Relationship between the high frequency of 23S rRNA point mutations in Treponema pallidum and low serological response rate to azithromycin treatment in China
Rui-Li Zhang,Qian-Qiu Wang*,Zhi-Ju Zheng,Guo-Jun Liang,Li-Jia Yang,Li-Gang Yang,Dong-Nu Pei,Shao-Chun LinDepartment of Dermatology,Affiliated Wuxi No. People’s Hospital of Nanjing Medical University,Wuxi,Jiangsu 00,China.National Center for STD Control and Prevention,China Centers for Diseases Control and Prevention,Hospital for Skin Diseases(Institute of Dermatology),Chinese Academy of Medical Sciences and Peking Union Medical College,Nanjing,Jiangsu 00,China.Department of Dermatology,Dermatology Hospital of Southern Medical University,Guangzhou,Guangdong 009,China.Department of Dermatology,Hainan Provincial Dermatology Hospital,Haikou,Hainan 7000,China.Department of Dermatology,Institute of Dermatology,Sichuan Provincial People’s Hospital,Chengdu,Sichuan 600,China.
【Abstract】Objective Although azithromycin is effective against Treponema pallidum(T.pallidum),the causative agent of syphilis,recent reports indicate that the prevalence of azithromycin resistance in China is very high,which may result in the failure of treatment.In this study,we aimed to investigate the association between azithromycin resistance and therapeutic outcomes in early syphilis patients.Methods Between February 2010 and December 2014,patients aged 18-65 years with early syphilis were enrolled.T.pallidum DNA were extracted to test the presence of A2058G and A2059G mutations.Then,eligible patients were randomly assigned to receive oral azithromycin(0.5 g,once daily for 15 days)or intramuscular BPG(2.4 million units,once weekly for 3 weeks).All patients were followed up in 2 weeks and 3,6,9,and 12 months after treatment to collect demographic and clinical characteristics and laboratory results.The differences on serological response,serological failure and serofast rate were compared between the two groups by Chi-square test.Results Among the 187 T.pallidum-infected patients enrolled,172(92.0%)cases had a mutation associated with azithromycin resistance(A2058G,153 cases;A2059G,19 cases).During the 5-year study period,the percentage of cases enrolled with these mutations steadily increased,from 90.9%in 2010 to 95.3%in 2014.Of the 172 patients presenting with these mutations,only 78(45.3%;all benzathine penicillin G[BPG]-treated)obtained a serological response to treatment;32.6%and 22.1%of patients presented with serological failure and serofast results,respectively.For azithromycin-treated cases,66.3%and 33.7%had serological failure and serofast results,respectively,in contrast with 1.1%and 11.3%of BPG-treated cases.However,among the A2058G-and A2059G-negative patients,the serological response rates between the two treatment groups were similar.In multivariate analyses,patients with lower rapid plasma reagin(RPR)titers(RPR,≤ 1∶8;odds ratio[OR],0.23;95%confidence interval[CI],0.09-0.37)or who received azithromycin treatment(OR,121.50;95%CI,35.38-386.17)were more likely to display serological failure and serofast results.Conclusion This prospective study found that the 23S rRNA A2058G and A2059G point mutations in T.pallidum are currently circulating with high frequency in China,suggesting a correlation between the high prevalence of macrolide resistance and a lower serological response rate to azithromycin treatment.
【Keywords】 Syphilis;Azithromycin;Mutation;Serological response;Treponema pallidum.
Introduction
The incidence of syphilis,which is caused by the spirochete Treponema pallidum(T.pallidum),has increased rapidly worldwide.In China,its rate of increase has been particularly rapid,with a 16.3%annual percentage increase since 2004[1].In 2017,a total of 475,860 patients in China were newly diagnosed with syphilis.Syphilis remains a significant burden of disease.
Azithromycin,a macrolide antibiotic,has been proven efficient against T.pallidum in trials[2-4],is recommended to treat early syphilis patients and recommended as an alternative antibiotic for patients allergic to penicillin,particularly in developing countries,because its effectiveness and well-tolerated singledose oral therapy.Moreover,because azithromycin is active against chlamydia and Gram-positive bacteria,this drug is frequently used to treat sexually transmitted diseases(STDs)and other bacterial infections in China[5-6].
