Inhibition of Sirtuin 2 exerts neuroprotection in aging rats with increased neonatal iron intake
Xijin Wang, Meihua Wang, Liu Yang, Jie Bai, Zhiqiang Yan, Yuhong Zhang, Zhenguo Liu
1 Department of Neurology, Xinhua Hospital Af fi liated to Shanghai Jiao Tong University School of Medicine, Shanghai, China
2 Shanghai Laboratory Animal Center, Chinese Academy of Sciences, Shanghai, China
3 Department of Neurology, Shanghai Tenth People’s Hospital, Tongji University, Shanghai, China
Inhibition of Sirtuin 2 exerts neuroprotection in aging rats with increased neonatal iron intake
Xijin Wang1, Meihua Wang1, Liu Yang1, Jie Bai1, Zhiqiang Yan2, Yuhong Zhang3, Zhenguo Liu1
1 Department of Neurology, Xinhua Hospital Af fi liated to Shanghai Jiao Tong University School of Medicine, Shanghai, China
2 Shanghai Laboratory Animal Center, Chinese Academy of Sciences, Shanghai, China
3 Department of Neurology, Shanghai Tenth People’s Hospital, Tongji University, Shanghai, China
Impaired iron homeostasis may cause damage to dopaminergic neurons and is critically involved in the pathogenesis of Parkinson’s disease. At present, very little is understood about the effect of neonatal iron intake on behavior in aging animals. Therefore, we hypothesized that increased neonatal iron intake would result in signi fi cant behavior abnormalities and striatal dopamine depletion during aging, and Sirtuin 2 contributes to the age-related neurotoxicity. In the present study, we observed that neonatal iron intake (120 μg/g per day) during postnatal days 10-17 resulted in significant behavior abnormalities and striatal dopamine depletion in aging rats. Furthermore, after AK-7 (a selective Sirtuin 2 inhibitor) was injected into the substantia nigra at postnatal 540 days and 570 days (5 μg/side per day), striatal dopamine depletion was signi fi cantly diminished and behavior abnormality was improved in aging rats with neonatal iron intake. Experimental fi ndings suggest that increased neonatal iron intake may result in Parkinson’s disease-like neurochemical and behavioral de fi cits with aging, and inhibition of Sirtuin 2 expression may be a neuroprotective measure in Parkinson’s disease.
nerve regeneration; Parkinson’s disease; iron homeostasis disruption; aging; dopamine; corpus striatum; neurotoxicity; Sirtuin; AK-7; NSFC grants; neural regeneration
Funding:This study was supported by the National Natural Science Foundation of China, No. 81171204, 81171203, 30772280, 81200871, and 81200921; a grant from the Project of Shanghai Municipal Education Commission of China, No. 14YZ046; a grant from the Project of Shanghai Municipal Health and Family Planning Commission of China, No. 20134049; a grant from the Project of Shanghai Jiao Tong University of China, No. YG2013MS22; and a grant from the Projects of Shanghai Committee of Science and Technology of China, No. 11nm0503300 and 12XD1403800.
Wang XJ, Wang MH, Yang L, Bai J, Yan ZQ, Zhang YH, Liu ZG. Inhibition of Sirtuin 2 exerts neuroprotection in aging rats with increased neonatal iron intake. Neural Regen Res. 2014;9(21):1917-1922.
Introduction
Parkinson’s disease is one of the most prevalent age-related neurodegenerative diseases. Its main clinical features are resting tremor, rigidity, bradykinesia, and abnormal postural re fl exes. Increasing evidence indicates that the causes of Parkinson’s disease are multifactorial, including aging, genetic predisposition, exposure to environmental toxins, immune/ inflammatory factors, and innate characteristics of the nigrostriatal dopaminergic system in the brain (Olanow and Tatton, 1999; Kidd, 2000; Gao et al., 2003; Wang et al., 2005a, b, 2007a, b, 2011; Connolly and Lang, 2014). Among multiple factors suspected to play a role in Parkinson’s disease, aging is a major risk factor for idiopathic Parkinson’s disease (Dillin and Kelly, 2007; Yankner et al., 2008; Gureviciene et al., 2009; Hindle, 2010). Epidemiological studies show that Parkinson’s disease affects approximately 1-2% of the population over the age of 65 years, with incidence and prevalence further increasing with advancing age (von Campenhausen et al., 2005; Yankner et al., 2008; Hindle, 2010).
