Genistein suppresses the mitochondrial apoptotic pathway in hippocampal neurons in rats with Alzheimer’s disease
Yan Wang , Biao Cai , , Jing Shao , Ting-ting Wang , Run-ze Cai Chang-ju Ma Tao Han Jun Du
1 School of Integrated Chinese and Western Medicine, Anhui University of Chinese Medicine, Hefei, Anhui Province, China
2 Institute of Integrated Chinese and Western Medicine, Anhui Academy of Chinese Medicine, Hefei, Anhui Province, China
Genistein suppresses the mitochondrial apoptotic pathway in hippocampal neurons in rats with Alzheimer’s disease
Yan Wang1,2, Biao Cai1,2,*, Jing Shao1,2, Ting-ting Wang1,2, Run-ze Cai1, Chang-ju Ma1, Tao Han1, Jun Du1
1 School of Integrated Chinese and Western Medicine, Anhui University of Chinese Medicine, Hefei, Anhui Province, China
2 Institute of Integrated Chinese and Western Medicine, Anhui Academy of Chinese Medicine, Hefei, Anhui Province, China
Graphical Abstract

*Correspondence to: Biao Cai, caibiao9035@163.com.
orcid: 0000-0002-3375-0073 (Biao Cai)
Genistein is effective against amyloid-β toxicity, but the underlying mechanisms are unclear. We hypothesized that genistein may protect neurons by inhibiting the mitochondrial apoptotic pathway, and thereby play a role in the prevention of Alzheimer's disease. A rat model of Alzheimer's disease was established by intraperitoneal injection of D-galactose and intracerebral injection of amyloid-β peptide (25—35). In the genistein treatment groups, a 7-day pretreatment with genistein (10, 30, 90 mg/kg) was given prior to establishing Alzheimer's disease model, for 49 consecutive days. Terminal deoxyribonucleotidyl transferase-mediated dUTP nick end labeling assay demonstrated a reduction in apoptosis in the hippocampus of rats treated with genistein. Western blot analysis showed that expression levels of capase-3, Bax and cytochrome c were decreased compared with the model group. Furthermore, immunohistochemical staining revealed reductions in cytochrome c and Bax immunoreactivity in these rats. Morris water maze revealed a substantial shortening of escape latency by genistein in Alzheimer's disease rats. These findings suggest that genistein decreases neuronal loss in the hippocampus, and improves learning and memory ability. The neuroprotective effects of genistein are associated with the inhibition of the mitochondrial apoptotic pathway, as shown by its ability to reduce levels of caspase-3, Bax and cytochrome c.
nerve regeneration; genistein; Alzheimer's disease; mitochondrion; apoptosis; hippocampus; Bax; cytochrome c; caspase-3; learning; memory; neural regeneration
Introduction
Alzheimer's disease (AD) is a deadly progressive neurodegenerative disease, the clinical symptoms of which include poor perception and memory abilities, and a progressive decline in the quality of daily living associated with neurological and behavioral dysfunction (Bhattacharya and Montag, 2015). The pathological changes in AD include a reduction in neuronal numbers, and the appearance of senile plaques and neurofibrillary tangles (Baruch et al., 2015; Calafate et al., 2015).
Although estrogen therapy plays an important role in AD prevention, additional drugs are needed for a cure. Genistein (GS; 4′,5,7-trihydroxyisoflavone) is an isoflavone compound rich in soybean, oat, rye and maize. GS has therapeutic effects against cancer, cardiovascular disease and osteoporosis, likely through its ability to modulate the immune system (Ma et al., 2010; Rahman et al., 2012; Behloul et al., 2013; Tae et al., 2013; Kinoshita et al., 2014). It has been reported that GS reduces apoptosis of hippocampal and cortical neurons in vitro, and that it crosses the blood-brain barrier to influence brain morphology and reduce amyloid-β (Aβ) toxicity in the brain (Liu et al., 2008; Liao et al., 2013; Wang et al., 2014; Aras et al., 2015). GS also has phytoestrogen-like properties, and it can prevent the severe side effects caused by estrogen replacement therapy (Reiter et al., 2009). However, the underlying mechanisms of action of GS are not well understood.
