Latest Update of Cosmetic Regulations in China Since 2017
CIRS
Great changes happened to Chinese cosmetics regulations in 2017. This article focuses on the latest update of cosmetics regulations in China since 2017 and assesses how those changes will impact the import of cosmetics to China.
Data of registered or recorded cosmetics with CFDA or local FDAs
Import cosmetics by CFDA
For imported non special use cosmetics, the average number of approval imported non special use cosmetics is more than 11,000 from 2013 to 2017. In 2017, the number of approval has a slight growth with reach to 13,785.Nevertheless, the number of approval imported special use cosmetics is about 15% of approval imported non special use cosmetics (Figure 1).
Import non special use cosmetics by Pudong new area
According to the pilot policy from 1 Mar 2017 to 21Dec 2018, non-special use cosmetics, imported via Pudong new area, can be made record keeping with Shanghai FDA besides CFDA registration (Figure 2). So far, there are about 30%imported cosmetics recorded with Shanghai FDA.
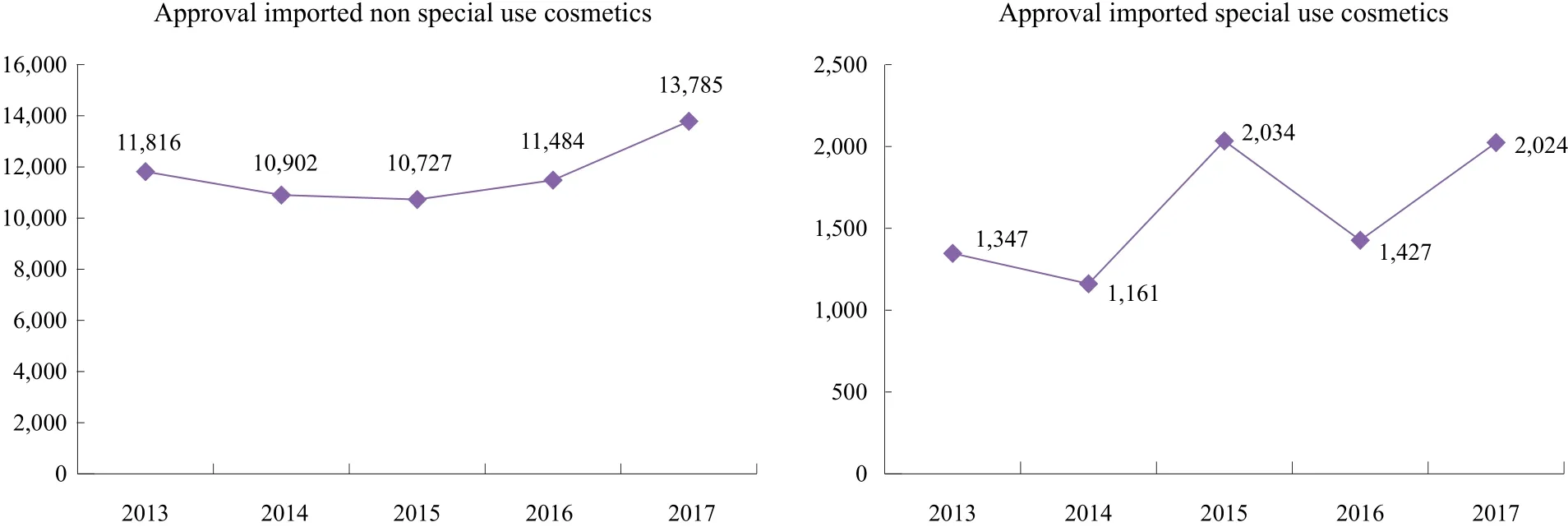
Figure 1. Registration data of imported cosmetics approved by CFDA, 2013~2017
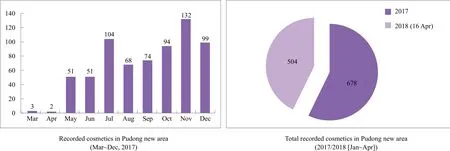
Figure 2. Record-keeping data of imported non special use cosmetics by Pudong new area
Domestic cosmetics by CFDA and local FDA
Compared with the imported special use cosmetics, the number of approval domestic special use cosmetics is similar. But there is a fast decline in 2016 and 2017. As we can see that the number of recorded domestic non special use cosmetics in 2017 will be closed to 300,000 (Figure 3). There is no technical review process for the record keeping of local non special use cosmetics.