Unfortunately,the emergence of T.pallidum strains with a 23S rRNA point mutation(either A2058G or A2059G)have been described in recent studies performed in various countries[7-9],including China[10-12].In addition,azithromycin treatment failure in patients with syphilis,first reported in San Francisco in 2004[13],has been reported worldwide,giving rise to concerns about azithromycin resistance,however,there are still limited studies on the association between T.pallidum carrying a 23S rRNA mutation and failure of responce to azithromycin treatment.Herein we conduct this prospective study to test the hypothesis that T.pallidum carrying a 23S rRNA mutation contribute to high serological failure and serofast results following azithromycin treatment.
Materials and methods
Participants
This multi-center,prospective survey was conducted in the STD clinics of following five major hospitals:(1)Hospital for Skin Diseases(Institute of Dermatology),Chinese Academy of Medical Sciences and Peking Union Medical College,located in Nanjing,Jiangsu Province(2)Affiliated Wuxi No.2 People’s Hospital of Nanjing Medical University located in Wuxi,Jiangsu Province,(3)Dermatology Hospital of Southern Medical University located in Guangzhou,Guangdong Province,(4)Hainan Provincial Dermatology Hospital,located in Haikou,Hainan Province,and(5)Institute of Dermatology,Sichuan Provincial People’s Hospital,located in Chengdu,Sichuan Province.
Inclusion criteria are:(1)patients aged 18-65 years with early syphilis(primary and secondary)between February 2010 and December 2014;(2)residence in the local city until the study was finished.Exclusion criteria were(1)pregnancy,(2)HIV-positive status,(3)penicillin allergy,(4)previous treatment with antibiotics for syphilis during the past 2 years,and(5)cardiovascular disease.All eligible patients were asked to complete a questionnaire including questions about demographics(age,marital status,and sexual orientation),sexual behavior,and HIV status.
The study design was approved by the Ethics Committee of the National Center for STD Control and Prevention,China Centers for Diseases Control and Prevention,Chinese Academy of Medical Sciences and Peking Union Medical College.All participants signed the informed consent before the beginning of study.
Reagents
Nontreponemal rapid plasma reagin(RPR)test was bought from Rongshang Biotech Inc.,Shanghai,China).T.pallidum particle agglutination(TPPA)test was purchased from Fujirebio Inc.,Tokyo,Japan).HIV serologic status was determined via an enzyme-linked immunosorbent assay(ELISA,Wansheng Biotech Inc.,Beijing,China)and confirmed through a western blotting assay(Genelabs Diagnostics Inc.,Singapore).
Screening and diagnosis of syphilis
Primary and secondary syphilis were diagnosed based on clinical presentations and positive serological treponemal test(TPPA)and nontreponemal test(RPR)based on the Chinese guidelines for syphilis[14].
Clinical presentations of primary syphilis include genital,anal,and/or oral ulcers with or without regional lymphadenopathy.Clinical presentations of secondary syphilis include generalized rashes,lymphadenopathy,mucosal erosion,or other signs.
Serological response was defined as either a negative RPR titer or a≥4-fold(two dilutions)decrease in RPR titer over 12 months relative to baseline[15].Serological failure was defined as a≥4-fold increase in RPR titer at 12 months after treatment,and serofast status was defined as either no change in RPR test results or a<2-fold decrease or increase in RPR titers at 12 months after treatment[15].All patients exhibiting serological failure after treatment were retreated with BPG and tested again for HIV infection.
Collection of clinical specimens
All patients were sampled only once before treatment.Exudates of moist lesions from syphilis patients were absorbed onto cotton swabs and stored in special medium(saline solution containing 20%rabbit serum and 20%glycerol)at-70℃.A 5-ml blood sample was obtained from each patient for serologic analysis before treatment.These samples were transported monthly to the central laboratory at the National Center for STD Control and Prevention,China Centers for Diseases Control and Prevention.The laboratory technicians were blinded to the clinical characteristics of patients,who provided the samples.For RPR tests,any test for which the result was in disaccord with the clinical manifestations was repeated by two different technicians.