Apoptosis is in large part responsible for the massive loss of neurons in AD patients (Wu et al., 2013; Obulesu et al., 2014). Therefore, reducing neuronal apoptosis should improve the symptoms of AD.
There are two major pathways of apoptosis, i.e., caspase-dependent apoptosis and caspase-independent apoptosis. Caspase-dependent apoptosis can be divided into the exogenous pathway initiated by death receptors and the endogenous mitochondrial pathway. In the endogenous pathway, under the action of various apoptosis stimulating factors, the integrity of mitochondria was damaged and the permeability of mitochondria was increased. Mitochondria release cytochrome c and other apoptotic factors after receiving apoptotic signals. Cytochrome c forms an apoptotic complex with Apaf-1 and caspase-9 precursor. Caspase-9, caspase-3 and caspase-7 are successively activated, inducing apoptosis. The release of apoptotic factors is closely associated with mitochondrial membrane permeability, which is regulated mainly by Bcl-2 and Bax, which are members of the Bcl-2 family of proteins. Bcl-2 inhibits apoptosis, while Bax promotes apoptosis (Tan et al., 2006; Fennell et al., 2008; Malla et al., 2010; Zhao et al., 2014).
In this study, we investigate the effect of GS on apoptosis in hippocampal neurons in a rat model of AD, and we examine the underlying mechanisms of action. We focus on whether the neuroprotective effects of GS are associated with inhibition of the mitochondrial apoptotic pathway.
Materials and Methods
Animals
A total of 120 healthy female Sprague-Dawley rats, 10 months old and weighing 250—350 g, were provided by the Animal Experiment Center of Anhui Medical University in China (certification No. SXCK (Wan) 2011-002). This experiment was approved by the Center of Scientific Research of Anhui University of Chinese Medicine.
The rats were randomly allocated to the following six groups: sham-operated (sham group), AD (model group), GS-low (GS-L group; 10 mg/kg), GS-moderate (GS-M group; 30 mg/kg), GS-high (GS-H group; 90 mg/kg), and estradiol valerate (EV group; 0.3 mg/kg).
Establishment of a rat model of AD and drug treatment
For the establishment of AD model, 2 mg/mL Aβ25—35(Sigma, St. Louis, MO, USA) was prepared using sterile normal saline and incubated at 37°C for 7 days. Except for the sham group, the rats in the other five groups were intraperitoneally injected with 100 mg/kg D-galactose (Sinopharm Chemical Reagent, Shanghai, China) once per day for 42 days. On the twenty-first day of the injection with D-galactose, rats were then intraperitoneally administered 10% chloral hydrate (380 mg/kg) and immobilized on a brain stereotaxic apparatus (Stoelting, Wood Dale, IL, USA). The center of the vertex was cut to expose the bregma, and then a hole was drilled in the skull, 4.4 mm posterior and 2.2 mm lateral to the bregma. The needle was inserted 3.0 mm deep from the brain surface into the hippocampus. 5 μL Aβ25—35(10 μg) was injected slowly using a microsyringe over a period of 5 minutes, and maintained in place for 5 minutes. The injection was performed bilaterally. Rats in the sham group were injected with the same volume of normal saline (5 μL). After needle withdrawal, the incision was coated lightly with penicillin sodium powder, sutured, and sterilized with iodine. Penicillin sodium was injected in the abdominal cavity to prevent infection for 3 days postoperatively. Morris water maze test indicted that the rat model of AD was established successfully (Fine et al., 1985; Irie et al., 1996; Sigurdsson et al., 1996).
Drug treatment was started on pre-operative day 7, and was given for 49 consecutive days. GS (Sigma) was prepared at concentrations of 1 mg/mL (GS-L group), 3 mg/mL (GS-M group) and 9 mg/mL (GS-H group) in 0.5% carboxymethyl cellulose sodium. EV (Delpharm Lille SAS, ZI de Roubaix-Est, France) was prepared to a final concentration of 0.03 mg/mL in distilled water. The three GS groups and EV group were intragastrically administered the respective drug after weighing (1 mL/100 g), once a day. The rats in the model group were given the same amount of 0.5% carboxymethyl cellulose sodium, and those in the sham group were given normal feed.