Sirtuins are NAD+-dependent protein deacetylases that regulate a variety of cellular functions (Saunders and Verdin, 2007; Milne and Denu, 2008; Outeiro et al., 2008; Finkel et al., 2009; Kalle et al., 2010). Sirtuin 2 is mainly distributed in the brain (Dillin and Kelly, 2007; Gan and Mucke, 2008; Finkel et al., 2009) and has been shown to be associated with the aging process and age-related neurodegeneration (Longo and Kennedy, 2006; Dillin and Kelly, 2007; Gan and Mucke, 2008; de Oliveira et al., 2010). AK-7 is a cell- and brain-permeable selective Sirtuin 2 inhibitor (Taylor et al., 2011) and was used to investigate the effects of Sirtuin 2 inhibition on striatal dopamine depletion and behavioral abnormalities in aging rats with increased neonatal iron intake.
Iron is an essential trace metal. It plays an important role in electron transfer, oxygen transport, neurotransmitter synthesis, and myelin production in the central nervous system (Stankiewicz et al., 2007; Xiong et al., 2012). However, impaired iron homeostasis may be harmful to neurons, especially dopaminergic neurons (Stankiewicz et al., 2007; Snyder and Connor, 2009; Lee and Andersen, 2010). Iron dyshomeostasis is associated with the etiopathogenesis of Parkinson’s disease (Barnham and Bush, 2008; Rhodes and Ritz, 2008; Bolognin et al., 2009; Snyder and Connor, 2009).Insufficient iron content can lead to iron-deficient anemia (Anand et al., 2014), and severe iron de fi ciency early in life can result in impaired brain development (Lozoff and Georgieff, 2006; Radlowski and Johnson, 2013). Children who are not breast-fed or who are partially breast-fed should be given an iron-fortified formula. For these reasons, it is of interest to investigate the long-ranging effects of neonatal iron intake in adulthood and senescence. Kaur et al. (2007) reported that elevated neonatal iron intake in mice contributed to age-related neurodegeneration similar to Parkinson’s disease. However, little is known about the effect of neonatal iron treatment on motor behavior in aging animals. Thus, we hypothesized that increased neonatal dietary iron may result in behavior abnormalities and striatal dopamine depletion during aging, and Sirtuin 2 may be involved in the age-related neurotoxicity.
Materials and Methods
Iron intake and Sirtuin 2 inhibitor intervention
All animals were provided from Sino-British SIPPR/BK Lab Animal, Shanghai, China. All experiments were performed according to the Guide forthe Care and Use of Laboratory Animalspublished by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996) and the Guideline for Animal Experimentation of Shanghai Jiao Tong University School of Medicine (China). Seventy male and female, specific-pathogen free, Sprague-Dawley rat pups were maintained in a temperature-controlled (21-22°C) room with a 12-hour light/dark cycle (lights on: 06:00-18:00). Ambient humidity was set between 30% and 70%. Sprague-Dawley rat pups were fed either saline vehicle (n= 20) or carbonyl iron (n= 50) daily by oral gavage from postnatal days 10 to 17. Previous studies (Kaur et al., 2007) demonstrated that increased murine neonatal iron intake (120 μg/g per day) resulted in Parkinson’s disease-like neurodegeneration during aging, so the rat pups in this study were fed an increased iron diet (120 μg/g per day; Sigma-Aldrich, St. Louis, MO, USA). Rats were assigned to young (n= 20, 10 non-fed rats and 10 high iron-fed rats) and aging (n= 50, 10 non-fed rats and 40 high iron-fed rats) groups. The rats in the young and aging groups were aged to 170 days and 615 days, respectively, and behavior tests were conducted on the rats. The rats were then sacri fi ced for further experiments.