DNA extraction and macrolide resistance testing
Total DNA was extracted from specimens using QIAamp DNA minikits(Qiagen,GmbH,Germany).The presence of T.pallidum DNA was detected using PCR amplification of the tpp47 gene.Specimen DNA that was positive for tpp47 was stored at-70℃until macrolide resistance screening and strain subtyping were performed.
To test the presence of A2058G and A2059G mutations,the extracted T.pallidum DNA was amplified by PCR and then digested with the enzymes MboII(for A2058G)or BsaI(for A2059G)[16].The digested DNA was electrophoresed on agarose gels.The two mutations were analyzed by comparison with amplified and digested DNA purified from the Nichols and Street 14 strains,which contain the A2058G mutation,and DNA purified from a clinical isolate that contains the A2059G mutation.
Teatment and follow?up
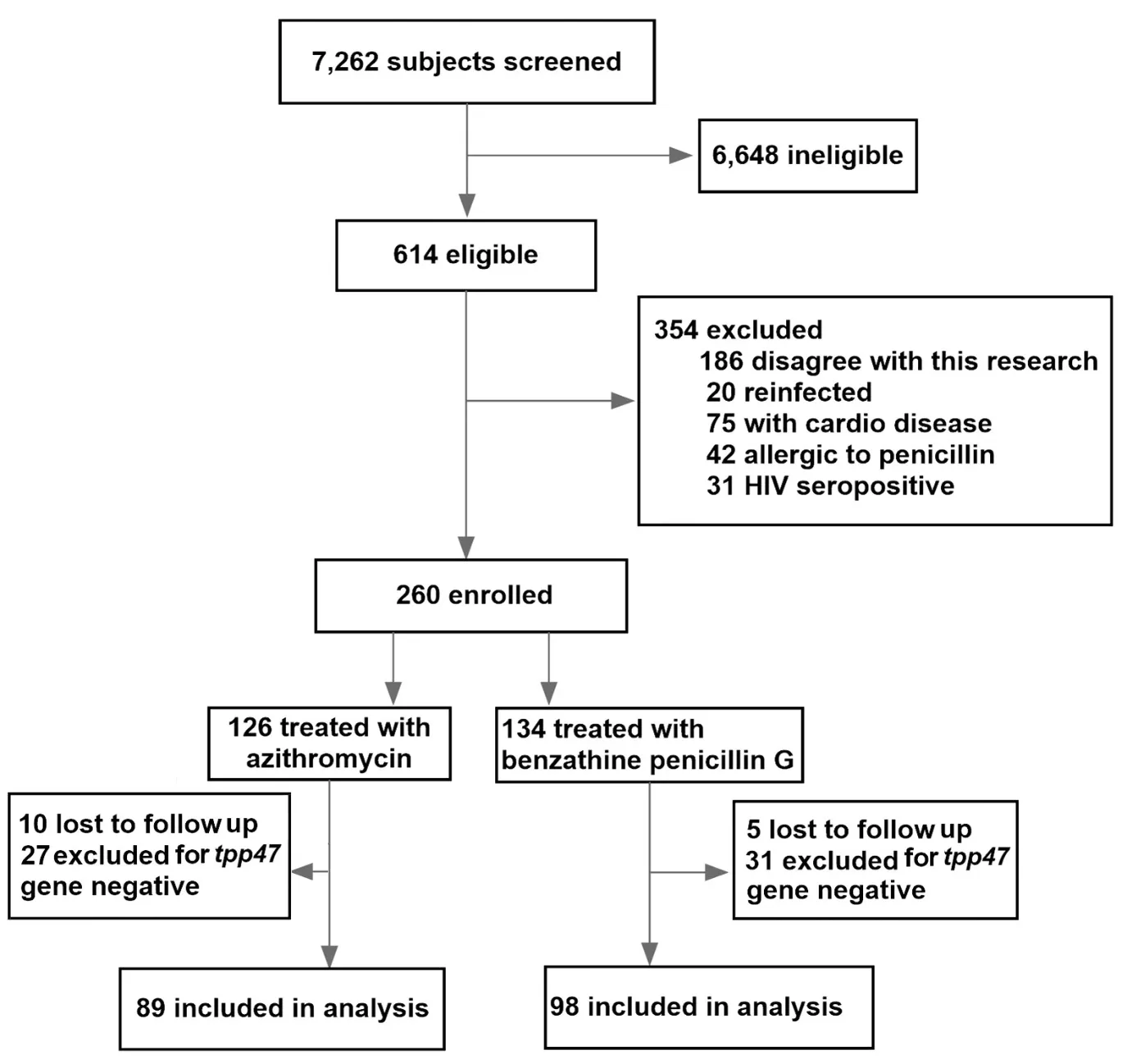
Figure 1 Flow chart of subject selection in the study.