Sample preparation
After drug treatment, 10 rats from each group were anesthetized by intraperitoneal injection of 10% chloral hydrate (380 mg/kg) and decapitated. The brain tissues were taken out on ice and washed with 4°C normal saline to clear the tissue of blood. The hippocampi were dissected out and stored at -80°C to detect caspase-3, Bax and cytochrome c by western blot assay. The remaining 10 rats in each group were subjected to cardiac perfusion and fixation. The rats were anesthetized as above and perfused through the heart with 100—200 mL normal saline, followed by 300—350 mL 4% paraformaldehyde, until the body was stiff. Each rat was decapitated, and the brain removed and fixed in 4% paraformaldehyde for observation of apoptosis by terminal deoxyribonucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay and for assessing Bax and cytochrome c expression by immunohistochemistry.
Morris water maze test
Morris water maze test was carried out on day 33 postoperatively to evaluate learning and memory ability. The rats were tested once a day for 6 days. In the test, the rats were put into the water facing the pool wall. The time for searching and climbing the platform (escape latency) was recorded. If the rat did not find the platform within 120 seconds, it was led to the platform, and the escape latency was recorded as 120 seconds. The temperature of the water was kept at 26 ± 1°C.
The escape latency was monitored and analyzed with the Morris water maze image automatic monitoring and processing system (ZH, Huaibei, Anhui Province, China).
TUNEL assay for apoptosis in hippocampal cells
The fixed brain tissue was dehydrated, embedded, sectioned, dewaxed, and stained by TUNEL according to the manufacturer's instructions (Roche, Basel, Switzerland). One 400× field was randomly chosen for observing the CA1 region of the hippocampus with a microscope (BX51; Olympus, Tokyo, Japan). A cell clearly displaying tan-colored particles in the nucleus was defined as a TUNEL-positive cell.
Western blot assay
Caspase-3, Bax and cytochrome c were detected by western blot assay in rat hippocampal homogenates. Protein samples were separated by 10% sodium dodecylsulfate-polyacrylamide gel electrophoresis. The separated proteins were then transferred from the gel onto a nitrocellulose membrane. The membranes were blocked in 5% fat-free milk prepared in phosphate-buffered saline/Tween-20 (PBST) buffer for 1 hour, and incubated with rabbit primary antibodies against activated caspase-3, Bax and cytochrome c (1:500; Santa Cruz Biotechnology, Dallas, TX, USA) overnight at 4°C. The membranes were washed three times with PBST, and then incubated with goat anti-rabbit IgG (1:20,000; ZSGB-BIO, Beijing, China) for 2 hours at room temperature. Blots were developed using a 3,3′-diaminobenzidine kit (ZSGB-BIO). Semiquantitative analysis of the blots was performed with a FM 0442 gel imaging system (ProteinSimple, Hercules, CA, USA). Each experiment was performed in triplicate. The final optical density value was the average of the three separate analyses. Protein levels were expressed as the optical density ratio of the target protein to β-actin (reference).
Evaluation of Bax and cytochrome c levels by immunohistochemistry
Both Bax and cytochrome c were evaluated by immunohistochemistry in the rat hippocampus. Citrate solution (0.01 M, pH 6.0) was used for antigen retrieval in a microwave. After cooling to room temperature, the tissue was washed three times with PBS, incubated in 3% H2O2for 10 minutes, and blocked in goat serum. The tissue section was then incubated with 50 μL of primary antibody (rabbit anti-Bax and cytochrome c antibodies; Santa Cruz Biotechnology; 1:150) overnight at 4°C, washed three times with PBS, and incubated with 50 μL secondary antibody (goat anti-rabbit IgG; ZSGB-BIO) at 37°C for 30 minutes. The sample was treated with 50 μL streptavidin-horseradish peroxidase, developed with 3,3′-diaminobenzidine, counterstained with hematoxylin for 1 minute, differentiated with hydrochloric acid and alcohol for a few seconds, mounted, and baked in neutral resin. Each section was observed under a microscope (Olympus) to photograph CA1 regions of the hippocampus after randomly selecting fields. Brown staining of the neuronal cytoplasm and processes indicates positive expression. The JEDA 801D image collection and processing system (Jieda, Jiangsu Province, China) was used to assess mean optical density.