At the age of 540 and 570 days, respectively, 20 aging rats received intranigral injections of a selective Sirtuin 2 inhibitor, 3-(1-azepanylsulfonyl)-N-(3-bromphenyl) benzamide (AK-7) (Sigma-Aldrich) in both hemispheres, 1 μg/side per day (n= 10) or 5 μg/side per day (n= 10), respectively. The aging rats were anesthetized with ketamine and xylazine (60 mg/kg and 3 mg/kg, respectively; Sigma-Aldrich)viaintramuscular injection and were positioned in a stereotaxic apparatus (Narishige Scientific Instrument Lab, Tokyo, Japan). Then, AK-7 (2 or 10 μg, respectively) was dissolved in DMSO (4 μL) or vehicle (4 μL of DMSO), respectively, and was injected into the substantia nigra at a fl ow rate of 1 μL/min using a 10-μL microsyringe (Hamilton, Bonaduz, Switzerland), with 2 μL volume of intranigral injection per hemisphere. The following coordinates were used: anterior-posterior -5.4 mm, medial-lateral ±2.1 mm, dorsal-ventral -7.8 mm (Manfredsson et al., 2009; Klein et al., 2010). The needle was left in place for 5 minutes to avoid reflux along the injection track prior to being withdrawn.
Behavior tests
Rotarod performance test and open field test were conducted to evaluate rat behaviors during the light period (Graham and Sidhu, 2010). The basic requirements for the rotarod test consisted of a power source, a roller, and four separators dividing the roller into equal-sized compartments (IITC Life Science, Woodland Hills, CA, USA). Following training, the rats were tested three times at rotarod speeds of 5, 10, and 15 rotations per minute (r/min), respectively. The latency time to fall was recorded for each test. For locomotor activity, each rat was placed into an open field chamber made of wood covered with impermeable Formica. The chamber had a white fl oor (100 cm × 100 cm) divided into 25 squares (20 cm × 20 cm) and 50-cm-high walls. Before testing, each animal was placed in the center of the open field and habituated for 10 minutes. Rat motor behavior was recorded for 30 minutes. The following parameters were evaluated: (1) number of crossings: entering of another square with all four paws; (2) number of rearings: rearing with and without wall contact (standing only on hind legs).
High-pressure liquid chromatography-ECD analysis of dopamine content
High-pressure liquid chromatography-ECD was used to assay neurotransmitter content in the rat striata. Briefly, rat striata were dissected on ice and weighed. The striata were then homogenized (10%, w/v) through sonication in ice-cold homogenization buffer containing perchloric acid (0.1 mol/L). 3,4-Dihydroxybenzylamine was used as the internal standard. Obtained samples were centrifuged at 25,000 ×gfor 10 minutes at 4°C and the supernatants were collected. Dopamine and serotonin (5-hydroxytryptamine) content were detected by high-pressure liquid chromatography (Eicom, Kyoto, Japan) with an electrochemical detector, equipped with a column of 5 μm spherical C18 particles. The mobile phase was composed of 0.1 mol/L phosphate buffer (pH 2.6) containing 2.5% methanol, 0.2 mmol/L octane sulfonic acid, and 4.5% acetonitrile. Dopamine content was expressed as ng/g equivalent striatal tissue. The percentage of the detected concentrations of dopamine and serotonin to baseline levels was de fi ned as contents of dopamine and serotonin in the striata of aging rats.
Statistical analysis
The GraphPad Prism 5.0 (GraphPad software, San Diego, CA, USA) program was used for statistical analyses. Data were expressed as the mean ± SEM. Differences were determined using the two-tailed Student’st-test for comparison between two groups and an analysis of variance and Bonferronipost hoctest for comparison between more than two groups. Normality of sample distribution and homogeneity of variances were tested before each analysis of variance. Values ofP< 0.05 were considered statistically signi fi cant.
