Cholecystokinin octapeptide antagonizes apoptosis in human retinal pigment epithelial cells
Yuan Liu, Yueling Zhang, Zhaohui Gu, Lina Hao, Juan Du, Qian Yang, Suping Li, Liying Wang, Shilei Gong
1 Department of Ophthalmology, First Central Hospital of Baoding, Baoding, Hebei Province, China
2 Department of Ophthalmology, Hebei Province People’s Hospital, Shijiazhuang, Hebei Province, China
3 Department of Endoscope Room, First Central Hospital of Baoding, Baoding, Hebei Province, China
Cholecystokinin octapeptide antagonizes apoptosis in human retinal pigment epithelial cells
Yuan Liu1, Yueling Zhang1, Zhaohui Gu1, Lina Hao2, Juan Du1, Qian Yang1, Suping Li1, Liying Wang1, Shilei Gong3
1 Department of Ophthalmology, First Central Hospital of Baoding, Baoding, Hebei Province, China
2 Department of Ophthalmology, Hebei Province People’s Hospital, Shijiazhuang, Hebei Province, China
3 Department of Endoscope Room, First Central Hospital of Baoding, Baoding, Hebei Province, China
Although cholecystokinin octapeptide-8 is important for neurological function, its neuroprotective properties remain unclear. We speculated that cholecystokinin octapeptide-8 can protect human retinal pigment epithelial cells against oxidative injury. In this study, retinal pigment epithelial cells were treated with peroxynitrite to induce oxidative stress. Peroxynitrite triggered apoptosis in these cells, and increased the expression of Fas-associated death domain, Bax, caspase-8 and Bcl-2. These changes were suppressed by treatment with cholecystokinin octapeptide-8. These results suggest that cholecystokinin octapeptide-8 can protect human retinal pigment epithelial cells against apoptosis induced by peroxynitrite.
nerve regeneration; retinal pigment epithelial cells; peroxynitrite; cholecystokinin
octapeptide; apoptosis; Fas-associated death domain; Bax; Caspase-8; Bcl-2; neural regeneration
Liu Y, Zhang YL, Gu ZH, Hao LN, Du J, Yang Q, Li SP, Wang LY, Gong SL. Cholecystokinin octapeptide antagonizes apoptosis in human retinal pigment epithelial cells. Neural Regen Res. 2014;9(14):1402-1408.
Introduction
Retinal pigment epithelial (RPE) cells are a monolayer of cuboidal cells located between the photoreceptors of the neurosensory retina and the choroidal capillary bed. RPE cells are involved in visual signal processing. Age-related macular degeneration is an idiopathic retinal degenerative disease, and is the leading cause of irreversible vision loss among people over the age of 65. RPE cell apoptosis is an important feature of the advanced forms of age-related macular degeneration (Yang et al., 2005; Koyama et al., 2008).
Oxidative stress may cause RPE cell apoptosis (Sinha et al., 2013). RPE cells are exposed to continual oxidative stress throughout life (Rodriguez and Beconi, 2009; Sankaralingam et al., 2010; Agbani et al., 2011a, b; Guven et al., 2011). Previous investigations on oxidative stress injury caused by oxygen free radicals emphasized the contribution of hydrogen peroxide (Wijeratne et al., 2005), nitric oxide (Jang et al., 2010; Ru et al., 2011) and superoxide anion. Nitric oxide and superoxide react to produce peroxynitrite, which, along with its derivatives, are strong oxidants (Drake et al., 2002; Gebicka and Didik, 2010).
Cholecystokinin octapeptide (CCK) is a peptide originally discovered in the gastrointestinal tract, and subsequently found in the mammalian brain. The C-terminal sulfated octapeptide fragment of cholecystokinin (CCK-8) constitutes one of the major neuropeptides in the brain. CCK-8 contributes to numerous physiological functions. For example, CCK is involved in the neurobiology of anxiety, depression, psychosis, cognition, nociception and feeding behavior (Noble, 2007; Oz et al., 2007; Merino et al., 2008; Hamamura et al., 2010). In addition, CCK can protect cholinergic neurons against basal forebrain lesion caused by brain injury (Sugaya et al., 1992). In this study, we treated human RPE cells with the oxidative stress inducer peroxynitrite, and evaluated the neuroprotective effects of CCK-8.
Materials and Methods
Synthesis of peroxynitrite
Peroxynitrite was obtained by reacting ice-cold solutions of sodium nitrite (0.6 mol/L) and H2O2(0.7 mol/L) in acidic medium (0.6 mol/L HCl) and rapidly quenching the reaction in NaOH (1.5 mol/L), as described previously (Koppenol et al., 1996; Thiagarajan et al., 2004). The reaction mixture solution was frozen at -20°C, and the peroxynitrite concentrated in the upper layer was collected. Concentration was measured at 302 nm using a molar extinction coefficient of 1,670/mol/cm (Koppenol et al., 1996; Thiagarajan et al., 2004).
RPE cell culture and intervention
Human eyes from eight donors (26-56 years of age) were obtained following eyeball rupture in our hospital. The eyes had an intact posterior segment and RPE layers. For RNA extraction, tissues were suspended in RNA preservation solution (Ambion; Austin, TX, USA) and stored at 4°C until processing. The experimental procedures complied with the Declaration of Helsinki.
For RPE isolation, the anterior segment, iris, lens and vitreous of each eye were carefully extracted. After removal of the tissue punches, posterior poles were cut into quadrants.Each quadrant was rinsed with sterile PBS, and the neural retina was gently teased away from the RPE. After removal of the retina, RPE cells free of choroidal contamination were collected. They were cultured in Dulbecco’s modi fi ed Eagle medium/F-12 human amniotic membrane nutrient mixture (DMEM/F-12; Sigma-Aldrich, St. Louis, MO, USA) with 10% fetal bovine serum (Sigma-Aldrich) in a humidified incubator at 37°C and 5% CO2. The medium was changed every 3 days.
RPE cells were obtained from the second or third generation, and then divided into control, peroxynitrite and CCK-8 groups. The control group was treated with saline; the peroxynitrite group was treated with peroxynitrite, 16 nmol/L; the CCK-8 group was treated with CCK-8, 10 nmol/L, after addition of peroxynitrite, 16 nmol/L. All changes were observed at 6, 12 and 24 hours after treatment.