Statistical analysis
The data, presented as the mean ± SD, were analyzed with SPSS 13.0 software (SPSS, Chicago, IL, USA). Data were tested for homogeneity of variances. Differences among groups were assessed by one-way analysis of variance and the least significant difference test. A value of P < 0.05 was considered statistically significant.
Results
GS improved learning and memory abilities in AD rats
Learning and memory abilities were evaluated with the Morris water maze. Compared with the sham group, the escape latency was remarkably longer in the model group (P < 0.01). In comparison with the model group, the escape latencies were shortened dramatically in the GS-M, GS-H and EV groups (Figure 1).
GS reduced cell apoptosis in the hippocampus of AD rats TUNEL assay was used to observe changes in cell apoptosis in the hippocampus of AD rats. Compared with the sham group, the number of apoptotic cells was increased in the model group. The number of apoptotic cells was substantially reduced in the GS-L, GS-M, GS-H and EV groups, compared with the model group (Figure 2).
GS reduced cytochrome c, Bax and caspase-3 expression in the hippocampus of AD rats
Expression levels of caspase-3, Bax and cytochrome c were assessed by western blot assay. Caspase-3 expression was highest in the model group, and was significantly higher than in the sham group. Caspase-3 expression was significantly reduced in the GS-M, GS-H and EV groups, compared with the model group (Figure 3A).
Bax expression was highest in the model group, and was significantly higher than in the sham group. Bax expression was significantly reduced in the GS-M, GS-H and EV groups, compared with the model group (Figure 3B).
Cytochrome c expression was highest in the model group, and was significantly higher than in the sham group. Cytochrome c expression was significantly reduced in the GS-M and GS-H groups, compared with the model group (Figure 3C).
GS reduced cytochrome c and Bax immunoreactivity in the hippocampus of AD rats
Bax and cytochrome c immunoreactivities were evaluated by immunohistochemistry. Bax immunoreactivity was highest in the model group, and the mean optical density for Bax was higher in the model group than in the sham group (P < 0.01). Bax immunoreactivity was significantly reduced in the GSL, GS-M, GS-H and EV groups, compared with the model group. The mean optical density for Bax was significantly lower in the GS-H group than in the model group (P < 0.01; Figure 4).
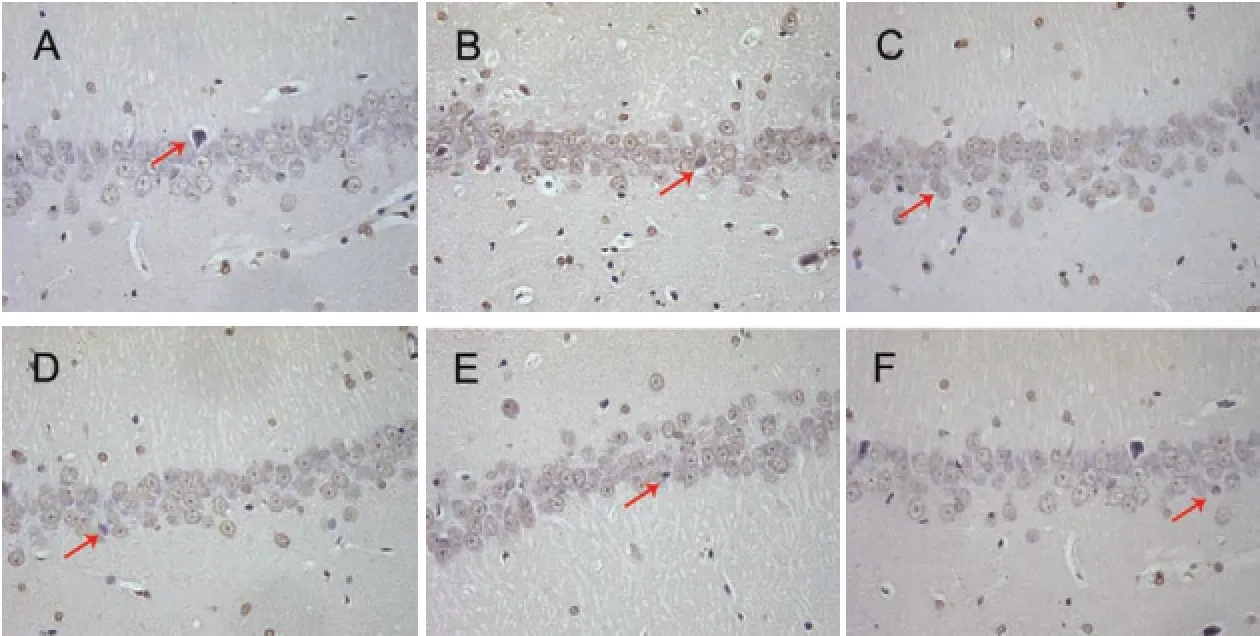
Figure 2 Effect of GS on celll apoptosis in the hippocampal CA1 region in AD rats (TUNEL staining, × 400).