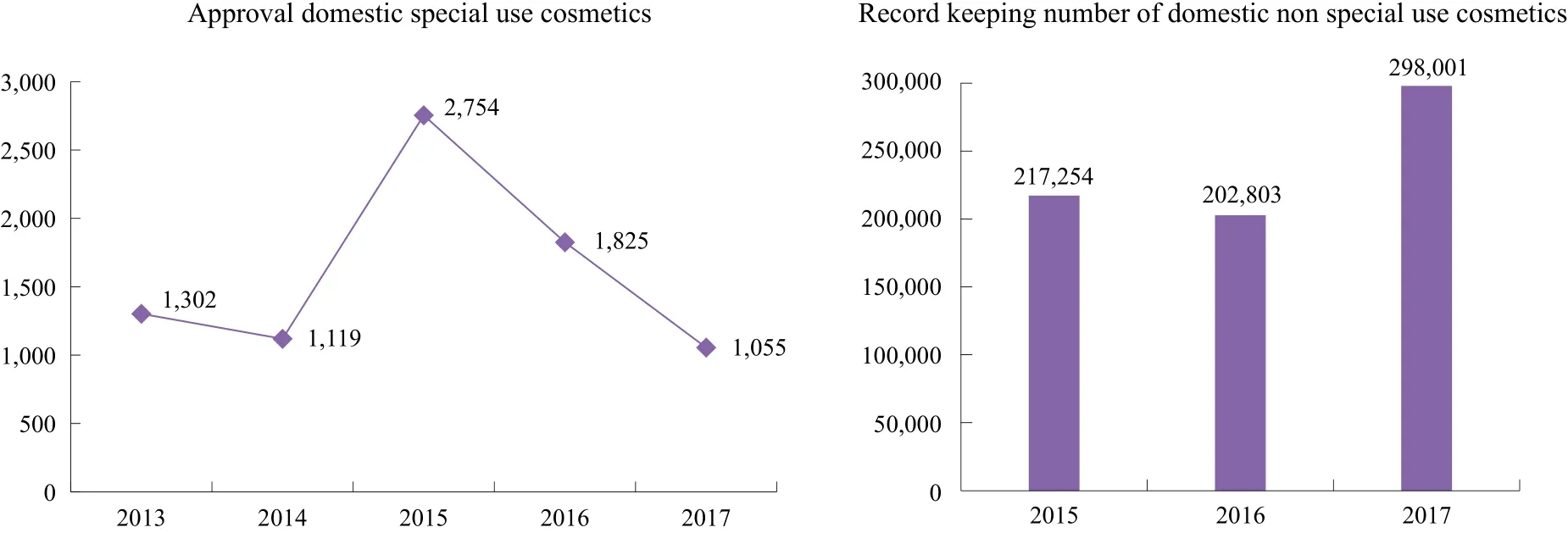
Figure 3. Registration or record-keeping data of domestic cosmetics
Updates of cosmetic regulations in China
New record-keeping system for imported non special use cosmetics in Pudong new area
The main differences between CFDA registration and Record-keeping in Shanghai FDA can be found in Table 1.