Results
Increased neonatal iron intake resulted in age-related behavior abnormalities and striatal dopamine depletion in rats
The rotarod performance test and open field test were performed to evaluate the effect of neonatal iron intake on motor behavior in young and aging rats. As shown in Figure 1, neonatal iron intake had no impact on behavior changes in young rats compared with the vehicle-treated rats. However, significant decreases in latency and the number of crossings and rearings were observed in aging rats with neonatal intake of the same dose of iron compared with the vehicle-treated rats (P< 0.01; Figure 1). In agreement with the behavioral tests, neonatal iron intake did not result in signi fi cant striatal dopamine depletion in young rats compared with the vehicle-treated rats (Figure 2A). However, significantly decreased striatal dopamine content was observed in aging rats with neonatal iron intake compared with the vehicle-treated rats (P< 0.01; Figure 2A). No signi fi cant change in striatal serotonin level was observed in aging rats with neonatal iron intake compared with vehicle-treated rats (P> 0.05; Figure 2B).
AK-7 was neuroprotective in aged rats with increased neonatal iron intake
As shown in Figure 3, although intranigral injection of AK-7 did not signi fi cantly change behavior abnormalities in aging rats with increased neonatal iron intake compared with vehicle-treated rats at a dose of 1 μg/side per day, behavior abnormalities in aging rats with increased neonatal iron intake at 5 μg/side per day were signi fi cantly improved (P< 0.01). In agreement with behavior tests, neurochemical analysis results also showed that AK-7 administration significantly diminished striatal dopamine depletion in aging rats with increased neonatal iron intake compared with vehicle-treated rats (P< 0.05: 1 μg/side per day,P< 0.01: 5 μg/side per day; Figure 4).
Discussion
Many studies have shown that aging is one of the strongest risk factors for idiopathic Parkinson’s disease (Dillin and Kelly, 2007; Yankner et al., 2008; Gureviciene et al., 2009; Hindle, 2010; Lu et al., 2013). Parkinson’s disease is rarely seen before 50 years of age and its incidence and prevalence increase with aging. Aging people gradually manifest pathological features of Parkinson’s disease, such as Lewy bodies, striatal dopamine reduction, and motor signs similar to those observed in Parkinson’s disease (Guang et al., 2012; Yu et al., 2012). In mice with elevated neonatal dietary iron feeding, Kaur et al. (2007) observed increased substantia nigra iron content at 3 months of age, as well as increased markers of oxidative stress and reduced striatal dopamine content at 12, 16, and 24 months, but not at 2 months, when compared with vehicle-treated animals. They also observed significantly decreased tyrosine hydroxylase-immunoreactive neurons in iron-fed mice compared with vehicle-treated mice at 24 months of age, but not at 2, 12, or 16 months. In the present study, we observed that elevated neonatal iron (120 μg/g per day) resulted in signi fi cant behavior abnormalities and striatal dopamine depletion in aging rats, while there was no change in young rats. No signi fi cant change in striatal serotonin content was observed in aging rats with the same amount of neonatal iron supplementation. These data support and extend previous findings showing that increased neonatal iron supplementation, given enough time, might cause some features of Parkinson’s disease. In addition, striatal serotonin content was not significantly affected in these aging rats, showing the selective neurotoxicity. Our results indicate that increased neonatal iron intake has long-lasting effects and could potentially represent a novel risk factor for age-related dopaminergic neurodegeneration. The potential toxic effects of elevated dietary iron in early life, as revealed by our study, should be taken into consideration and certainly warrant further studies in humans, especially as they impact neurological function in aging individuals. It is of interest to develop effective therapeutic strategies to attenuate age-related dopaminergic neurotoxicity as a consequence of increased neonatal iron exposure.