Figure 3 Effect of GS on the expression levels of caspase-3, Bax and Cyt c in AD rats.
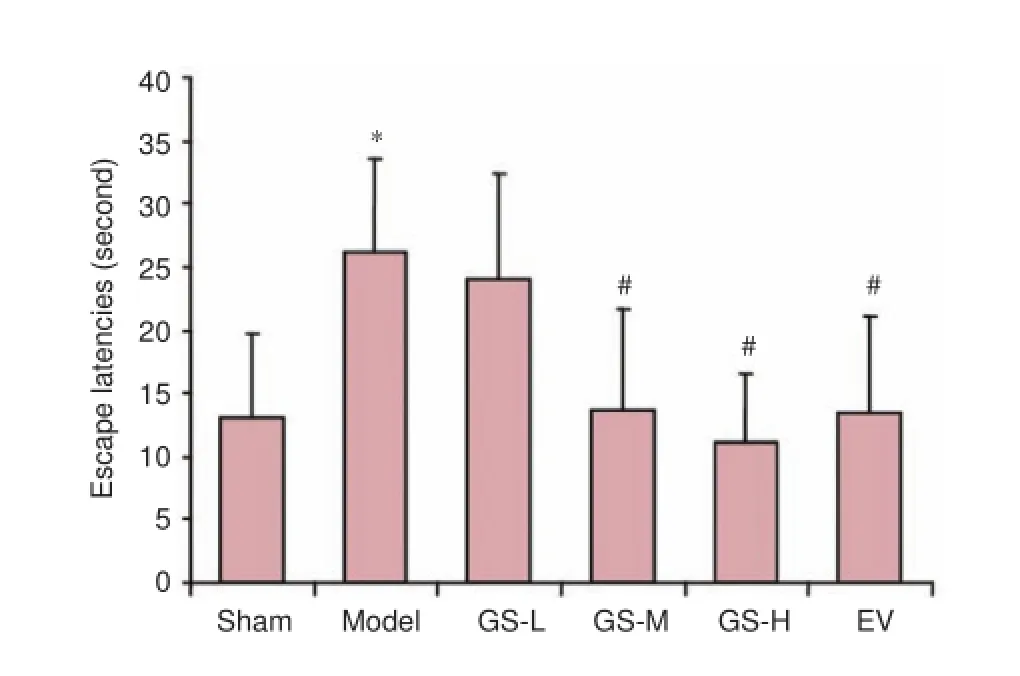
Figure 1 Effect of GS on learning and memory abilities in AD rats.
Cytochrome c immunoreactivity was highest in the model group, and the mean optical density for cytochrome c was higher in the model group than in the sham group (P < 0.01). Cytochrome c immunoreactivity was significantly reduced in the GS-L, GS-M, GS-H and EV groups, compared with the model group. The mean optical density for cytochrome c was significantly lower in the GS-H group than in the model group (P < 0.01; Figure 4).
Discussion
AD, also called senile dementia, has a relatively high incidence in China (Chan et al., 2013). The pathological features of AD include Aβ deposits, neurofibrillary tangles consisting of aggregated abnormally phosphorylated tau protein, and the loss of neuronal cells in the cortex and hippocampus (Liu et al., 2014; Bass et al., 2015). The main cause of neuronal death appears to be an abnormal increase and accumulation of Aβ in brain tissue (Huang and Jiang 2008; Duyckaerts et al., 2009; Rugarli and Langer 2012).
