 g of root of red-rooted salvia, 12 g of acorus tatarinowii, 2 g of scorpion, and 6 g of green tea leaves. It is boiled into a decoction at a concentration of 5.18 g/mL, and is processed by Shanghai Lei Yun Shang Pharmaceutical Co. Ltd. (Shanghai, China).
Methods
Establishing the model of levodopa-induced dyskinesia
The model of levodopa-induced dyskinesia was established by the well-accepted method of Ungerstedt[25]. In brief, after rats were confirmed to lack abnormal rotational behavior before surgery, they were anesthetized by intraperitoneal injection of 3% pentobarbital sodium (50 mg/kg; Shanghai Chinese and Western Pharmaceutical Co., Ltd., Shanghai, China) and fi xed on a brain stereotaxic instrument (TOW-3A; Shantou Education and Medical Instrument Factory, Shantou, Guangdong Province, China). The heads were shaved and routine disinfection with Bromo Geramine was performed. Under sterile conditions, we made an incision in the skin over the cranium along the midline and stripped the periosteum to expose the bregma. The substantia nigra was located with the brain stereotaxic instrument based on the diagram of Bao et al.[26]. The two coordinates for targeting the substantia nigra were as follows: (1) 5.2 mm pos-terior to the bregma, 1.0 mm right of the midline, 9.0 mm below the dura mater; (2) 5.2 mm posterior to the bregma, 2.5 mm right of the midline, 8.5 mm below the dura mater. After precisely locating the coordinates, we carefully drilled a hole and injected 6-hydroxydopamine (diluted in normal saline containing 0.2% ascorbic acid) into the right substantia nigra with a 5 μL micro-injector (the insertion speed was 1.0 mm/min). The volume per hole was 3 μL, injected at a speed of 1 μL/min. The retention time of the needle was 5 minutes, and the speed of needle withdrawal was 1.0 mm/min. Finally, the holes were sealed with medical gelatin sponge, and the skin incision was sutured. The rats were returned to their cages when they awoke and were given an intramuscular injection of gentamycin for 7 days. The sham-operated animals were only injected with saline containing 0.2% ascorbic acid. In the control group, the animals were fixed in the apparatus for the same amount of time without any additional procedure.
At 10 days, rats were given intraperitoneal injection of apomorphine, 0.5 mg/kg, to induce rotation to one side. The number of rotations was recorded over a period of 30 minutes. If the frequency met or exceeded seven rotations per minute, the rat was considered to successfully model Parkinson’s disease[27].
Parkinson’s disease model rats were given injection of 50 mg/kg of levodopa and 12.5 mg/kg of benserazide (containing 0.05% ethanol and 0.1% ascorbic acid), twice a day for 2 weeks. Both levodopa and benserazide were provided by Sigma, St. Louis, MO, USA. The sham surgery group was injected with 72.5 mg/kg of 0.05% ethanol and 0.1% ascorbic acid via intraperitoneal injection for 2 weeks. Only rats with an AIM score > 20 and an increased number of contralateral rotations induced by apomorphine were considered to have levodopa-induced dyskinesia[28-29].
Intragastric administration of Compound Formula Rehmannia
At the same time that they were given intraperitoneal injection of levodopa and benserazide, the rats in the Compound Formula Rehmannia group also received 2 mL of Compound Formula Rehmannia via intragastric administration. The drug dosage was calculated according to the method of Sun[30], and given once per day. The rats in the control, sham surgery and model groups were given 2 mL of physiological saline via intragastric administration.
Observation of neurological behavior in rats with levodopa-induced dyskinesia
Behavioral observation was conducted 1 day after the rats were given intraperitoneal injection of levodopa. The time to the start of rotational behavior, number of rotations/5 min, number of peak rotations and rotation duration (rotational behavior was triggered with apomorphine) were recorded after the usage of levodopa[13-16]. AIM scores were calculated after 4 and 6 weeks of levodopa injection[13-16]. The total AIM score was calculated every 35 minutes after injection. Four sets of data were recorded continuously. The duration was 140 minutes and the fi nal score was the sum of the four evaluations.