Changes in RPE cell morphology observed by electron microscopy
Cell samples were fixed in 2.5% glutaraldehyde in PBS, postfixed in 2% buffered osmium tetroxide for 2 hours, and then dehydrated in a graded ethanol series. Specimens were embedded in Epon. Thin sections were cut on an ultra microtome and double stained with uranyl acetate and lead citrate. Electron micrographs were taken on a JEM-2000 electron microscope (JEOL, Tokyo, Japan) operating at 80 kV.
Assessment of RPE cell apoptosis by detecting DNA laddering
Agarose gel electrophoresis was used to detect DNA laddering, an indicator of apoptosis, as described previously (Herrmann et al., 1994).
Assessment of RPE cell apoptosis by fl uorescence activated cell sorting
RPE cells were collected, washed with PBS, and adjusted to 1 × 106cells/mL. 5 μL Annexin V-FITC and 10 μL propidium iodide (10 μg/mL, Sigma) were added to a 100-μL aliquot of suspended cells, and then incubated for 15 minutes in a dark room at room temperature. 1 × 104cells were collected and analyzed using Cell Quest software 3.0 (Becton Dickinson, San Jose, CA, USA).
Expression of Fas-associated death domain (FADD), Bax, caspase-8 and Bcl-2 in RPE cells detected by western blot analysis
RPE cells were washed twice with cold Hanks’ balanced salt solution and lysed with RIPA buffer (50 mmol/L Tris-HCl, 150 mmol/L NaCl, 1% NP-40, 0.5% deoxycholate and 0.1% SDS, pH 8.0) supplemented with a protease inhibitor cocktail (Roche, Indianapolis, IN, USA). Lysates were cleared by centrifugation. Total protein in the supernatants was measured using Bradford assay (Bio-Rad, Hercules, CA, USA), with bovine serum albumin used to generate the standard curve, according to the manufacturer’s instructions. Protein (30 g) was electrophoresed on a 12.5% SDS-polyacrylamide gel overlaid with a 3.6% polyacrylamide stacking gel. The proteins were transferred to a nitrocellulose membrane (Bio-Rad) with a Mini Trans-Blot apparatus (Bio-Rad), according to the manufacturer’s directions. Mouse anti-human β-actin monoclonal antibody (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) was used as positive control. The filters were blocked in 0.1 mol/L PBS containing 5% skim milk and 0.05% Tween-20 for 1 hour at room temperature. They were then incubated overnight at 4°C with mouse anti-human FADD (1:1,000, Abnova, Taipei, Taiwan, China), Bax (1:800, Abnova) or caspase-8 (1:600, Abnova) monoclonal antibody or rabbit anti-Bcl-2 polyclonal antibody (1:200, Santa Cruz Biotechnology). After fi ve washes in 0.1 mol/L PBS containing 0.05% Tween 20, the fi lters were incubated for 1 hour at room temperature with a horseradish peroxidase-conjugated goat anti-mouse IgG (1:1,000; Cell Signaling, Beverly, MA, USA) and goat anti-rabbit IgG (1:1,000, Cell Signaling), washed, visualized in ECL solution (Amersham Biosciences, Arlington Heights, IL, USA) for 10 minutes, and exposed to fi lm (X-Omat, Fuji, Kanagawa, Japan) for 7 to 10 minutes. Finally, the fi lters were incubated in a stripping buffer (2% SDS, 0.7% 2-mercaptoethanol, 62.5 mmol/L Tris-HCl, pH 6.8) for 30 minutes at 65°C. Protein levels were quanti fi ed by absorbance.
Expression of caspase-8 and bcl-2 mRNA in RPE cells evaluated with real-time polymerase chain reaction (RTPCR)
Total RNA was isolated from cultured RPE cells (RNeasy; Qiagen Inc., Valencia, CA, USA) according to the manufacturer’s protocols. After isolation and DNase treatment (DNA-free; Ambion), RNA quantitation was performed (RiboGreen RNA, Molecular Probes, Eugene, OR, USA). Equal amounts of RNA were then used to synthesize fi rststrand cDNAs with a cDNA synthesis kit (iScript; Bio-Rad). RT-PCR for caspase-8 and bcl-2 mRNA was performed with a detection system (iCycler IQ; Bio-Rad). Cycling parameters for caspase-8: denaturation at 94°C for 1 minute; ampli fi cation for 34 cycles at 95°C for 10 seconds, 60°C for 15 seconds and 72°C for 1 minute. Cycling parameters for Bcl-2: denaturation at 95°C for 2 minutes; ampli fi cation for 50 cycles at 95°C for 15 seconds, 60°C for 15 seconds and 72°C for 15 seconds. Primers for RT-PCR are given inTable 1.
The presence of a single melting temperature peak per primer pair and 2% agarose gel analysis confirmed the identity of the PCR products. Each RT-PCR experiment was repeated at least three times.
Statistical analysis
Data were expressed as mean ± SD. Statistical signi fi cance was determined by one-way analysis of variance, followed by the Fisher post hoc test for multiple comparisons. A P <0.05 value was considered statistically signi fi cant difference.
Results
Effect of peroxynitrite and CCK-8 on the morphology of RPE cells
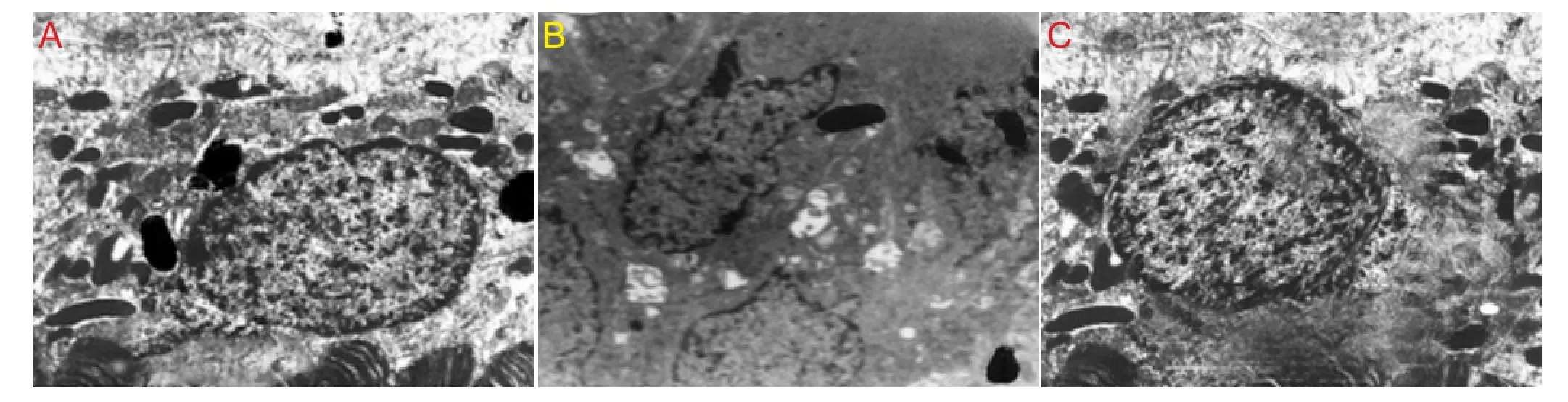
Figure 1 Effect of peroxynitrite and cholecystokinin octapeptide-8 (CCK-8) on the morphology of human retinal pigment epithelial cells (transmission electron microscopy).
