Hepatocellular carcinoma and type 2 diabetes mellitus: cytokeratin 8/18 expression in hepatocellular carcinoma and glycogen-storing hepatocytes
Kunio Takegoshi, Eikichi Okada, Qin Su
1Takegoshi Internal Medicine Clinic, Takaoka, Toyama 933-0014, Japan.
2Pathological Division of Takaoka City Hospital, Takaoka, Toyama 933-8550, Japan.
3Cell Marque, Sigma-Aldrich, Rocklin, CA 95677, USA.
Letter to Editor
Hepatocellular carcinoma and type 2 diabetes mellitus: cytokeratin 8/18 expression in hepatocellular carcinoma and glycogen-storing hepatocytes
Kunio Takegoshi1, Eikichi Okada2, Qin Su3
1Takegoshi Internal Medicine Clinic, Takaoka, Toyama 933-0014, Japan.
2Pathological Division of Takaoka City Hospital, Takaoka, Toyama 933-8550, Japan.
3Cell Marque, Sigma-Aldrich, Rocklin, CA 95677, USA.
10.20517/2394-5079.2016.26
Sir,
We have reported two patients with hepatocellular carcinoma (HCC) and type 2 diabetes mellitus (T2DM), who showed abundant glycogen in their liver parenchyma but a marked reduction of glycogen content in HCC.[1]It was suggested that the latter was associated with appearance of a Warburg type glycolysis[1]and discussed in some detail.[2]
Cytokeratins (CKs), the intermediate filament (IF) proteins of epithelia, are sub-divided into type I (CK9-20) and type II (CK1-8) and expressed as type I/II pairs in a cell differentiation manner. In adult liver, hepatocyte IF comprise only CK8/18.[3]CK8/18 expression in normal and diseased liver has been reported, including positive expression in alcoholic steatohepatitis (ASH) and/or non-alcoholic steatohepatitis (NASH) and HCC.[3]
We examined the expression of CK8/18 in the liver to investigate cytoskeletal alterations in hepatocytes, possibly related to changes in hepatocellular glycogen content during hepatocarcinogenesis. Our studies revealed that immunoreactivity for CK8/18 was reduced or frequently even negative in glycogen-rich hepatocytes of background liver [Figure 1b and d], but moderately positive in normal hepatocytes and glycogen-poor cells in HCC [Figure 1a, c, e and f]. Overexpression of CK8/18, as Malory Denk bodies, which are hallmark lesions in ASH and NASH,[3]was not detected [Figure 1b and d]. The results provide evidence for reduced to negative CK8/18 expression in glycogen-rich hepatocytes.
The mechanism of alteration of CK8/18 expression in glycogen-rich hepatocytes has not been elucidated. Su et al.[4]demonstrated that CK8/18 expression was reduced in excessively glycogen-storing (glycogenotic) clear hepatocytes, which also showed a relative reduction of cytoplasmic organelles as demonstrated by electron-microscopic studies. Given simple CK8/18 expression patterns, hepatocytes are sensitive to alterations of cytokeratin architecture.[3]Using hepatic cell culture systems, Mathew et al.[5]reported recently that CK8/18 is involved in the interplay between glucose utilization and insulin signaling. The authors demonstrated that insulin stimulates glucose uptake, glucose-6-phosphatase formation, lactate release, and glycogen formation in hepatocytes via the PI-3 kinase dependent signaling pathway, and that CK8/18 IF loss makes them more efficient glycogen producers.