There are several rat models of AD, including the APP transgenic rat model, D-galactose-induced rat AD model and the Aβ-induced rat AD model (Alkadhi et al., 2012;
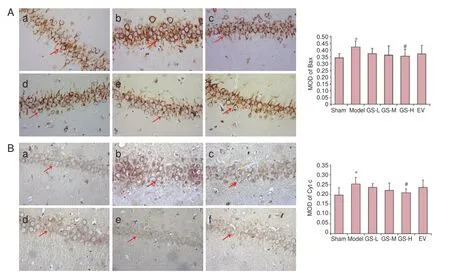
Figure 4 Effect of GS on Bax and Cyt c immunoreactivity in AD rats (immunohistochemical staining, ×400).
Hanzel et al., 2014; Gao et al., 2015). At present, the intracerebral Aβ25—35injection-induced AD model is very popular. However, this model is complicated by self-healing, which might affect the experimental results. Therefore, in the present study, a rat model of AD was established by intraperitoneal injection of D-galactose combined with intracerebral injection of Aβ25—35.
Caspase-3, Bax and cytochrome c are key components of the mitochondrial apoptotic pathway (Huttemann et al., 2011; Stevens, 2011). In our rat AD model, neurons in the hippocampus exhibited increased apoptosis, and the expression levels of caspase-3, Bax and cytochrome c were significantly elevated. After treatment with GS, hippocampal neuronal apoptosis was significantly reduced, and the expression levels of caspase-3, Bax and cytochrome c were significantly decreased. Both western blot assay and immunohistochemical staining were performed in this study, with each method confirming the results of the other, thereby enhancing the validity of the findings.
Our findings provide novel insight into the effectiveness and mechanisms of action of GS for the treatment of AD. Future studies should focus on the neuroprotective mechanisms of GS, and on processes upstream of the mitochondrial apoptotic pathway, such as endoplasmic reticulum stress.
Author contributions: YW, BC and JS analyzed data and wrote the paper. TTW gave technical support. RZC, CJM, TH and JD performed the experiments. BC designed study and revised the paper. All authors approved the final version of the paper.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Alkadhi KA, Alzoubi KH, Srivareerat M, Tran TT (2012) Elevation of BACE in an Aβ rat model of Alzheimer's disease: exacerbation by chronic stress and prevention by nicotine. Int J Neuropsychopharmacol 15:223-233.
Aras AB, Guven M, Akman T, Alacam H, Kalkan Y, Silan C, Cosar M (2015) Genistein exerts neuroprotective effect on focal cerebral ischemia injury in rats. Inflammation 38:1311-1321.
Baruch K, Rosenzweig N, Kertser A, Deczkowska A, Sharif AM, Spinrad A, Tsitsou-Kampeli A, Sarel A, Cahalon L, Schwartz M (2015) Breaking immune tolerance by targeting Foxp3(+) regulatory T cells mitigates Alzheimer's disease pathology. Nat Commun 6:7967.
Bass B, Upson S, Roy K, Montgomery EL, Jalonen TO, Murray IV (2015) Glycogen and amyloid-beta: key players in the shift from neuronal hyperactivity to hypoactivity observed in Alzheimer's disease?. Neural Regen Res 10:1023-1025.
Behloul N, Wu G (2013) Genistein: a promising therapeutic agent for obesity and diabetes treatment. Eur J Pharmacol 698:31-38.
Bhattacharya S, Montag D (2015) Acetylcholinesterase inhibitor modifications: a promising strategy to delay the progression of Alzheimer's disease. Neural Regen Res 10:43-45.
Calafate S, Buist A, Miskiewicz K, Vijayan V, Daneels G, de Strooper B, de Wit J, Verstreken P, Moechars D (2015) Synaptic contacts enhance cell-to-cell tau pathology propagation. Cell Rep 11:1176-1183.