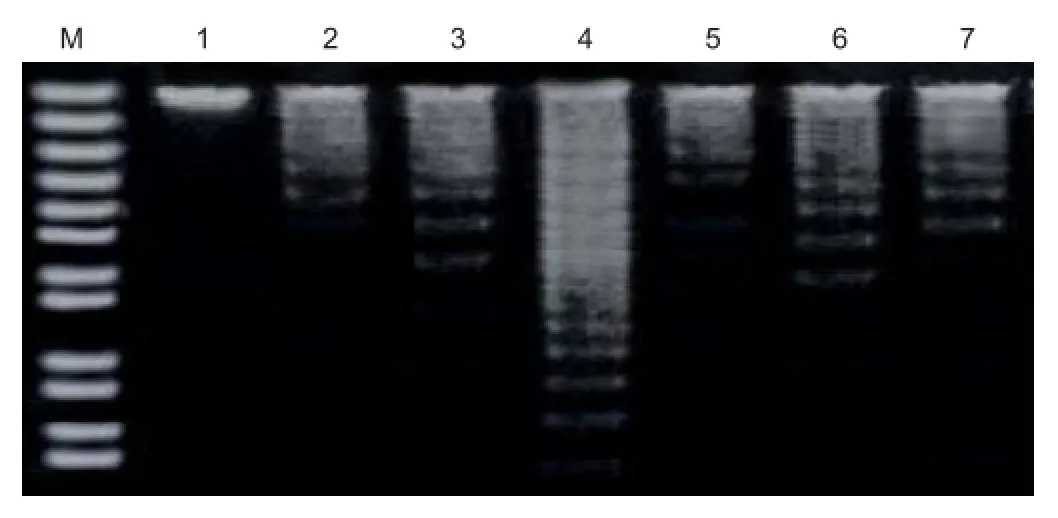
Figure 2 DNA ladder assay for detection of apoptosis in human retinal pigment epithelial cells.
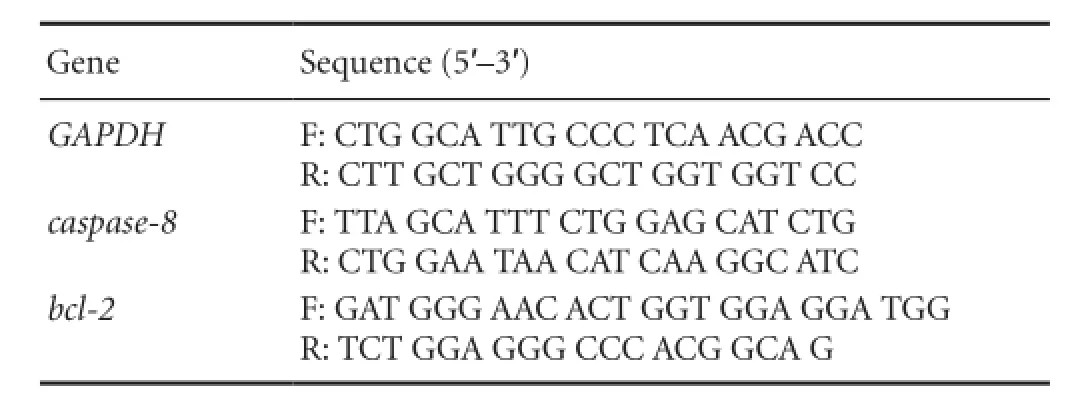
Table 1 Primers for real-time polymerase chain reaction
Under the transmission electron microscope, we observed nuclear fragmentation and chromatin marginalization induced by peroxynitrite in RPE cells. Compared with the peroxynitrite group, apoptotic features (loss of microvilli and chromatin condensation, fragmentation and marginalization) were less apparent in the CCK-8 group (Figure 1).
Effect of peroxynitrite and CCK-8 on apoptosis in RPE cells
Agarose gel electrophoresis revealed no DNA laddering in the control group, while there was typical DNA laddering in the peroxynitrite group. Compared with the peroxynitrite group, DNA laddering in the CCK-8 group was signi fi cantly weaker (Figure 2).
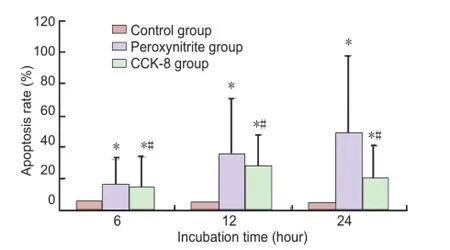
Figure 3 Apoptosis in human retinal pigment epithelial cells in each group.
Fluorescence activated cell sorting analysis showed that the number of apoptotic RPE cells in the peroxynitrite group was increased at 6, 12 and 24 hours compared with the control group (P < 0.05). RPE cell apoptosis in the CCK-8 group was decreased at 6, 12 and 24 hours compared with the peroxynitrite group (P < 0.05;Figure 3).
Effect of peroxynitrite and CCK-8 on protein levels of FADD, Bax, caspase-8 and Bcl-2 in RPE cells
Western blot analysis showed that the expression of the apoptosis-related proteins FADD, Bax, caspase-8 and Bcl-2 was up-regulated in a time-dependent manner in the peroxynitrite group compared with the control group (P < 0.01). CCK-8 suppressed the changes induced by peroxynitrite (P <0.01;Figure 4).
Effect of peroxynitrite and CCK-8 on caspase-8 and bcl-2 mRNA expression in RPE cells
RT-PCR showed that peroxynitrite markedly upregulated caspase-8 and bcl-2 mRNA expression in the RPE cells, compared with the control group (P < 0.01). CCK-8 suppressed the changes induced by peroxynitrite (P < 0.01;Figure 5).