Expression of NMDA R1, NMDA R2 and GABA RB1 mRNA in the corpus striatum of rats as detected by real-time PCR
After neurobehavioral observation was performed at 4 and 6 weeks, the rats were anesthetized with pentobarbital sodium (50 mg/kg) and their heads were cut off to harvest brain tissues, then the corpus striatum was separated and weighed. Brain tissues (50–100 mg) were pulverized with liquid nitrogen in a mortar, and the pulverized powder was homogenized with 1 mL Trizol (Invitrogen Company, Carlsbad, CA, USA) in a glass homogenizer. Subsequently, the specimens were placed into a 1.5-mL centrifuge tube, mixed with a 5-mL syringe needle, incubated on ice for 5 minutes, and centrifuged at 4°C for 5 minutes (12,000 × g). The supernatant was then harvested and transferred into a 1.5-mL centrifuge tube, gently shaken after the addition of 200 μL chloroform (Shanghai Shiyi Chemicals Reagent Co., Ltd., Shanghai, China) for 15 seconds, kept at room temperature for 15 minutes, and fi nally centrifuged at 4°C for 15 minutes (12,000 × g). The supernatant was transferred to an RNAsefree 1.5-mL tube, gently mixed with 0.5 mL isopropyl alcohol (Shanghai Shiyi Chemicals Reagent Co., Ltd.), incubated at room temperature for 10 minutes, and centrifuged at 4°C for 10 minutes (12,000 × g). After the supernatant was discarded, the RNA precipitate was rinsed with 1 mL 75% ethanol, followed by centrifugation at 4°C for 5 minutes (7,500 × g). The centrifuge tube was inverted and dried on a clean bench, and 30 μL of DEPC-treated water was added to dissolve RNA. The absorption of the RNA sample at 260 and 280 nm was determined with a spectrophotometer (Thermo, Boston, MA, USA). RNA concentration was then measured. The primers were designed using Primer Premier 5 (Gen-Script Real-time PCR Primer Design, https://www.genscript. com/ssl-bin/app/primer) and DNAStar software (DNAStar company, Madison, WI, USA), and subjected to BLAST analysis (NCBI).
RT-PCR amplification protocol: 1 cycle of 50°C for 2 minutes, 95°C for 10 minutes; 40 cycles of 95°C for 15 seconds, 60°C for 1 minute. Real-time fl uorescent quantitative PCR (ABI, Foster City, CA, USA) was used to determine expression of NMDA-R1, NMDA-R2 and GABA-RB1 in the striatum of rats. The mRNA expression was represented as 2–△△CT. Primer sequences of genes are shown as below:
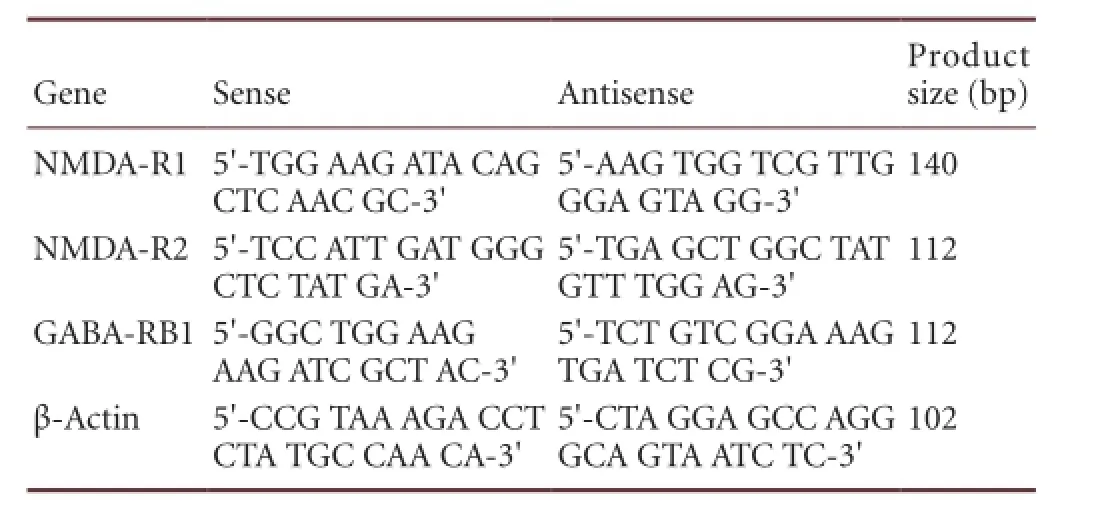
Gene Sense Antisense Product size (bp) NMDA-R15′-TGG AAG ATA CAG CTC AAC GC-3′140 NMDA-R2 5′-TCC ATT GAT GGG CTC TAT GA-3′5′-AAG TGG TCG TTG GGA GTA GG-3′112 GABA-RB15′-GGC TGG AAG AAG ATC GCT AC-3′5′-TGA GCT GGC TAT GTT TGG AG-3′112 β-Actin 5′-CCG TAA AGA CCT CTA TGC CAA CA-3′5′-TCT GTC GGA AAG TGA TCT CG-3′5′-CTA GGA GCC AGG GCA GTA ATC TC-3′102
Statistical analysis
Data are expressed as mean ± SD and were analyzed using SPSS 16.0 Software (SPSS, Chicago, IL, USA). Inter-group differences were compared with one-way analysis of variance and pairwise comparison was performed using the least signi fi cant difference test. A P < 0.05 was considered statistically signi fi cant.