Eligible patients were assigned to receive oral azithromycin(0.5 g,once daily for 15 days)or intramuscular BPG(2.4 million units,once weekly for 3 weeks)accoridng to a random number table created by a computor.All patients in the BPG group underwent penicillin skin testing before treatment. Oral doxycycline(100 mg,twice daily for 15 days)was used to treat patients who presented a positive penicillin skin reaction.
All patients were followed-up at 2 weeks and 3,6,9,and 12 months after treatment.Each follow-up included an assessment of the patient’s clinical characteristics and laboratory test results,especially their RPR titer.
Statistical analysis
All data were analyzed with the Statistical Package for Social Sciences(SPSS19.5?,SPSS Inc.,Chicago,USA).Associations between categorical variables were determined using the Chi-squared test.Odds ratios(ORs)and 95%confidence intervals(CIs)were determined,and logistic regressions were conducted to adjust theORsas needed.AP-value of<0.05 was considered to indicate statistical significance.
Results
Characteristics of study participants
Figure 1illustrates the process of selecting eligible subjects for this study.Of the 7,262 subjects,who visited the STD clinics in the paticipateing hospitals to be screened for syphilis between February 2010 and December 2014,614 were confirmed as having early syphilis,and 260 of them were initially enrolled into this study.Of these 260 patients,126 were treated with azithromycin and 134 with BPG according to the protocol.Ten azithromycin-treated and five BPG-treated patients were lost to follow-up,including six who did not return for any follow-up visit and nine who failed to return after the second follow-up visit.Among the remaining 245 samples,there were 27 azithromycin-treated cases and 31 BPG-treated cases subsequently assessed astpp47gene-negative and excluded from the study.Thus,after the final followup visit,a total of 187 patients remained to be analyzed by this study.Of these patients,89 patients were treated with azithromycin and 98 with BPG.
Table 1summarizes the demographic and clinical characteristics of the enrolled patients.The 187 patients included 130(69.5%)men and 57(30.5%)women,with a mean age of 32.8 years(range,18-65 years).Of these 187 patients,103(55.1%)presented with primary syphilis and 84(44.9%)with secondary syphilis.The RPR titer was≤ 1∶8 in 50(26.7%)patients,1∶16-1∶32 in 95(50.8%)patients,and ≥ 1∶64 in 42(22.4%)patients.There were no significant differences in gender distribution,age,number of sexual partners,syphilis stage,and baseline RPR titers between the azithromycin and BPG groups(allP>0.05).
Prevalence of A2058G and A2059G mutations in T.pallidum

Table 1 Baseline of early syphilis patients treated with azithromycin or BPG
Over the 5-year study period,the percentage ofT.pallidumwith one of these two mutations(A2058G and A2059G)steadily increased,from 90.9%in 2010 to 95.3% in 2014(Figure 2).Of the total 187 specimens,172(92.0%)had either an A2058G(153 cases,89.0%)orA2059G(19 cases,11.0%)mutation.