Discussion
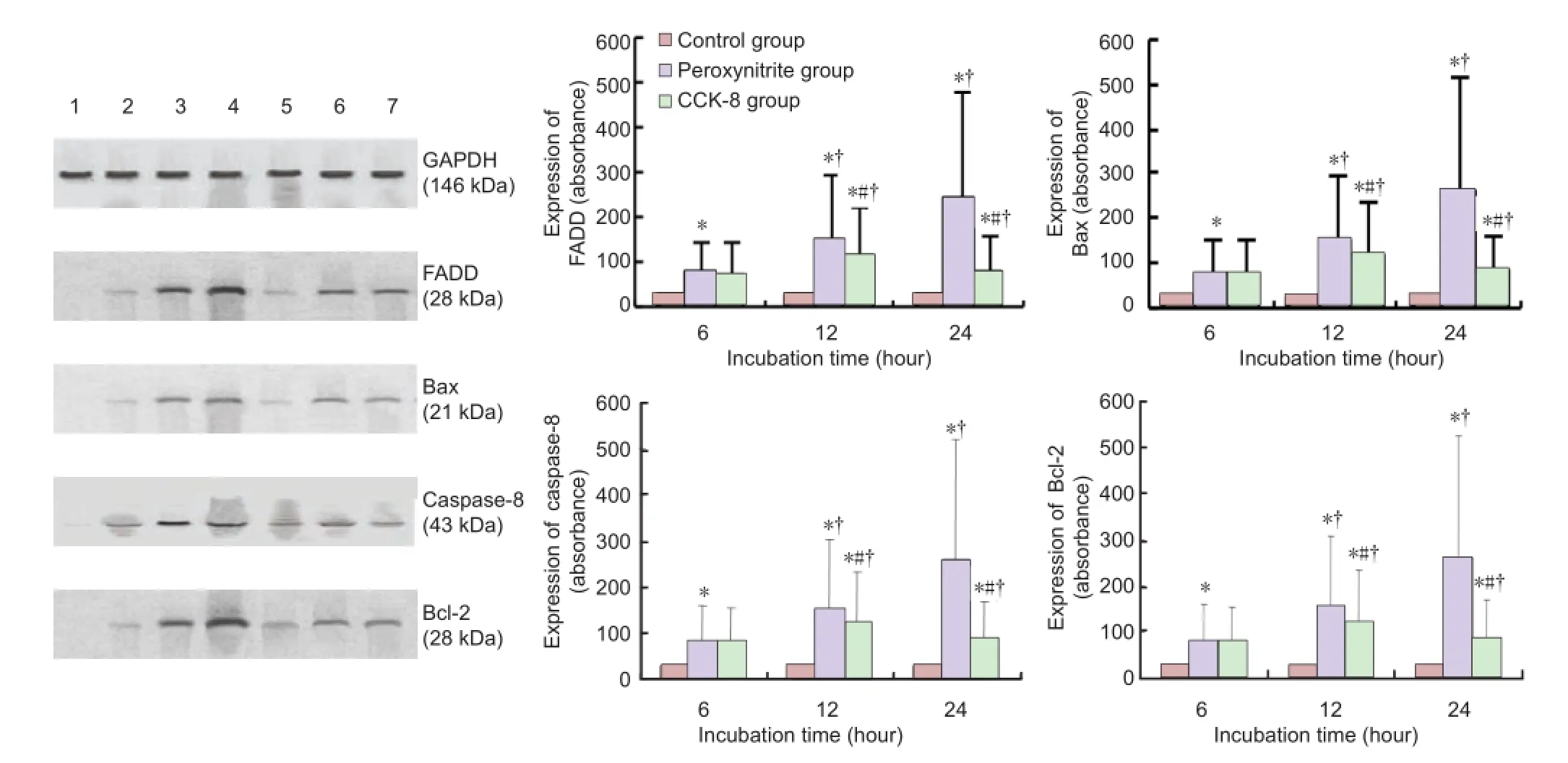
Figure 4 Effect of peroxynitrite and cholecystokinin octapeptide-8 (CCK-8) on protein levels of Fas-associated death domain (FADD), Bax, caspase-8 and bcl-2 in human retinal pigment epithelial cells.
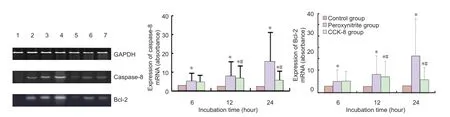
Figure 5 Effect of peroxynitrite and cholecystokinin octapeptide-8 (CCK-8) on caspase-8 and bcl-2 mRNA expression in human retinal pigment epithelial cells.
Recently, we demonstrated that Fas/FasL interactions are critical for maintaining immune privilege in the eye. Cell death induced by Fas/FasL is important for the induction of apoptosis in RPE cells (Hao et al., 2010, 2011a, c, d). Because apoptosis in the eye is a rapid, yet tightly modulated process, we examined whether signals in the ocular microenvironment regulate apoptosis in RPE cells. There is very little information on RPE cell signaling through the Fas/ FasL-FADD-caspase-8 pathway, despite being very important for eye diseases such as age-related macular degeneration and proliferative vitreoretinopathy (Kroll et al., 2007; Stone, 2007; Meleth et al., 2011; Querques et al., 2011). Consequently, it is crucial to determine how apoptosis in the eye is regulated by Fas/FasL-FADD-caspase-8 signaling, and whether anti-apoptotic factors can counteract cell death. To better understand the mechanisms regulating apoptosis in the eye, in the present study, we focused on the effects of FADD and caspase-8 signaling in RPE cells.
It is thought that the relative balance between anti- and pro-apoptotic signaling determines the viability of a cell. RPE cells are critically important for neural retinal function. Thus, RPE cells exposed to oxidative stress are likely to express many anti-apoptotic proteins and genes. Indeed, we found that apoptosis-related proteins and genes were expressed in cultured RPE cells. Expression of FADD, caspase-8 and Bax were upregulated by peroxynitrite. These changes were suppressed by CCK-8. In general, the expression of the apoptosis-related proteins under basal conditions mirrored mRNA expression as determined by RT-PCR. In addition, caspase-8 protein levels paralleled caspase-8 mRNA expres-sion after peroxynitrite stimulation, and Bcl-2 protein levels paralleled bcl-2 mRNA expression after CCK-8 administration. To our knowledge, these data are the first to demonstrate an involvement of FADD and caspase-8 in RPE cell apoptosis. The pattern of FADD and caspase-8 expression under basal conditions and after exposure to peroxynitrite stimulus is very cell type speci fi c.