Acknowledgments:We thank Professor Tian ZZ from Fudan University, China, for guiding on experimental design.
Author contributions:Teng L was responsible for writing the manuscript. All authors participated in the conception and design of the study, data integration, and data analysis, and approved the final version of the manuscript.
Con fl icts of interest:None declared.
Peer review:This study tried to apply Compound Formula Rehmannia for treating Parkinson’s disease and levodopa-induced dyskinesia. The results showed that Compound Formula Rehmannia alleviates levodopa-induced dyskinesia in Parkinson’s disease by modulating the expression of inhibitory and excitatory amino acid.
[1] Pezzoli G, Zini M. Levodopa in Parkinson’s disease: from the past to the future. Expert Opin Pharmacother. 2010;11(4):627-635.
[2] Sethi KD. The impact of levodopa on quality of life in patients with Parkinson disease. Neurologist. 2010;16(2):76-83.
[3] He JC, Yuan CX, Wei HC, et al. Effects of Chinese drugs for nourishing the liver and kidney and removing obstruction in collaterals and detoxication on tyrosine hydroxylase and its mRNA in Parkinson’s disease model rats. Zhongyi Zazhi. 2004;54(2):140-142.
[4] Sheng HM, He JC, Wang WW, et al. The literature research of syndromes of TCM about Parkinson’s disease. Shizhen Guoyi Guoyao. 2011;22(4):967-969.
[5] He HC. Re-thinking of treatment of Parkinson’s disease in traditional Chinese medicine--discussion on three basic therapeutic rules of the disease, reinforcing kidney & smoothing liver, activating blood & dissolving stasis and dispelling phlegm & clearing nodules. Zhongyiyao Tongbao. 2005;4(1):12-14.
[6] Ge J, He JC, Yuan CX. Clinical observation on treatment of Parkinson’s disease by nourishing liver and kidney. Shizhen Guoyi Guoyao. 2012;23(12):3070-3072.
[7] Wu ZE, Wang DQ, Li P, et al. The method of simultaneous measurement of the plasma concentration of L-DOPA and extracellular amino acids in striatum of the Parkinson’s disease rats induced by 6-OHDA. Zhongguo Yaoli Xue Tongbao. 2010;26(10):1395-1399.
[8] Turski L, Bressler K, Rettig KJ, et al. Protection of substantia nigra from MPP+neurotoxicity by N-methyl-D-aspartate antagonists. Nature. 1991;349(6308):414-418.
[9] Lozáno AM, Lang AE, Hutchison WD, et al. Microelectrode recording-guided posteroventral pallidotomy in patients with Parkinson’s disease. Adv Neurol. 1997;74:167-174.
[10] Obeso JA, Rodriguez MC, DeLong MR. Basal ganglia pathophysiology. A critical review. Adv Neurol. 1997;74:3-18.
[11] Blandini F, Greenamyre JT. Prospects of glutamate antagonists in the therapy of Parkinson’s disease. Fundam Clin Pharmacol. 1998;12(1):4-12.
[12] Zhang CG, He JC. Dynamic changes of amino acid content in the LID model of Yin-de fi ciency and Wind-agitation in Parkinson’s disease and intervening effects of the compound rehmannia formula. Xi’an Jiaotong Daxue Xuebao: Yixue Ban. 2012;33(4):501-506.
[13] Ran Q, He JC. Study on the traditional Chinese medicine syndrome properties of the rat models of Parkinson’s disease and“combining syndrome and disease” induced by 6-hydroxydopamine. Zhongguo Shiyan Dongwu Xuebao. 2011;19(6):465-471.
[14] Ding HJ. The research on authentication of syndrome attribute and changes of TH and GDNF of LD rats with endogenous wind syndrome caused by deficiency of Yin of Parkinson’s disease. Shanghai: Shanghai University of Traditional Chinese Medicine, 2010.