Chan KY, Wang W, Wu JJ, Liu L, Theodoratou E, Car J, Middleton L, Russ TC, Deary IJ, Campbell H, Wang W, Rudan I (2013) Epidemiology of Alzheimer's disease and other forms of dementia in China, 1990-2010: a systematic review and analysis. Lancet 381:2016-223.
Duyckaerts C, Delatour B, Potier MC (2009) Classification and basic pathology of Alzheimer disease. Acta Neuropathol 118:5-36.
Fennell DA, Chacko A, Mutti L (2008) BCL-2 family regulation by the 20S proteasome inhibitor bortezomib. Oncogene 27:1189-1197.
Fine A, Dunnett SB, Bj?rklund A, Iversen SD (1985) Cholinergic ventral forebrain grafts into the neocortex improve passive avoidance memory in a rat model of Alzheimer disease. Proc Natl Acad Sci U S A 82:5227-5230.
Gao J, He H, Jiang W, Chang X, Zhu L, Luo F, Zhou R, Ma C, Yan T (2015) Salidroside ameliorates cognitive impairment in a d-galactose-induced rat model of Alzheimer's disease. Behav Brain Res 293:27-33.
Hanzel CE, Pichet-Binette A, Pimentel LS, Iulita MF, Allard S, Ducatenzeiler A, Do Carmo S, Cuello AC (2014) Neuronal driven pre-plaque inflammation in a transgenic rat model of Alzheimer's disease. Neurobiol Aging 35:2249-2262.
Huang H, Jiang Z (2008) Accumulated amyloid-beta peptide and hyperphosphorylated tau protein: relationship and links in Alzheimer's disease. J Alzheimers Dis 16:15-27.
Huttemann M, Pecina P, Rainbolt M, Sanderson TH, Kagan VE, Samavati L, Doan J.W, Lee I (2011) The multiple functions of cytochrome c and their regulation in life and death decisions of the mammalian cell: from respiration to apoptosis. Mitochondrion 11:369-381.
Irie T, Fukushi K, Namba H, Iyo M, Tamagami H, Nagatsuka S, Ikota N (1996) Brain acetylcholinesterase activity: validation of a PET tracer in a rat model of Alzheimer's disease. J Nucl Med 37:649-655.
Kinoshita S, Noda K, Tagawa Y, Inafuku S, Dong Y, Fukuhara J, Dong Z, Ando R, Kanda A, Ishida S (2014) Genistein attenuates choroidal neovascularization. J Nutr Biochem 25:1177-1182.
Liao W, Jin G, Zhao M, Yang H (2013) The effect of genistein on the content and activity of α- and β-secretase and protein kinase C in Aβ-injured hippocampal neurons. Basic Clin Pharmacol Toxicol 112:182-185.
Liu J, Ma Y, Tian S, Zhang L, Zhao M, Zhang Y, Xu D (2014) T cells promote the regeneration of neural precursor cells in the hippocampus of Alzheimer's disease mice. Neural Regen Res 9:1541-1547.
Liu LX, Chen WF, Xie JX, Wong MS (2008) Neuroprotective effects of genistein on dopaminergic neurons in the mice model of Parkinson's disease. Neurosci Res 60:156-161.
Ma W, Yuan L, Yu H, Ding B, Xi Y, Feng J, Xiao R(2010) Genistein as a neuroprotective antioxidant attenuates redox imbalance induced by beta-amyloid peptides 25—35 in PC12 cells. Int J Dev Neurosci 28:289-295.
Malla R, Gopinath S, Alapati K, Gondi CS, Gujrati M, Dinh DH, Mohanam S, Rao JS (2010) Downregulation of uPAR and cathepsin B induces apoptosis via regulation of Bcl-2 and Bax and inhibition of the PI3K/Akt pathway in gliomas. PLoS One 5:e13731.
Obulesu M, Lakshmi MJ (2014) Apoptosis in Alzheimer's disease: an understanding of the physiology, pathology and therapeutic avenues. Neurochem Res 39:2301-2312.
Rahman S, Islam R, AM Swaraz, Anesa Ansari, Anowar Khasru Parvez, Depak Kumar Paul (2012) An insight on genistein as potential pharmacological and therapeutic agent. Asian Pac J Trop Biomed 2:1924-1937.