Sirtuins are a family of seven distinct NAD+-dependent deacetylase enzymes with homology to the yeast SIR2 (Outeiro et al., 2008; Finkel et al., 2009; Harting and Kn?ll, 2010). Growing evidence indicates that sirtuins participate in the regulation of a variety of biological activities (Saunders and Verdin, 2007; Milne and Denu, 2008; Outeiro et al., 2008; Finkel et al., 2009; Kalle et al., 2010). Recent studies have suggested a role for sirtuins during the aging process and age-related neurodegeneration (Longo and Kennedy, 2006; Dillin and Kelly, 2007; Gan and Mucke, 2008; de Oliveira et al., 2010). Among the seven sirtuins, Sirtuin 2 is strongly expressed in the brain (Dillin and Kelly, 2007; Southwood et al., 2007; Gan and Mucke, 2008; Pandithage et al., 2008; Finkel et al., 2009; Maxwell et al., 2011). Sirtuin 2 has been shown to be expressed in the cytoplasm, and not the nucleus, of neurons and oligodendrocytes (Li et al., 2007; Southwood et al., 2007; Werner et al., 2007; Pandithage et al., 2008), and Sirtuin 2 overexpression decreases survival of healthy neurons (Pfister et al., 2008). Sirtuin 2 inhibition was identi fi ed as a promising approach for treating Huntington’s disease (Luthi-Carter et al., 2010; Taylor et al., 2011; Chopra et al., 2012). Outeiro et al. (2007) reported that Sirtuin 2 inhibition ameliorates α-synuclein-induced toxicity in three different models (in vitroandin vivo) relevant to Parkinson’s disease. AK-7 is a brain-penetrating, selective, Sirtuin 2 inhibitor (Taylor et al., 2011), which has been shown to attenuate mutant Huntingtin fragment-induced neurodegeneration (Taylor et al., 2011). AK-7 treatment results in decreased brain atrophy, extended survival, and improved motor behavior in two genetic mouse models of Huntington’s disease (Chopra et al., 2012). Based on its properties and results from previous studies (Taylor et al., 2011; Chopra et al., 2012), AK-7 was used as a Sirtuin 2 inhibitor in the present study. The results showed that selective Sirtuin 2 inhibition signi fi cantly diminished striatal dopamine depletion and improved behavior abnormality in aging rats with increased neonatal iron intake, suggesting the potential dopaminergic neuroprotection of AK-7 in Parkinson’s disease.

Figure 1 Increased neonatal iron intake resulted in age-related behavior abnormalities.

Figure 2 Increased neonatal iron intake in rat pups resulted in age-related striatal dopamine (DA) depletion in rats.
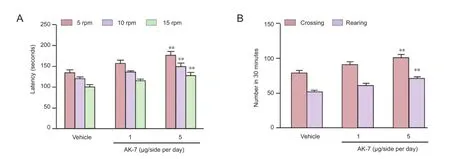
Figure 3 Effect of AK-7 treatment on motor behavior of aging rats with increased neonatal iron intake in rotarod test (A) and open field test (B).
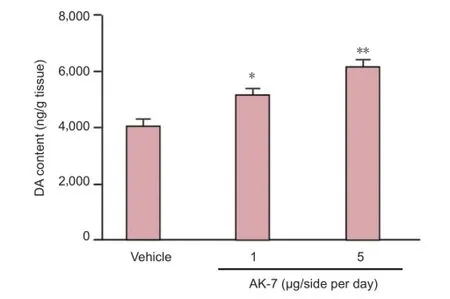
Figure 4 Effect of AK-7 treatment on striatal dopamine (DA) content of aging rats with increased neonatal iron intake.
In summary, the results from our study suggest that increased neonatal iron intake may result in Parkinson’s disease-like neurochemical and behavioral de fi cits with aging, and AK-7 may be neuroprotective in Parkinson’s disease. We will further investigate this age-related neurotoxicity and its underlying mechanisms through other detection methods in our future research. Further studies will bring us a greater understanding of the potential role of Sirtuin 2 in the aging process and Parkinson’s disease, as well as the development of effective therapeutic strategies to slow the progression of aging and Parkinson’s disease neurodegeneration (Lavu et al., 2008; Outeiro et al., 2008; Han, 2009; Donmez and Outeiro, 2013).
Author contributions:Wang XJ and Liu ZG designed the study and wrote the paper. Wang XJ, Wang MH, Yang L, Bai J, Yan ZQ and Zhang YH performed the experiments and data analysis. All authors approved the final version of the manuscript.
Con fl icts of interest:None declared.
Anand T, Rahi M, Sharma P, Ingle GK (2014) Issues in prevention of iron de fi ciency anemia in India. Nutrition 30:764-770.
Barnham KJ, Bush AI (2008) Metals in Alzheimer’s and Parkinson’s Diseases. Curr Opin Chem Biol 12:222-228.
Bolognin S, Messori L, Zatta P (2009) Metal ion physiopathology in neurodegenerative disorders. Neuromolecular Med 11:223-238.