[15] Cao LS, He JC, Zhuang YH, et al. Different treatment methods on dynamic changes of D2 receptors in Parkinson’s disease rats in PD rats. Shizhen Guoyi Guoyao. 2011;22(12):3006-3008.
[16] Luo RJ. Study of the DAT dynamic change of LD rat with “Yin Yacuity Stirring Wind” pattern of Parkinson’s disease. Shanghai: Shanghai University of Traditional Chinese Medicine, 2011.
[17] Shen Y, Zhao YB. Motor symptoms of Parkinson’s disease and its mechanism of motor symptoms. Nao yu Shenjing Jibing Zazhi. 2011;19(1):79-81.
[18] Fabbrini G, Brotchie JM, Grandas F, et al. Levodopa-induced dyskinesias. Mov Disord. 2007;22(10):1379-1389,1523.
[19] Obeso JA, Rodriguez-Oroz MC, Rodriguez M, et al. Pathophysiology of levodopa-induced dyskinesias in Parkinson’s disease: problems with the current model. Ann Neurol. 2000;47(4 Suppl 1):S22-34.
[20] Groc L, Heine M, Cousins SL, et al. NMDA receptor surface mobility depends on NR2A-2B subunits. Proc Natl Acad Sci U S A. 2006;103(49):18769-18774.
[21] Duty S. Targeting glutamate receptors to tackle the pathogenesis, clinical symptoms and levodopa-induced dyskinesia associated with Parkinson’s disease. CNS Drugs. 2012;26(12):1017-1032.
[22] Zhu HL, Wang DS, Li JS. The excitatory role of GABA during the early development of the central nervous system. Shengli Kexue Jinzhan. 2003;34(1):60-63.
[23] Sun FJ, Chen L. Effects of destruction of subthalamic nucleus on spontaneous discharge of globus pallidus neurons in rats. Qingdao Daxue Yixueyuan Xuebao. 2013;49(1):1-3.
[24] The Ministry of Science and Technology of the People’s Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006-09-30.
[25] Ungerstedt U. 6-Hydroxy-dopamine induced degeneration of central monoamine neurons. Eur J Pharmacol. 1968;5(1):107-110.
[26] Bao XM, Shu SY. The Stereotaxic Atlas of Rat Brain. Beijing: People’s Medical Publishing House. 1991.
[27] Carman LS, Gage FH, Shults CW. Partial lesion of the substantia nigra: relation between extent of lesion and rotational behavior. Brain Res. 1991;553(2):275-283.
[28] Ding HH, He JC, Wang WW. Effects of different doses of levodopa on neuroethology of rats with Parkinson’s disease. Xi’an Jiaotong Daxue Xuebao: Yixue Ban. 2011;32(1):93-96,106.
[29] Ding HJ, He JC, Zhou AG. The Research on Changes of TH and GDNF of LD Rats with Endogenous Wind Syndrome Caused by De fi ciency of Yin of Parkinson’s disease. Hohhot: The fourth national conference of integrated traditional Chinese and Western medicine diagnosis, 2010.
[30] Sun RY. Quantitative Pharmacology. Beijing: People’s Medical Publishing House. 1987.
Copyedited by Patel B, Huang SJ, Shang HF, Yu J, Yang Y, Li CH, Song LP, Zhao M
10.4103/1673-5374.128246
Jiancheng He, M.D., Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China, hejc8163@163.com. Haiying Wang, M.D., Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China, wanghaiying _7@hotmail.com.
http://www.nrronline.org/
Accepted: 2014-01-18
 中國(guó)神經(jīng)再生研究(英文版)2014年4期
中國(guó)神經(jīng)再生研究(英文版)2014年4期
- 中國(guó)神經(jīng)再生研究(英文版)的其它文章
- Posterior quadrantic disconnection maintains the activity of isolated temporal-parietal-occipital nerve tissue: neuroprotective measures in the surgical treatment of epilepsy
- Examination of Huntington’s disease in a Chinese family
- Circadian fl uctuations in three types of sensory modules in healthy subjects
- 7.0T nuclear magnetic resonance evaluation of the amyloid beta (1–40) animal model of Alzheimer’s disease: comparison of cytology veri fi cation
- Local inhibition of GABA affects precedence effect in the inferior colliculus
- The Pael-R gene does not mediate the changes in rotenone-induced Parkinson’s disease model cells