Normal cell growth requires a precisely controlled balance between cell death and survival. This involves activation of different types of intracellular signaling cascades within the cell. While some types of signaling proteins promote apoptosis, or programmed cell death, other proteins within the cell can promote survival. Bcl-2 can protect RPE cells from apoptosis in response to several different types of stimuli. We infer one way that Bcl-2 may promote cell survival is by phosphorylating and thereby inhibiting the proapoptotic protein Bad. This leads in turn to the inhibition of effector caspases such as caspase-8. Under these conditions, Bcl-2 inhibits apoptosis early in the caspase cascade, antagonizing the activation of the apoptotic initiator, caspase-8. This inhibition of apoptosis may involve suppression of caspase-8 recruitment to the death domain receptors. This role in regulating initiator caspases is an entirely novel role for the Bcl-2 proteins and suggests a new mechanism by which these proteins promote cell survival.
Taken together, our fi ndings show that Bcl-2 overexpression suppresses oxidative stress events. Our data suggest that Bcl-2, rather than Bax, is an important endogenous RPE cell anti-apoptotic factor, which is consistent with other reports (Banga et al., 2007; Ploner et al., 2008; Teijido and Dejean, 2010; Willimott and Wagner, 2010; Vogler et al., 2011). Further studies are needed to provide further support for this contention. These results highlight the importance of celltype speci fi city in therapeutic strategies for inducing apoptotic cell death. Members of the Bcl-2 protein family are crucial apoptosis regulators. We evaluated the expression of Bcl-2 mRNA by RT-PCR, which showed that levels were regulated by CCK-8. An increase in Bcl-2 expression prevented RPE cell apoptosis in an oxidative stress model. In addition, we readily detected Bax protein in cultured human RPE cells by western blot analysis. Similarly, other investigators have identi fi ed Bax protein in RPE cells (Letai, 2009; Lalier et al., 2011; Robinson et al., 2011).
Peroxynitrite upregulated FADD, Bax and caspase-8, indicating that it modulates RPE cell apoptosis (Jung et al., 2009; Hirschberg et al., 2010; Holthoff et al., 2010; Gaupels et al., 2011; Juhász et al., 2011). Oxidative stress-induced RPE cell apoptosis has been proposed as a major pathophysiological mechanism in age-related macular degeneration and proliferative vitreoretinopathy (Agrawal et al., 2007; Coleman et al., 2008). Apoptosis is the result of a cascade of gene expression. Numerous genes have been found to contribute to the regulation of apoptosis. It is thought that apoptosis is regulated by an interaction between gene expression and signaling cascades initiated at the cell surface (Ferrington et al., 2006; Zhou et al., 2010; Hao et al., 2011b; DiBaise et al., 2012). The multifunctional protein FADD and caspase-8 could participate in the mechanisms of RPE cell apoptosis induced by peroxynitrite. The death inducing signaling complex formed by Fas receptor, FADD and caspase-8 is a pivotal trigger of apoptosis. The Fas-FADD death inducing signaling complex represents a receptor platform, which once assembled, initiates apoptosis. A highly oligomeric network of homotypic protein interactions comprised of the death domains of Fas and FADD is at the centre of the death inducing signaling complex. Thus, characterizing the Fas-FADD interaction is crucial for understanding cell death induction. Scott et al. (2009) successfully isolated the human Fas-FADD death domain complex and reported its crystal structure. The complex has a tetrameric arrangement of four FADD death domains bound to four Fas death domains. An opening of the Fas death domain exposes the FADD binding site and simultaneously generates a Fas-Fas bridge. The result is a regulatory Fas-FADD complex bridge governed by weak protein-protein interactions revealing a model where the complex itself functions as a mechanistic switch. This switch prevents accidental death inducing signaling complex assembly, yet allows for assembly and clustering upon a suffi cient stimulus.
In addition to revealing a previously unknown mode of death domain interactions, these results further uncover a mechanism for receptor signaling solely by oligomerization and clustering events (álvaro-Bartolomé et al., 2010; Thorenoor et al., 2010). Accumulating evidence suggests that apoptosis plays an important role in numerous pathophysiological processes (Matsuda et al., 2009; Drakos et al., 2011). A better understanding of the molecular mechanisms involved in the pathogenesis of RPE cell death is required to develop new therapeutic approaches.
FADD and its apoptotic partner, caspase-8, have also been implicated in necrosis. FADD is intriguing in that T-cell receptor-induced proliferation is blocked in FADD-defective T cells. FADD appears to help keep T-cell receptor-induced programmed necrotic signaling in check during early phases of T-cell clone expansion (Osborn et al., 2010; Ikner and Ashkenazi, 2011). Death domain complexes are key protein arrangements in the regulation of various cellular signaling events. One of the most prominent death domain complexes fi rst described in the initiation of apoptosis is formed by the transmembrane receptor Fas, the cytosolic adaptor protein FADD and caspase-8, and is referred to as the Fas/FADD/ caspase-8 death inducing signaling complex. The recent structure of the Fas/FADD death domain complex reveals how formation of this signaling platform can be stringently regulated by Fas receptor clustering to form a death domain network (Salvesen and Riedl, 2009; Li et al., 2010; Wang et al., 2010). The formation of the death-inducing signaling complex is a direct indicator of the activation of the Fascaspase-8 signaling pathway. The production of cytokines such as type I interferon is an essential component of innate immunity. A reduction in FADD and TRIM21 (TRIM21 is a member of a large family of proteins that can impart ubiquitin modi fi cation onto its cellular targets) levels leads to higher interferon-α induction, IRF7 phosphorylation, and lower titers of RNA virus in infected cells (García-Fuster et al., 2009; Matsumura et al., 2009; Young et al., 2011).Caspases are a family of aspartate-speci fi c cysteine proteases responsible for the biochemical and morphological changes that occur during the execution phase of apoptosis. The hierarchical ordering of caspases has been clearly established using dATP-activated cell lysates to model the intrinsic pathway induced by initial mitochondrial perturbation. In the model, caspase-9, the initiator caspase, directly processes and activates the effector caspases, caspase-3 and caspase-7. Active caspase-3 then processes caspase-2 and caspase-6, and subsequently, the activated caspase-6 processes caspase-8 and caspase-10. The processing of caspase-2 and caspase-6 occurs within the cytoplasm and active caspase-6 is then responsible for both the processing of caspase-8 and the cleavage of caspase-6 substrates (Inoue et al., 2009). Gene-targeted mice show that caspase-8 is essential for hepatocyte killing (O’Reilly et al., 2004; Kaufmann et al., 2009). Furthermore, L. interrogans-induced apoptosis in macrophages is mediated by caspase-3 and caspase-6 activation through a FADD-caspase-8-dependent pathway, independently of mitochondrial cytochrome c-caspase-9 signaling (Jin et al., 2009).