Chopra V, Quinti L, Kim J, Vollor L, Narayanan KL, Edgerly C, Cipicchio Patricia M, Lauver Molly A, Choi Soo H, Silverman Richard B, Ferrante Robert J, Hersch S, Kazantsev Aleksey G (2012) The sirtuin 2 inhibitor AK-7 is neuroprotective in Huntington’s disease mouse models. Cell Reports 2:1492-1497.
Connolly BS, Lang AE (2014) Pharmacological treatment of Parkinson disease: a review. JAMA 311:1670-1683.
de Oliveira RM, Pais TF, Outeiro TF (2010) Sirtuins: common targets in aging and in neurodegeneration. Curr Drug Targets 11:1270-1280.
Dillin A, Kelly JW (2007) Medicine. The yin-yang of sirtuins. Science 317:461-462.
Donmez G, Outeiro TF (2013) SIRT1 and SIRT2: emerging targets in neurodegeneration. EMBO Mol Med 5:344-352.
Finkel T, Deng CX, Mostoslavsky R (2009) Recent progress in the biology and physiology of sirtuins. Nature 460:587-591.
Gan L, Mucke L (2008) Paths of convergence: sirtuins in aging and neurodegeneration. Neuron 58:10-14.
Gao HM, Hong JS, Zhang W, Liu B (2003) Synergistic dopaminergic neurotoxicity of the pesticide rotenone and in fl ammogen lipopolysaccharide: relevance to the etiology of Parkinson’s disease. J Neurosci 23:1228-1236.
Graham DR, Sidhu A (2010) Mice expressing the A53T mutant form of human alpha-synuclein exhibit hyperactivity and reduced anxiety-like behavior. J Neurosci Res 88:1777-1783.
Guang K, Yang MQ, Lu ZY, Ding FX, Wang M (2012) Discharge pattern changes of the subthalamic nucleus and primary motor cortex in Parkinson’s disease rats. Zhongguo Zuzhi Gongcheng Yanjiu 16:2757-2761.
Gureviciene I, Gurevicius K, Tanila H (2009) Aging and α-synuclein affect synaptic plasticity in the dentate gyrus. J Neural Transm 116:13-22.
Han SH (2009) Potential role of sirtuin as a therapeutic target for neurodegenerative diseases. J Clin Neurol 5:120-125.
Harting K, Kn?ll B (2010) SIRT2-mediated protein deacetylation: An emerging key regulator in brain physiology and pathology. Eur J Cell Biol 89:262-269.
Hindle JV (2010) Ageing, neurodegeneration and Parkinson’s disease. Age Ageing 39:156-161.
Kalle AM, Mallika A, Badiger J, Alinakhi, Talukdar P, Sachchidanand (2010) Inhibition of SIRT1 by a small molecule induces apoptosis in breast cancer cells. Biochem Biophys Res Commun 401:13-19.
Kaur D, Peng J, Chinta SJ, Rajagopalan S, Di Monte DA, Cherny RA, Andersen JK (2007) Increased murine neonatal iron intake results in Parkinson-like neurodegeneration with age. Neurobiol Aging 28:907-913.
Kidd PM (2000) Parkinson’s disease as multifactorial oxidative neurodegeneration: implications for integrative management. Altern Med Rev 5:502-529.
Klein RL, Dayton RD, Diaczynsky CG, Wang DB (2010) Pronounced microgliosis and neurodegeneration in aged rats after tau gene transfer. Neurobiol Aging 31:2091-2102.
Lavu S, Boss O, Elliott PJ, Lambert PD (2008) Sirtuins--novel therapeutic targets to treat age-associated diseases. Nat Rev Drug Discov 7:841-853.
Lee DW, Andersen JK (2010) Iron elevations in the aging Parkinsonian brain: a consequence of impaired iron homeostasis? J Neurochem 112:332-339.
Li W, Zhang B, Tang J, Cao Q, Wu Y, Wu C, Guo J, Ling EA, Liang F (2007) Sirtuin 2, a mammalian homolog of yeast silent information regulator-2 longevity regulator, is an oligodendroglial protein that decelerates cell differentiation through deacetylating alpha-tubulin. J Neurosci 27:2606-2616.