Rajasekharan SK, Ramesh S, Bakkiyaraj D (2015) Synergy of flavonoids with HDAC inhibitor: new approach to target Candida tropicalis biofilms. J Chemother 27:246-249.
Reiter E, Reiter E, Beck V, Medjakovic S, Jungbauer A (2009) Isoflavones are safe compounds for therapeutical applications-evaluation of in vitro data. Gynecol Endocrinol 25:554-580.
Rugarli EI, Langer T (2012) Mitochondrial quality control: a matter of life and death for neurons. EMBO J 31:1336-1349.
Sigurdsson EM, Lorens SA, Hejna MJ, Dong XW, Lee JM (1996) Local and distant histopathological effects of unilateral amyloid-beta 25-35 injections into the amygdal of young F344 rats. Neurobiol Aging 17:893-901.
Stevens JM (2011) Cytochrome c as an experimental model protein. Metallomics 3:319-322.
Son TG, Gong EJ, Bae MJ, Kim SD, Heo K, Moon C, Yang K, Kim JS (2013) Protective effect of genistein on radiation-induced intestinal injury in tumor bearing mice. BMC Complement Altern Med 13:103. Sun J, Li Z, Chu H, Guo J, Jiang G, Qi Q (2015) Candida albicans amphotericin b-tolerant persister formation is closely related to surface adhesion. Mycopathologia 181:41-49.
Tan C, Dlugosz PJ, Peng J, Zhang Z, Lapolla SM, Plafker SM, Andrews DW, Lin J (2006) Auto-activation of the apoptosis protein Bax increases mitochondrial membrane permeability and is inhibited by Bcl-2. J Biol Chem 281:14764-14775.
Wang TJ, Chen JR, Wang WJ, Wang YJ, Tseng GF(2014) Genistein partly eases aging and estropause-induced primary cortical neuronal changes in rats. PLoS One 9:e89819.
Wu Q, Tang ZH, Peng J, Liao L, Pan LH, Wu CY, Jiang ZS, Wang GX, Liu LS (2014) The dual behavior of PCSK9 in the regulation of apoptosis is crucial in Alzheimer's disease progression. Biomed Rep 2:167-171.
Zhao G, Zhu Y, Eno CO, Liu Y, Deleeuw L, Burlison JA, Chaires JB, Trent JO, Li C (2014) Activation of the proapoptotic Bcl-2 protein Bax by a small molecule induces tumor cell apoptosis. Mol Cell Biol 34:1198-1207.
Copyedited by Patel B, Frenchman B, Wang J, Qiu Y, Li CH, Song LP, Zhao M
How to cite this article: Wang Y, Cai B, Shao J, Wang TT, Cai RZ, Ma CJ, Han T, Du J (2016) Genistein suppresses the mitochondrial apoptotic pathway in hippocampal neurons in rats with Alzheimer's disease. Neural Regen Res 11(7)∶1153-1158.
Funding: This work was supported by the National Natural Science Foundation of China, No. 81202941; the Key Project Foundation of Oversea Visiting and Research for the Excellent Young and Middle-aged Faculties in Universities of Anhui Province in China, No. gxfxZD2016119; the Key Project Foundation of Natural Science Research in Universities of Anhui Province in China, No. KJ2016A406.
10.4103/1673-5374.187056
2015-12-22
We thank Lei Lv from the Laboratory of Pathology and Chang-an Liu from the Laboratory of Neurobiology, Anhui University of Chinese Medicine in China for their technical support in this study.
RESEARCH ARTICLE
- 中國神經(jīng)再生研究(英文版)的其它文章
- Recovery of an injured corticospinal tract by subcortical peri-lesional reorganization in a patient with intracerebral hemorrhage
- Dose response and time course of manganeseenhanced magnetic resonance imaging for visual pathway tracing in vivo
- Low-power laser therapy for carpal tunnel syndrome: effective optical power
- Extracellular matrix from human umbilical cordderived mesenchymal stem cells as a scaffold for peripheral nerve regeneration
- Differential temporal expression of matrix metalloproteinases following sciatic nerve crush
- Protective effects of ginsenoside Rg1 against hydrogen peroxide-induced injury in human neuroblastoma cells