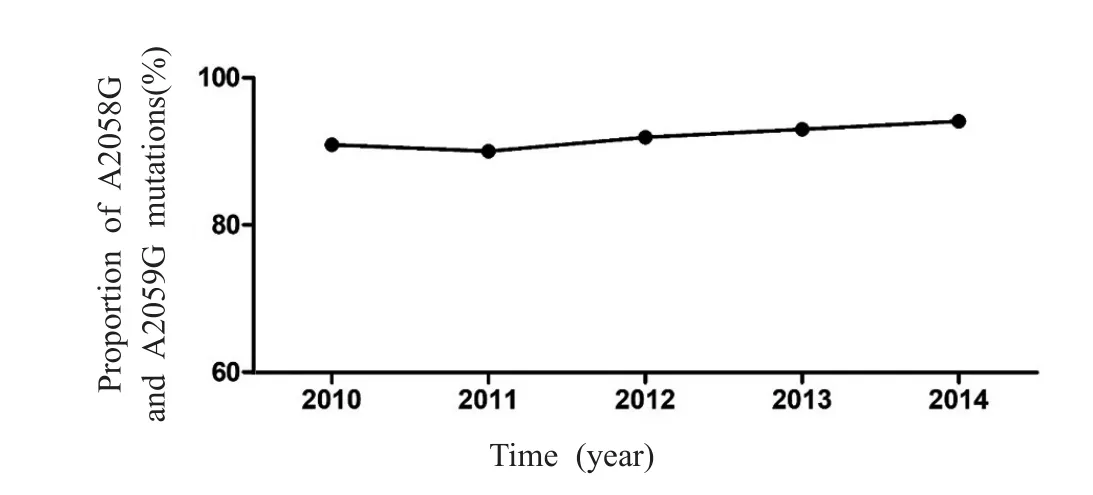
Figure 2 The proportion of A2058G and A2059G mutations in Treponema pallidum from 2010 to 2014.
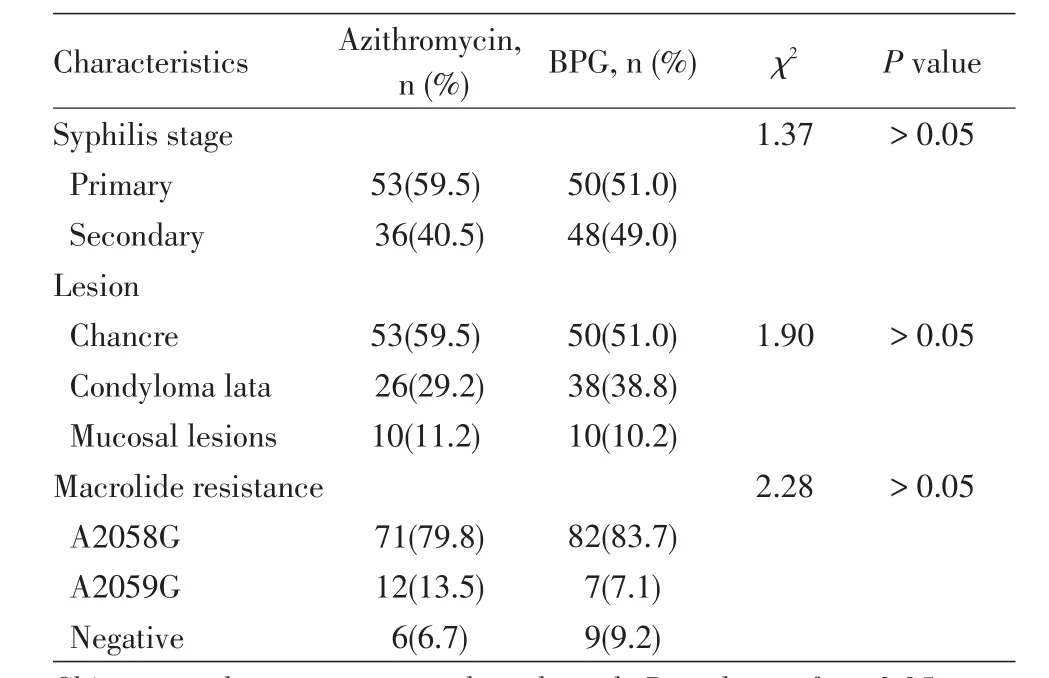
Table 2 Status of Treponema pallidum A2058G and A2059G mutations in azithromycin-or BPG-treated patients
A2058G and A2059G mutation statuses in the azithromycin and BPG groups
The A2058G and A2059G mutation statuses in the azithromycin and BPG groups are shown in Table 2.Of the 89 DNA samples obtained from the azithromycin group that were positive for thetpp47gene,83(93.3%)were positive for a mutation,including 71(79.8%)positive for A2058G and 12(20.2%)positive for A2059G.Of these 83 samples,51(61.4%)were collected from chancres and 32 from condyloma lata and mucosal lesions.Of the 98 equivalent samples obtained from the BPG group,89(90.8%)were positive for one of the two mutations;82(83.7%)of these 89 patients had an A2058G mutation,and 7(7.1%)had an A2059G mutation.There was no significant difference in the proportions of the two mutations between the azithromycin and BPG groups(P>0.05).