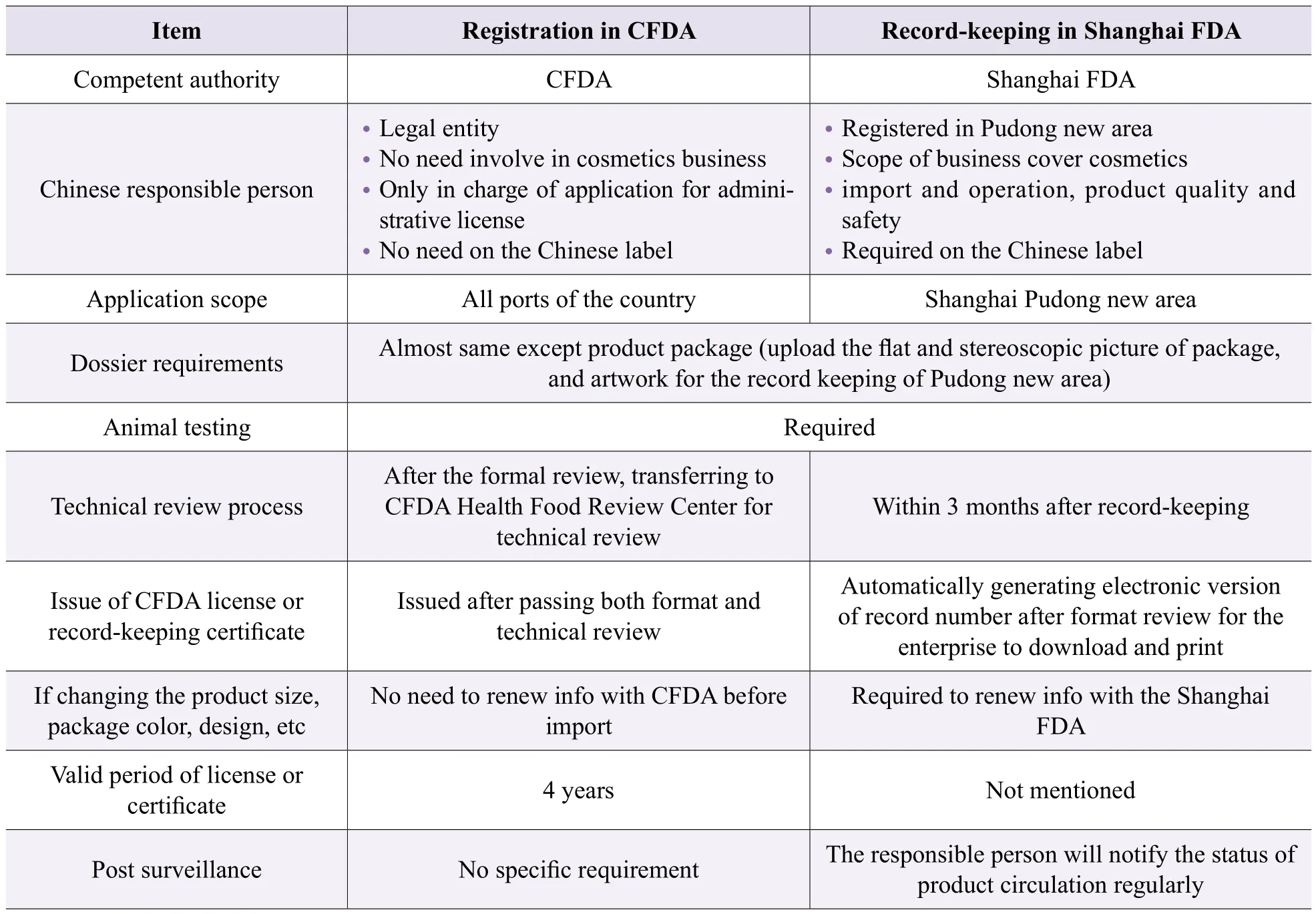
Table 1. Comparision of two ways of importing non special use cosmetics to China
Expanding 10 new free trade zones to implement new record keeping system of imported non special use cosmetics
This new pilot policy will be available from 8 Mar, 2018 to 21 Dec 2018. The 10 new free trade zones include Tianjin,Liaoning, Zhejiang, Fujian, Henan, Hubei, Guangdong,Chongqing, Sichuan and Shanxi. Zhejiang and Guangdong FDA are the first two government bodies to issue notices for the preparation work of implementing this new pilot policy.