Longo VD, Kennedy BK (2006) Sirtuins in aging and age-related disease. Cell 126:257-268.
Lozoff B, Georgieff MK (2006) Iron de fi ciency and brain development. Semin Pediatr Neurol 13:158-165.
Lu MJ, Wang SS, Zhu Y (2013) Microglia-mediated oxidative stress injury in a mouse model of Parkinson’s disease. Zhongguo Zuzhi Gongcheng Yanjiu 17:2001-2006.
Luthi-Carter R, Taylor DM, Pallos J, Lambert E, Amore A, Parker A, Mof fi tt H, Smith DL, Runne H, Gokce O, Kuhn A, Xiang Z, Maxwell MM, Reeves SA, Bates GP, Neri C, Thompson LM, Marsh JL, Kazantsev AG (2010) SIRT2 inhibition achieves neuroprotection by decreasing sterol biosynthesis. Proc Natl Acad Sci U S A 107:7927-7932.
Manfredsson FP, Tumer N, Erdos B, Landa T, Broxson CS, Sullivan LF, Rising AC, Foust KD, Zhang Y, Muzyczka N, Gorbatyuk OS, Scarpace PJ, Mandel RJ (2009) Nigrostriatal rAAV-mediated GDNF overexpression induces robust weight loss in a rat model of age-related obesity. Mol Ther 17:980-991.
Maxwell MM, Tomkinson EM, Nobles J, Wizeman JW, Amore AM, Quinti L, Chopra V, Hersch SM, Kazantsev AG (2011) The Sirtuin 2 microtubule deacetylase is an abundant neuronal protein that accumulates in the aging CNS. Hum Mol Genet 20:3986-3996.
Milne JC, Denu JM (2008) The Sirtuin family: therapeutic targets to treat diseases of aging. Curr Opin Chem Biol 12:11-17.
Olanow CW, Tatton WG (1999) Etiology and pathogenesis of Parkinson’s disease. Annu Rev Neurosci 22:123-144.
Outeiro TF, Marques O, Kazantsev A (2008) Therapeutic role of sirtuins in neurodegenerative disease. Biochim Biophys Acta 1782:363-369.
Outeiro TF, Kontopoulos E, Altmann SM, Kufareva I, Strathearn KE, Amore AM, Volk CB, Maxwell MM, Rochet JC, McLean PJ, Young AB, Abagyan R, Feany MB, Hyman BT, Kazantsev AG (2007) Sirtuin 2 inhibitors rescue alpha-synuclein-mediated toxicity in models of Parkinson’s disease. Science 317:516-519.
Pandithage R, Lilischkis R, Harting K, Wolf A, Jedamzik B, Lüscher-Firzlaff J, Vervoorts J, Lasonder E, Kremmer E, Kn?ll B, Lüscher B (2008) The regulation of SIRT2 function by cyclin-dependent kinases affects cell motility. J Cell Biol 180:915-929.
P fi ster JA, Ma C, Morrison BE, D’Mello SR (2008) Opposing effects of sirtuins on neuronal survival: SIRT1-mediated neuroprotection is independent of its deacetylase activity. PLoS One 3:e4090.
Radlowski EC, Johnson RW (2013) Perinatal iron de fi ciency and neurocognitive development. Front Hum Neurosci 7:585.
Rhodes SL, Ritz B (2008) Genetics of iron regulation and the possible role of iron in Parkinson’s disease. Neurobiol Dis 32:183-195.
Saunders LR, Verdin E (2007) Sirtuins: critical regulators at the crossroads between cancer and aging. Oncogene 26:5489-5504.
Snyder AM, Connor JR (2009) Iron, the substantia nigra and related neurological disorders. Biochim Biophys Acta 1790:606-614.
Southwood CM, Peppi M, Dryden S, Tainsky MA, Gow A (2007) Microtubule deacetylases, SirT2 and HDAC6, in the nervous system. Neurochem Res 32:187-195.
Stankiewicz J, Panter SS, Neema M, Arora A, Batt C, Bakshi R (2007) Iron in chronic brain disorders: imaging and neurotherapeutic implications. Neurotherapeutics 4:371-386.