Response to treatment
The serological response to azithromycin or BPG and distribution of the two mutations are shown in Figure 3 andTable 3.Of the 172 patients presenting as A2058G-or A2059G-positive,78(45.3%)patients,all of whom were in the BPG group,obtained a serological response,whereas 32.6%and 22.1%of these patients displayed serological failure and serofast results,respectively(Figure 3B).Among the A2058G-or A2059G-positive patients treated with azithromycin,55(66.3%)and 28(33.7%)patients,respectively,displayed serological failure and serofast results,in contrast with 1.1%and 11.3%of BPG-treated patients.This difference between the two treatment groups was significant(P<0.05).
Among the 15 patients who presented as negative for the A2058G and A2059G mutations,the serological response rate was 66.7%(10/15),with four patients in the azithromycin group and six in the BPG group.Within this subset,there was no significant difference in the serological response rate between the two groups(P>0.05,Figure 3C).
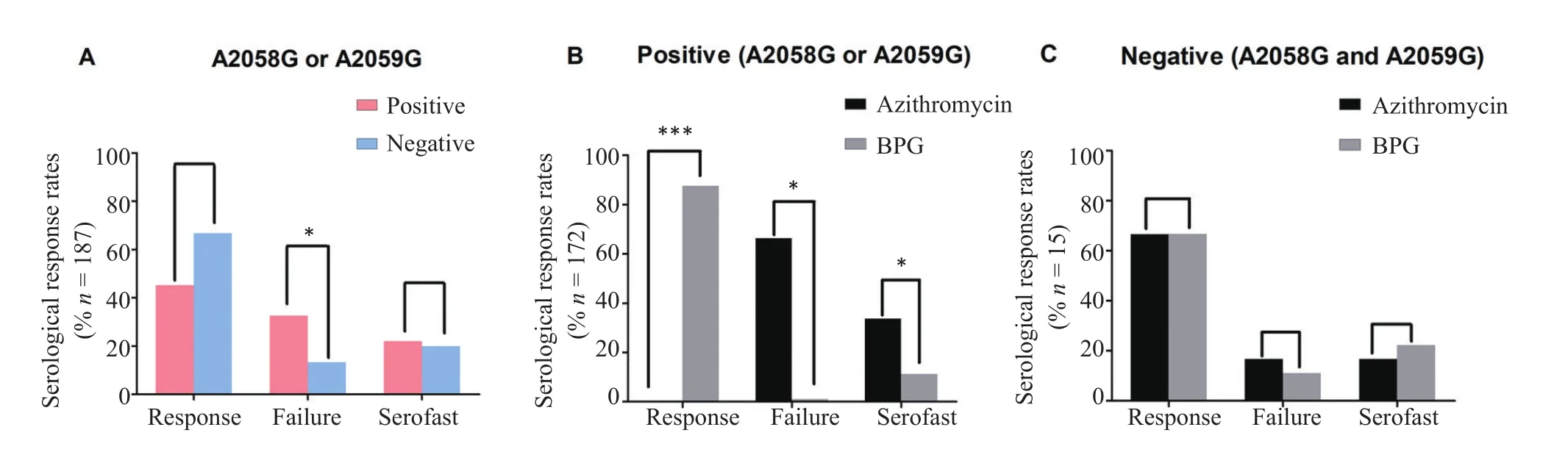
Figure 3The serological response rates in the azithromycin and BPG groups.A:The serological response rates among patients with(positive)or without(negative)an A2058G or A2059G mutation.B:The serological response rates among azithromycin-treated or BPG-treated patients who presented with an A2058G or A2059G mutation.C:The serological response rate among azithromycin-treated or BPG-treated patients who presented as negative for both A2058G and A2059G mutations.*P<0.05,**P<0.01,***P<0.001.