The Bax protein is pivotal for the apoptotic process. Bax, which resides in an inactive form in the cytosol of healthy cells, is activated during the early stages of apoptosis and becomes associated with mitochondria through poorly understood mechanisms. A cysteine is present in the loop between the two transmembrane alpha helices of Bax (Letai, 2009; Lalier et al., 2011; Robinson et al., 2011).
In conclusion, we speculate that CCK-8, along with antioxidant enzymes such as catalase, superoxide dismutase and glutathione peroxidase, protect RPE cells from oxidative stress-induced apoptotic cell death by modulating FADD and caspase-8 signaling.
Author contributions:All authors were responsible for the study design, implementing the experiment, and evaluating the results. Liu Y drafted the manuscript. All authors approved the final version of the manuscript.
Con fl icts of interest:None declared.
Agbani EO, Coats P, Wadsworth RM (2011a) Acute hypoxia stimulates intracellular peroxynitrite formation associated with pulmonary artery smooth muscle cell proliferation. J Cardiovasc Pharmacol 57:584-588.
Agbani EO, Coats P, Mills A, Wadsworth RM (2011b) Peroxynitrite stimulates pulmonary artery endothelial and smooth muscle cell proliferation: involvement of ERK and PKC. Pulm Pharmacol Ther 24:100-109.
Agrawal RN, He S, Spee C, Cui JZ, Ryan SJ, Hinton DR (2007) In vivo models of proliferative vitreoretinopathy. Nat Protoc 2:67-77.
álvaro-Bartolomé M, Esteban S, García-Gutiérrez MS, Manzanares J, Valverde O, García-Sevilla JA (2010) Regulation of Fas receptor/ Fas-asssociated protein with death domain apoptotic complex and associated signalling systems by cannabinoid receptors in the mouse brain. Brit J Pharmacol 160:643-656.
Banga S, Gao P, Shen X, Fiscus V, Zong WX, Chen L, Luo ZQ (2007) Legionella pneumophila inhibits macrophage apoptosis by targeting pro-death members of the Bcl2 protein family. Proc Natl Acad Sci U S A 104:5121-5126.
Coleman HR, Chan CC, Ferris FL, Chew EY (2008) Age-related macular degeneration. Lancet 372:1835-1845.
DiBaise JK, Richmond BK, Ziessman HA, Everson GT, Fanelli RD, Maurer AH, Ouyang A, Shamamian P, Simons RJ, Wall LA, Weida TJ, Tulchinsky M (2012) Cholecystokinin-cholescintigraphy in adults: consensus recommendations of an interdisciplinary panel. Clin Nucl Med 37:63-70.
Drake J, Kanski J, Varadarajan S, Tsoras M, Butter fi eld DA (2002) Elevation of brain glutathione by γ-glutamylcysteine ethyl ester protects against peroxynitrite-induced oxidative stress. J Neurosci Res 68:776-784.
Drakos E, Leventaki V, Atsaves V, Schlette EJ, Lin P, Vega F, Miranda RN, Claret F-X, Medeiros LJ, Rassidakis GZ (2011) Expression of serine 194-phosphorylated Fas-associated death domain protein correlates with proliferation in B-cell non-Hodgkin lymphomas. Hum Pathol 42:1117-1124.
Ferrington DA, Tran TN, Lew KL, Van Remmen H, Gregerson DS (2006) Different death stimuli evoke apoptosis via multiple pathways in retinal pigment epithelial cells. Exp Eye Res 83:638-650.
García-Fuster MJ, Clinton SM, Watson SJ, Akil H (2009) Effect of cocaine on Fas-associated protein with death domain in the rat brain: individual differences in a model of differential vulnerability to drug abuse. Neuropsychopharmacology 34:1123-1134.
Gaupels F, Spiazzi-Vandelle E, Yang D, Delledonne M (2011) Detection of peroxynitrite accumulation in Arabidopsis thaliana during the hypersensitive defense response. Nitric Oxide 25:222-228.
Gebicka L, Didik J (2010) Oxidative stress induced by peroxynitrite. Postepy Biochem 56:103-106.
Guven A, Uysal B, Caliskan B, Oztas E, Ozturk H, Korkmaz A (2011) Mercaptoethylguanidine attenuates caustic esophageal injury in rats: a role for scavenging of peroxynitrite. J Pediatr Surg 46:1746-1752.
Hamamura M, Ozawa H, Ozaki M, Shimazoe T, Terada Y, Fukumaki Y (2010) Repeated administration of methamphetamine blocked cholecystokinin-octapeptide injection-induced c-fos mRNA expression without change in capsaicin-induced junD mRNA expression in rat cerebellum. J Neural Transm 117:1041-1053.
Hao LN, Zhang YQ, Shen YH, Wang ZY, Wang YH (2011a) Inducible nitric oxide synthase and Fas/FasL with C3 expression of mouse retinal pigment epithelial cells in response to stimulation by peroxynitrite and antagonism of puerarin. Chin Med J (Engl) 124: 2522-2529.
Hao LN, Zhang XD, Wang M, Yang T, He SZ (2011b) Peroxynitrite-induced expression of inducible nitric oxide synthase and activated apoptosis via nuclear factor-kappa B pathway in retinal pigment epithelial cells and antagonism of cholecystokinin octapeptide-8 in vitro. Int J Ophthalmol 4:474-479.
Hao LN, He SZ, Shen YH, Zhang YQ, Wang ZY, Wang YH (2011c) Protective effects of puerarin on lens epithelial cells in rat diabetic cataract. Zhonghua Yan Ke Za Zhi 47:320-326.
Hao LN, Zhang YQ, Shen YH, Wang ZY, Wang YH, Zhang HF, He SZ (2010) Effect of puerarin on retinal pigment epithelial cells apoptosis induced partly by peroxynitrite via Fas/FasL pathway. Int J Ophthalmol 3:283-287.