Taylor DM, Balabadra U, Xiang Z, Woodman B, Meade S, Amore A, Maxwell MM, Reeves S, Bates GP, Luthi-Carter R, Lowden PAS, Kazantsev AG (2011) A brain-permeable small molecule reduces neuronal cholesterol by inhibiting activity of sirtuin 2 deacetylase. ACS Chem Biol 6:540-546.
von Campenhausen S, Bornschein B, Wick R, B?tzel K, Sampaio C, Poewe W, Oertel W, Siebert U, Berger K, Dodel R (2005) Prevalence and incidence of Parkinson’s disease in Europe. Eur Neuropsychopharm 15:473-490.
Wang X, Chen S, Ma G, Ye M, Lu G (2005a) Genistein protects dopaminergic neurons by inhibiting microglial activation. Neuroreport 16:267-270.
Wang X, Chen S, Ma G, Ye M, Lu G (2005b) Involvement of proin fl ammatory factors, apoptosis, caspase-3 activation and Ca2+disturbance in microglia activation-mediated dopaminergic cell degeneration. Mech Ageing Dev 126:1241-1254.
Wang XJ, Yan ZQ, Lu GQ, Stuart S, Chen SD (2007a) Parkinson disease IgG and C5a-induced synergistic dopaminergic neurotoxicity: Role of microglia. Neurochem Int 50:39-50.
Wang XJ, Liu WG, Zhang YH, Lu GQ, Chen SD (2007b) Effect of transplantation of c17.2 cells transfected with interleukin-10 gene on intracerebral immune response in rat model of Parkinson’s disease. Neurosci Lett 423:95-99.
Wang XJ, Zhang S, Yan ZQ, Zhao YX, Zhou HY, Wang Y, Lu GQ, Zhang JD (2011) Impaired CD200-CD200R-mediated microglia silencing enhances midbrain dopaminergic neurodegeneration: Roles of aging, superoxide, NADPH oxidase, and p38 MAPK. Free Radic Biol Med 50:1094-1106.
Werner HB, Kuhlmann K, Shen S, Uecker M, Schardt A, Dimova K, Orfaniotou F, Dhaunchak A, Brinkmann BG, M?bius W, Guarente L, Casaccia-Bonne fi l P, Jahn O, Nave KA (2007) Proteolipid protein is required for transport of sirtuin 2 into CNS myelin. J Neurosci 27:7717-7730.
Xiong P, Chen X, Guo C, Zhang N, Ma B (2012) Baicalin and deferoxamine alleviate iron accumulation in different brain regions of Parkinson’s disease rats. Neural Regen Res 7:2092-2098.
Yankner BA, Lu T, Loerch P (2008) The aging brain. Annu Rev Pathol 3:41-66.
Yu XH, Tian XL, Li YY, Jiang WW, Qian L (2012) Effects of repetitive transcranial direct current stimulation on praxiology of rats with Parkinson’s disease. Zhongguo Zuzhi Gongcheng Yanjiu 16:4471-4475.
Copyedited by Kuhn CC, Norman C, Yu J, Yang Y, Li CH, Song LP, Zhao M
Zhenguo Liu, M.D., Ph.D., Department of Neurology, Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, 1665 Kongjiang Road, Shanghai 200092, China, zhenguoliu2004@aliyun.com.
10.4103/1673-5374.145361
http://www.nrronline.org/
Accepted: 2014-09-28
- 中國神經(jīng)再生研究(英文版)的其它文章
- Hot spots and future directions of research on the neuroprotective effects of nimodipine
- Recovery of cerebellar peduncle injury in a patient with a cerebellar tumor: validation by diffusion tensor tractography
- Amyloid precursor-like protein 2 C-terminal fragments upregulate S100A9 gene and protein expression in BV2 cells
- Reversible lesions in the brain parenchyma in Wilson’s disease con fi rmed by magnetic resonance imaging: earlier administration of chelating therapy can reduce the damage to the brain
- Adult neurogenesis in the four-striped mice (Rhabdomys pumilio)
- The occurrence of diffuse axonal injury in the brain: associated with the accumulation and clearance of myelin debris