Factors associated with serological response
The factors associated with serological response at 12-months follow-up are shown in Table 4.Both in univariate and multivariate analyses,patients with higher RPR titers(RPR,≥ 1∶64)were more likely to
achieve serological response than those with lower RPR titers(RPR, ≤ 1∶8;OR,0.23;95%CI,0.09-0.37).Patients in the azithromycin group were less likely to achieve serological response than those in the BPG group(OR,121.50;95%CI,35.38-386.17).Additionally,subgroup analyses showed that there was no significant relationship between the serological response and the following demographic and clinical characteristics,including gender distribution,age,number of sexual partners,and syphilis stage.

Table 3 Comparison of serological response in azithromycin-or BPG-treated early syphilis patients at 12-months follow-up relative to the baseline

Table 4 Risk factors associated with serological response for early syphilis patients in the two groups at 12-months follow-up relative to the baseline time
Adverse events
Non-serious adverse events during the first month after treatment were reported by 9(10.1%)of the 89 patients treated with azithromycin and by 7(7.1%)of 98 patients treated with BPG.The most common adverse events reported by patients in the azithromycin group were gastrointestinal discomfort,including nausea(4 patients),diarrhea(2 patients),abdominal pain(1 patient),and abdominal bloating(2 patients).Specifically dizziness and headache were also reported by two patients,respectively.In the BPG group,administration-related adverse events were the most common type of adverse event.Seven(7.1%)of the 98 patients reported persistent injection-site pain.In addition,39 patients experienced Jarisch-Herxheimer reactions(azithromycin group,6[6.7%];BPG group,23[23.5%]).No serious adverse events were reported during the study,all adverse events were characterized by the patients as mild,and all resolved as treatment continued.
Discussion
To the best of our knowledge,this is the first study focusing on the potential associations between A2058G and A2059G mutations and the efficacy of azithromycin in early syphilis patients in China.Since azithromycin resistance among syphilis patients was firstly reported in 2004[13],several centers in different countries have screened for the presence of A2058G and A2059G mutations inT.pallidum[17-19].The rates of macrolide resistance have been found to vary worldwide:0.7%in Taiwan in 2009-2013[20],16.3%in Canada in 2007-2009[21],64.3%in the United States in 2007-2009[22],84.4%in Australia in 2004-2011[23],93.1%in Ireland in 2009-2010[24],and 100%in China in 2010-2012[25].Overall,the prevalence of macrolide resistance has increased over time and showed geographical differences within countries(e.g.,in relatively remote areas to large cities).In this prospective observational study,we determined the prevalence of A2058G and A2059G mutations inT.palliduminfections in China.In agreement with the findings of a previous survey[25],the proportion ofT.pallidumcontaining A2058G and A2059G mutations in China was very high.Over the past 5 years,the percent ofT.pallidumcases with one of these two mutations increased yearly,from 90.9%in 2010 to 95.3%in 2014.Thus,China is an area with a high prevalence of macrolide-resistantT.palliduminfection.