Hao LN, Zhang YQ, Shen YH, Li MQ, Yang T, Zhao ZH, Wang ZY, Wang YH, He SZ (2011d) Toxicity of endogenous peroxynitrite and effects of puerarin on transplanted retinal pigment epithelial sheets in the subretinal space in mice. Int J Ophthalmol 4:250-254.
Herrmann M, Lorenz HM, Voll R, Grünke M, Woith W, Kalden JR (1994) A rapid and simple method for the isolation of apoptotic DNA fragments. Nucleic Acids Res 22:5506-5507.
Hirschberg K, Radovits T, Korkmaz S, Loganathan S, Z?llner S, Seidel B, Páli S, Barnucz E, Merkely B, Karck M, Szabó G (2010) Combined superoxide dismutase mimetic and peroxynitrite scavenger protects against neointima formation after endarterectomy in association with decreased proliferation and nitro-oxidative stress. Eur J Vasc Endovasc Surg 40:168-175.
Holthoff JH, Woodling KA, Doerge DR, Burns ST, Hinson JA, Mayeux PR (2010) Resveratrol, a dietary polyphenolic phytoalexin, is a functional scavenger of peroxynitrite. Biochem Pharmacol 80:1260-1265.
Ikner A, Ashkenazi A (2011) TWEAK induces apoptosis through a death-signaling complex comprising receptor-interacting protein 1 (RIP1), Fas-associated death domain (FADD), and caspase-8. J Biol Chem 286:21546-21554.
Inoue S, Browne G, Melino G, Cohen GM (2009) Ordering of caspases in cells undergoing apoptosis by the intrinsic pathway. Cell Death Differ 16:1053-1061.
Jang HY, Ji SJ, Kim YH, Lee HY, Shin JS, Cheong HT, Kim JT, Park IC, Kong HS, Park CK, Yang BK (2010) Antioxidative effects of astaxanthin against nitric oxide-induced oxidative stress on cell viability and gene expression in bovine oviduct epithelial cell and the developmental competence of bovine IVM/IVF embryos. Reprod Domest Anim 45:967-974.
Jin D, Ojcius DM, Sun D, Dong H, Luo Y, Mao Y, Yan J (2009) Leptospira interrogans induces apoptosis in macrophages via caspase-8- and caspase-3-dependent pathways. Infect Immun 77:799-809.
Juhász L, Kiss A, Nyes? E, Kovács M, Seprényi G, Kaszaki J, Végh A (2011) Is there a trigger role of peroxynitrite in the anti-arrhythmic effect of ischaemic preconditioning and peroxynitrite infusion? Eur J Pharmacol 667:306-313.
Jung M, Hotter G, Vi?as JL, Sola A (2009) Cisplatin upregulates mitochondrial nitric oxide synthase and peroxynitrite formation to promote renal injury. Toxicol Appl Pharmacol 234:236-246.
Kaufmann T, Jost PJ, Pellegrini M, Puthalakath H, Gugasyan R, Gerondakis S, Cretney E, Smyth MJ, Silke J, Hakem R, Bouillet P, Mak TW, Dixit VM, Strasser A (2009) Fatal hepatitis mediated by tumor necrosis factor TNFalpha requires caspase-8 and involves the BH3-only proteins Bid and Bim. Immunity 30:56-66.
Koppenol WH, Kissner R, Beckman JS (1996) Syntheses of peroxynitrite: to go with the fl ow or on solid grounds? Methods Enzymol 269: 296-302.
Koyama Y, Matsuzaki S, Gomi F, Yamada K, Katayama T, Sato K, Kumada T, Fukuda A, Matsuda S, Tano Y, Tohyama M (2008) Induction of amyloid beta accumulation by ER calcium disruption and resultant upregulation of angiogenic factors in ARPE19 cells. Invest Ophthalmol Vis Sci 49:2376-2383.
Kroll P, Rodrigues EB, Hoerle S (2007) Pathogenesis and classi fi cation of proliferative diabetic vitreoretinopathy. Ophthalmologica 221:78-94.
Lalier L, Cartron PF, Olivier C, Logé C, Bougras G, Robert JM, Oliver L, Vallette FM (2011) Prostaglandins antagonistically control Bax activation during apoptosis. Cell Death Differ 18:528-537.
Letai A (2009) Puma strikes Bax. J Cell Biol 185:189-191.
Li P, Jayarama S, Ganesh L, Mordi D, Carr R, Kanteti P, Hay N, Prabhakar BS (2010) Akt-phosphorylated mitogen-activated kinase-activating death domain protein (MADD) inhibits TRAIL-induced apoptosis by blocking Fas-associated death domain (FADD) association with death receptor 4. J Biol Chem 285:22713-22722.
Matsuda N, Yamamoto S, Takano KI, Kageyama SI, Kurobe Y, Yoshihara Y, Takano Y, Hattori Y (2009) Silencing of Fas-associated death domain protects mice from septic lung in fl ammation and apoptosis. Am J Respir Crit Care Med 179:806-815.
Matsumura Y, Shimada K, Tanaka N, Fujimoto K, Hirao Y, Konishi N (2009) Phosphorylation status of Fas-associated death domain-containing protein regulates telomerase activity and strongly correlates with prostate cancer outcomes. Pathobiology 76:293-302.
Meleth AD, Wong WT, Chew EY (2011) Treatment for atrophic macular degeneration. Curr Opin Ophthalmol 22:190-193.
Merino B, Somoza B, Ruiz-Gayo M, Cano V (2008) Circadian rhythm drives the responsiveness of leptin-mediated hypothalamic pathway of cholecystokinin-8. Neurosci Lett 442:165-168.
Noble F (2007) Pharmacology of CCKRs and SAR studies of peptidic analog ligands. Curr Top Med Chem 7:1173-1179.
O’Reilly LA, Divisekera U, Newton K, Scalzo K, Kataoka T, Puthalakath H, Ito M, Huang DC, Strasser A (2004) Modi fi cations and intracellular traf fi cking of FADD/MORT1 and caspase-8 after stimulation of T lymphocytes. Cell Death Differ 11:724-736.
Osborn SL, Diehl G, Han SJ, Xue L, Kurd N, Hsieh K, Cado D, Robey EA, Winoto A (2010) Fas-associated death domain (FADD) is a negative regulator of T-cell receptor-mediated necroptosis. Proc Natl Acad Sci U S A 107:13034-13039.