New alternative methods available and consulted in public
On 21 Aug 2017, there are two alternative methods accepted by CFDA. They are the In Vitro Skin Corrosion: Transcutaneous Electrical Resistance Test(TERT) and Skin Photoallergy Test. Previously, only the In Vitro 3T3 Neutral Red Uptake Phototoxicity Assay (In Vitro 3T3 NRU PA) has been adopted on 11 Nov 2016. In 2018, another two alternative methods In Chemico Skin Sensitisation: Direct Peptide Reactivity Assay (DPRA) and Short Time Exposure In Vitro Test(STE) are consulted in public on 5 Feb. As we can see,CFDA has sped up the process of adopting non animal test method in cosmetics and cosmetic ingredients.
Extending transition period of cross-border e-commerce
On 20 Sep 2017, Premier Li Keqiang highlighted in the State Council executive meeting the transition period of supervising import business of cross-border e-commerce will be extended to the end of 2018.
Imported cosmetics tariffs adjusted
On November 22, 2017, the Tariff Commission of State Council released a Notice regarding the adjustment of import tariff to some consumer goods. The adjusted import tariffs will come into force on December 1, 2017. The imported cosmetics tax rate is dropped from the original 15%, 10%, 9%, 6.5% to 5% and 2%.
Public opinion on new Guidelines for Cosmetic Classifications
On 15 Mar 2016, the National Institutes for Food and Drug Control published the Standards of Cosmetic Classification(Draft version). On 17 Jan 2018, CFDA published the Guidelines for Cosmetic Classification (Draft version).According to the new guidelines, the classification code will consist of 4 layers. They are the efficacy claim, application site,product form and suitable users. There will be 26 codes of efficacy claim, 27 of application site, 18 of product form, and 7 of suitable user (Figure 4 and Table 2).