In China,macrolides including azithromycin were recommended as an alternative antibiotic for patients allergic to penicillin based on the findings that azithromycin is active against chlamydia and Gram-positive bacteria,as well as on the background that desensitization therapy to penicillin was never officially recommended for penicillin-allergic patients.Considerring the current high prevalence of macrolide-resistance,is azithromycin still effective as alternative antibiotic for syphilis?In this study,we found that among the subset of patients who presented as A2058G-or A2059G-positive,none of those in the azithromycin treatment group obtained a serological response.Statistical analysis revealed a high frequency of the two mutations and a low RPR titer as risk factors of a low serological response rate for azithromycin treatment.In contrast,among the subset of patients who were negative for both mutations,the serological response rate in the azithromycin group was similar to that in the BPG group.These results indicate that azithromycin is still an effective therapeutic alternative for patients,especially those co-infected with chlamydia and mycoplasma,if their infectedT.pallidumstains lack both the A2058G and A2059G mutations.A rapid and efficient method for detecting the presence of these two mutations prior to treatment with azithromycin would contribute to increasing the serological rate for early syphilis patients in China.
In agreement with previous results[26],we found that azithromycin was well tolerated by patients without serious adverse events.Rates of non-serious adverse events were similar in the azithromycin and BPG treatment groups.Most of the non-serious adverse events for azithromycin were gastrointestinal in nature and of mild intensity.The incidence of Jarisch-Herxheimer reaction in patients administrated with azithromycin was lower than that in patinets treated with BPG,and the result is consistent with a previous finding[27].Several hypotheses have been proposed to explain this difference[28-30].For example,the ability of BPG to reach its peak concentration more rapidly may stimulate the cytokine cascade and release ofT.pallidumlipoproteins,activating the onset of the Jarisch-Herxheimer reaction[31-32].Azithromycin,as a bacteriostatic agent,has long been recognized to exert anti-inflammatory effects,suppressing the production of inflammatory cytokines[33-34].Further investigations are warranted to elucidate the mechanisms underlying the Jarisch-Herxheimer reaction.
The present study has following several limitations.First,it was performed across five central cities in China,which might be different on economic and social development compared with other areas in China,particularly rural and remote areas.Second,the definition of serological cure for syphilis treatment varies among studies monitoring the serological response to treatment;as such,it should carefully compare our results with all relevant previous work.Third,only early syphilis patients with moist lesions were analyzed in this study,thus excluding those patients without moist lesions and those with latent syphilis,for which the bias in the analyses was inavoidable.Additionally,we did not investigate other factors,such as host’s immune that may affect the serological response after azithromycin treatment.Finally,because the prevalence of macrolide-resistantT.pallidumis particularly high in China,our results should not be generalized to areas with low prevalence of macrolide-resistantT.pallidum.Thus,the future application of our findings to similar problems should be undertaken with consideration of these limitations.This prospective study showed a high frequency of 23S rRNA A2058G and A2059G point mutations inT.pallidumcurrently circulating in China.In addition,a lower serological response rate for azithromycin treatment was found compared with that for BPG treatment. Together, these findings suggest a correlative relationship between the high prevalence of macrolide resistance and a lower serological response rate after azithromycin treatment.
Acknowledgements
This research was supported by the Mega Project of China National Science Research for the 11th Fiveyear Plan(No.2008ZX10001-005),Natural Science Foundation of Jiangsu Province of China (No.BK20150121),National Natural Science Foundation of China(No.81601804,8177220),and the Union Innovation Team Project of Chinese Academy of Medical Sciences(No.2016-I2M-3021).We thank the staff at the study sites for participants enrollment,specimens collection,and interview conduct,and thank all the participants for their cooperation.
- 國際皮膚性病學雜志的其它文章
- Paraneoplastic pemphigus comorbid with cardiac cancer and duodenal gastrointestinal stromal tumors:a rare case report
- Reactive perforating collagenosis
- Sun?protection knowledge and strategies of Chinese dermatologists:a nation?wide,questionnaire?based survey
- Initial presentation of acute myeloid leukemia in a patient with cutaneous myeloid sarcoma
- Persistent papules with adult?onset Still’s disease:a case report
- Primary vulvar melanoma in a 27?year?old pregnant woman:a case report and literature review