Oz M, Yang KH, Shippenberg TS, Renaud LP, O’Donovan MJ (2007) Cholecystokinin B-type receptors mediate a G-protein-dependent depolarizing action of sulphated cholecystokinin ocatapeptide (CCK-8s) on rodent neonatal spinal ventral horn neurons. J Neurophysiol 98:1108-1114.
Ploner C, Rainer J, Niederegger H, Eduardoff M, Villunger A, Geley S, Ko fl er R (2008) The BCL2 rheostat in glucocorticoid-induced apoptosis of acute lymphoblastic leukemia. Leukemia 22:370-377.
Querques G, Coscas F, Forte R, Massamba N, Sterkers M, Souied EH (2011) Cystoid macular degeneration in exudative age-related macular degeneration. Am J Ophthalmol 152:100-107.
Robinson KS, Clements A, Williams AC, Berger CN, Frankel G (2011) Bax inhibitor 1 in apoptosis and disease. Oncogene 30:2391-2400.
Rodriguez PC, Beconi MT (2009) Peroxynitrite participates in mechanisms involved in capacitation of cryopreserved cattle. Anim Reprod Sci 110:96-107.
Ru XC, Liang KY, Lei WH, Tan YN, Xia Q (2011) Bicyclol protects rat thoracic aorta from superoxide anion-induced inhibition of vascular relaxation. Zhongguo Ying Yong Sheng Li Xue Za Zhi 27:81-85.
Salvesen GS, Riedl SJ (2009) Structure of the Fas/FADD complex: a conditional death domain complex mediating signaling by receptor clustering. Cell Cycle 8:2723-2727.
Sankaralingam S, Lalu MM, Xu Y, Davidge ST (2010) Effect of peroxynitrite scavenging on endothelial cells stimulated by plasma from women with preeclampsia: a proteomic approach. Hypertens Pregnancy 29:419-428.
Scott FL, Stec B, Pop C, Dobaczewska MK, Lee JJ, Monosov E, Robinson H, Salvesen GS, Schwarzenbacher R, Riedl SJ (2009) The Fas-FADD death domain complex structure unravels signalling by receptor clustering. Nature 457:1019-1022.
Sinha K, Das J, Pal PB, Sil PC (2013) Oxidative stress: the mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch Toxicol 87:1157-1180.
Stone EM (2007) Macular degeneration. Annu Rev Med 58:477-490.
Sugaya K, Takahashi M, Kubota K (1992) Cholecystokinin protects cholinergic neurons against basal forebrain lesion. Jpn J Pharmacol 59:125-128.
Teijido O, Dejean L (2010) Upregulation of Bcl2 inhibits apoptosisdriven BAX insertion but favors BAX relocalization in mitochondria. FEBS Lett 584:3305-3310.
Thiagarajan G, Lakshmanan J, Chalasani M, Balasubramanian D (2004) Peroxynitrite reaction with eye lens proteins: alpha-crystallin retains its activity despite modi fi cation. Invest Ophthalmol Vis Sci 45:2115-2121.
Thorenoor N, Lee JH, Lee SK, Cho SW, Kim YH, Kim KS, Lee C (2010) Localization of the death effector domain of Fas-associated death domain protein into the membrane of Escherichia coli induces reactive oxygen species-involved cell death. Biochemistry 49:1435-1447.
Vogler M, Hamali HA, Sun XM, Bampton ET, Dinsdale D, Snowden RT, Dyer MJ, Goodall AH, Cohen GM (2011) BCL2/BCL-X(L) inhibition induces apoptosis, disrupts cellular calcium homeostasis, and prevents platelet activation. Blood 117:7145-7154.
Wang L, Yang JK, Kabaleeswaran V, Rice AJ, Cruz AC, Park AY, Yin Q, Damko E, Jang SB, Raunser S, Robinson CV, M SR, Walz T, Wu H (2010) The Fas-FADD death domain complex structure reveals the basis of DISC assembly and disease mutations. Nat Struct Mol Biol 17:1324-1329.
Wijeratne SS, Cuppett SL, Schlegel V (2005) Hydrogen peroxide induced oxidative stress damage and antioxidant enzyme response in Caco-2 human colon cells. J Agric Food Chem 53:8768-8774.
Willimott S, Wagner SD (2010) Post-transcriptional and post-translational regulation of Bcl2. Biochem Soc Trans 38:1571-1575.
Yang P, Wiser JL, Peairs JJ, Ebright JN, Zavodni ZJ, Bowes Rickman C, Jaffe GJ (2005) Human RPE expression of cell survival factors. Invest Ophthalmol Vis Sci 46:1755-1764.
Young JA, Sermwittayawong D, Kim HJ, Nandu S, An N, Erdjument-Bromage H, Tempst P, Coscoy L, Winoto A (2011) Fas-associated death domain (FADD) and the E3 ubiquitin-protein ligase TRIM21 interact to negatively regulate virus-induced interferon production. J Biol Chem 286:6521-6531.
Zhou Z, Wu M, Barrett RP, McClellan SA, Zhang Y, Hazlett LD (2010) Role of the Fas pathway in Pseudomonas aeruginosa keratitis. Invest Ophthalmol Vis Sci 51:2537-2547.
Copyedited by Patel B, Norman C, Yu J, Yang Y, Li CH, Song LP, Zhao M
10.4103/1673-5374.137596
Lina Hao, M.D., Department of Ophthalmology, Hebei Province People’s Hospital, Shijiazhuang, Hebei Province, China, 75765892@qq.com.
http://www.nrronline.org/
Accepted: 2014-05-24
- 中國神經(jīng)再生研究(英文版)的其它文章
- Heat shock protein 72 confers protection in retinal ganglion cells and lateral geniculate nucleus neurons via blockade of the SAPK/JNK pathway in a chronic ocular-hypertensive rat model
- Amplitude of sensory nerve action potential in early stage diabetic peripheral neuropathy: an analysis of 500 cases
- Ultrasound imaging of chitosan nerve conduits that bridge sciatic nerve defects in rats
- Green tea polyphenols protect spinal cord neurons against hydrogen peroxide-induced oxidative stress
- Dynamic culture of a thermosensitive collagen hydrogel as an extracellular matrix improves the construction of tissue-engineered peripheral nerve
- Electroacupuncture attenuates neuropathic pain after brachial plexus injury