[5]This is in line with the notion that an insulinomimetic effect of oncogenic agents is responsible for the preneoplastic hepatocellular glycogenosis,[2]which is associated with a reduced or negative expression of CK 8/18 in glycogenotic clear cells appearing in chronic human and woodchuck hepadnaviral infection.[4]CK8/18 immunohistochemistry may allow distinct recognition of the glycogen-rich hepatocytes as shown in glycogenotic clear cells under various conditions.[4]
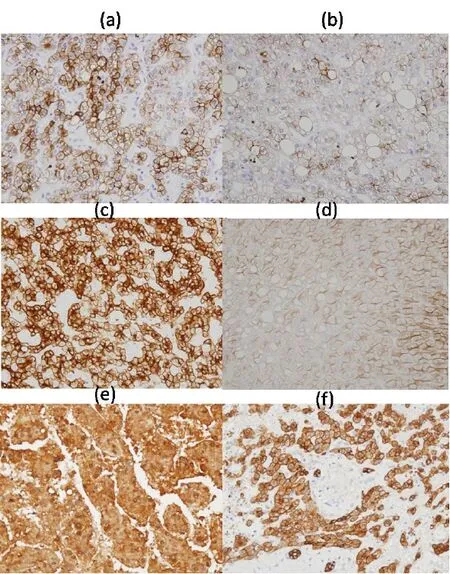
Figure 1:CK8/18 expression in hepatocellular carcinoma (a; case 1, c; case 2, e; control) and background liver (b; case1, d; case 2, f; control), demonstrated with mouse monoclonal antibodies B22.1/B23.1 (Cell Marque, USA) and visualized using the Envision method (Dako) (a-f, ×400). Control (a 79-year-old male, moderately differentiated adenocarcinoma in background of nearly normal liver)
Financial support and sponsorshipNil.
Conflicts of interest
There are no conflicts of interest.
1. Takegoshi K, Okada E, Nomoto K, Nobata K, Sawasaki T, Terada M, Terakawa H, Kobayashi T, Yabushita K, Sugimoto T, Terahata S. Hepatocellular carcinoma and type 2 diabetes mellitus: two cases highlighting changes in tumor glycogen content. Hepatoma Res 2016;2:26-30.
2. Bannasch P, Klimek F, Mayer D. Early bioenergetic changes in hepatocarcinogenesis: preneoplastic phenotypes mimic responses to insulin and thyroid hormone. J Bioenerg Biomembr 1997;29:303-13.
3. Strnad P, Paschke S, Jang KH, Ku NO. Keratins: markers and modulators of liver disease. Curr Opin Gastroenterol 2012;28:209-16.
4. Su Q, Zerban H, Otto G, Bannasch P. Cytokeratin expression is reduced in glycogenotic clear hepatocytes but increased in ground-glass cells in chronic human and woodchuck hepadnaviral infection. Hepatology 1998;28:347-59.
5. Mathew J, Loranger A, Gilbert S, Faure R, Marceau N. Keratin 8/18 regulation of glucose metabolism in normal versus cancerous hepatic cells through differential modulation of hexokinase status and insulin signaling. Exp Cell Res 2013;319:474-86.
Dr. Kunio Takegoshi, Takegoshi Internal Medicine Clinic, 377-7 Nomura, Takaoka, Toyama 933-0014, Japan. E-mail: kunio@takegoshi.jp
Received:04-07-2016;Accepted:08-07-2016
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
For reprints contact:service@oaepublish.com
How to cite this article:Takegoshi K, Okada E, Su Q. Hepatocellular carcinoma and type 2 diabetes mellitus: cytokeratin 8/18 expression in hepatocellular carcinoma and glycogen-storing hepatocytes. Hepatoma Res 2016;2:229-30.
- Hepatoma Research的其它文章
- Comment on “Preliminary outcome of microwave ablation of hepatocellular carcinoma: breaking the 3-cm barrier?”
- Detecting hepatic nodules and identifying feeding arteries of hepatocellular carcinoma: efficacy of cone-beam computed tomography in transcatheter arterial chemoembolization
- Efficacy of sorafenib therapy in patients with advanced hepatocellular carcinoma in Indian population
- Diet and nutrition therapy in pre-liver transplant patients
- AUTHOR INSTRUCTIONS
- Role of natural antioxidants in the therapeutic management of hepatocellular carcinoma