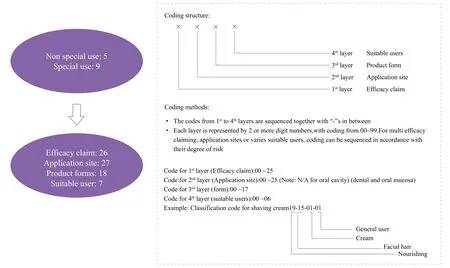
Figure 4. Coding structure and methods of new Guidelines for Cosmetic Classifications
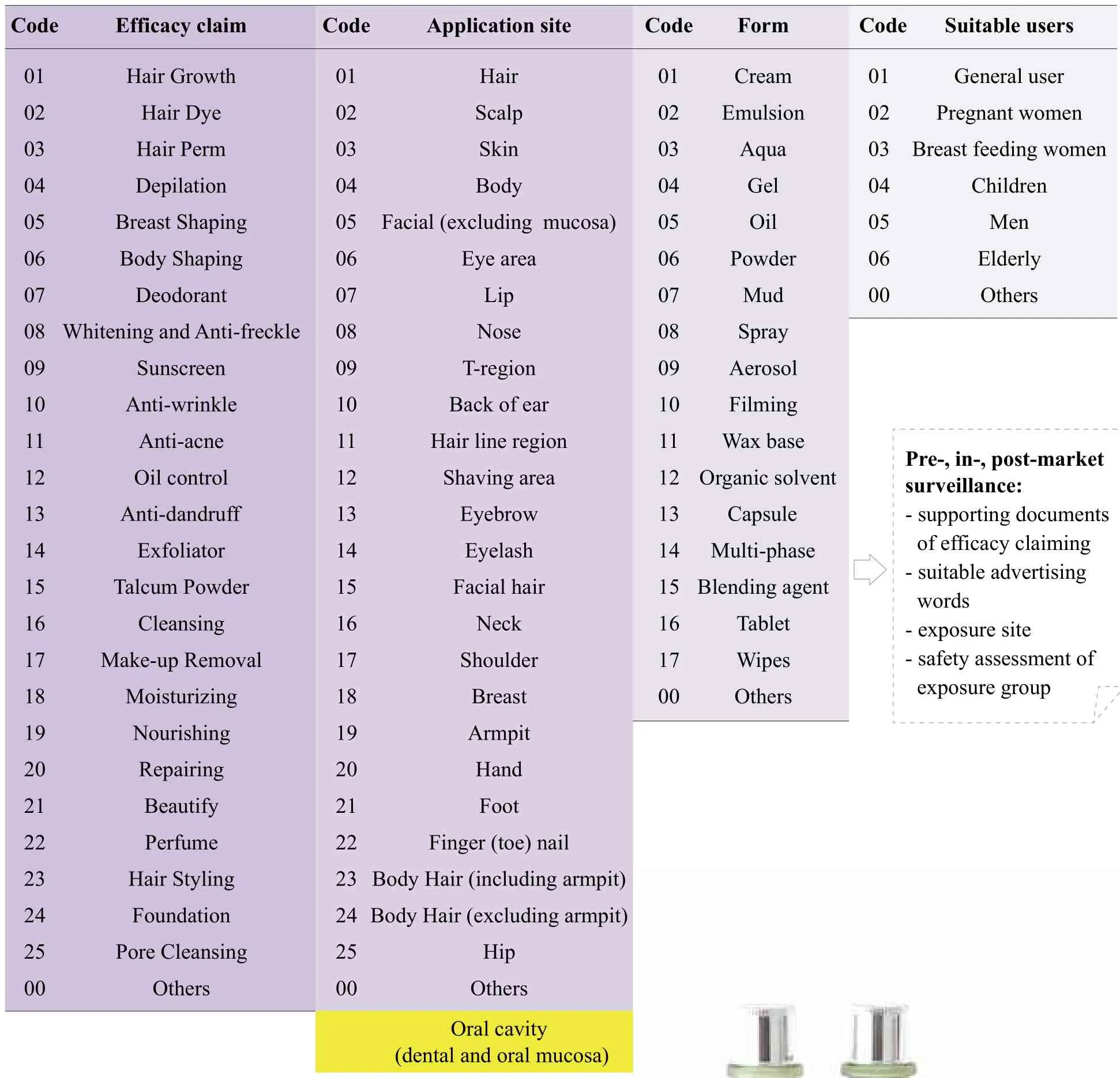
Table 2. The categories of new Guidelines for Cosmetic Classifications
Guidelines for the Evaluation of Cosmetic Claims (draft)
On 24 Jan, 2018, the National Institutes for Food and Drug Control published the Guidelines for the Evaluation of Cosmetic Claims for public opinion. It is required to evaluate the specific function like sunscreen, anti-freckle,hair growth, breast shaping, fitness, deodorant, anti-aging,anti-acne, oil control, anti-dandruff, repairing, moisturizing(>2 hs). The manufacturer can make the self-evaluation or appoint the third party lab to perform the evaluation. No matter whether the in vivo method on human/animal, or in vitro method, self-development method by verified lab would be acceptable.
 China Detergent & Cosmetics2018年2期
China Detergent & Cosmetics2018年2期
- China Detergent & Cosmetics的其它文章
- Sensory Evaluation of Hydroxy Ethyl Cellulose (HEC) in Facial Mask
- Application of “Youthful Curve of Ocular Region” in the Research of Asians Aging
- How to Comply with the Pilot Policy of China for the Import of Non-special Use Cosmetics
- 2017 Cosmetic Regulatory News in AP
- Analysis of Regulatory Policies and Registration / Record Keeping Status of Domestic Cosmetics in China
- Middle Class pivotal to China’s Beauty Market
