Frailty and Liver resection: where do we stand?
Georgios S. Sioutas, Ioannis A. Ziogas, Georgios Tsoulfas
1Department of Medicine, School of Health Sciences, Democritus University of Thrace, Alexandroupolis 68100, Greece.
2First Department of Surgery, Papageorgiou University Hospital, Aristotle University of Thessaloniki, Thessaloniki 54622, Greece.
#Authors contributed equally.
Abstract As the world population is continuously aging, the number of older patients requiring liver surgery is also on the rise. Data have shown that age should not be a limiting factor for liver resection, as it cannot accurately predict postoperative outcomes. Instead, frailty can serve as a more reliable measure of the patient’s overall health and functional reserves. Several frailty assessment tools have been implemented for preoperative risk stratification before liver surgery, and higher scores have commonly been associated with postoperative morbidity, mortality, and length of hospital stay. However, no consensus has been reached on the most useful screening tool. Future studies should focus on comparing the currently available assessment tools, constructing a liver resection-specific tool, and assessing the role of frailty assessment tools in preoperative patient optimization.
Keywords: Frailty, age, elderly, liver resection, liver surgery, morbidity, morbidity, complications
INTRODUCTION
Liver resection is the current standard of care for most patients with benign or malignant liver lesions and adequate liver function[1,2]. Advances in healthcare have led to a continually increasing life-expectancy, which consequently leads to a higher number of elderly patients (> 60 years) being offered liver surgery[2,3]. Several studies sought to compare liver resection in youngervs.older surgical candidates and reported varying yet acceptable outcomes in appropriately selected older individuals[2,4-8]. In fact, morbidity and mortality rates in patients undergoing liver resection for hepatocellular carcinoma (HCC) seem to range 9%-51% and 0%-42.9%, respectively[9]. Normal aging is associated with a gradual decline in the function of most organ systems, including the liver. The liver synthetic and metabolic activity, liver volume, and blood flow to the liver seem to be significantly affected in the elderly[10]. It is well known that the liver remnant regenerates after liver resection, and the final liver volume after regeneration does not seem to differ between younger and older individuals[9,11]. However, this process might be delayed in the elderly due to the liver’s decreased proliferative capacity in the early period after the loss of the liver mass[12]. Data have shown that liver regeneration after living donor liver transplantation can be delayed in older donors when compared to younger donors[13]. These findings indicate that physiological deconditioning and remaining organ function might have a more significant effect on clinical outcomes than the actual chronological age[14,15].
Frailty syndrome is defined as the increased vulnerability to stressors, loss of ability to adapt, and diminished resiliency secondary to an age-related decline in the physiological reserves and function of multiple organ systems[16,17]. It is essential to distinguish frailty from “comorbidity” and “disability”; although these three terms overlap to some extent and are often used interchangeably to predict patient outcomes, they represent entirely different entities[18]. As described by Feinstein, “comorbidity” is “any distinct additional entity that has existed or may occur during the clinical course of a patient who has the index disease under study”[19]. The term “disability” refers to the abnormal biological functioning or the defect that renders individuals inferior to the “normal” species around them leading to loss of social stability and survival[20]. Frailty should also not be considered synonymous to aging[21], but rather an intermediate clinical state between normal and pathological aging[22]. Frail patients commonly fail to return to their prior homeostasis after a stressor, resulting in adverse clinical outcomes[23,24]. Therefore, the need for developing accurate risk-stratification tools that can potentially identify older patients at risk for postoperative complications is apparent[25].
The aim of the present review is to summarize the impact of age on patient outcomes after liver surgery, describe the available frailty assessment tools, and discuss the impact of frailty on postoperative outcomes in patients undergoing liver resection.
LIVER RESECTION AND AGE
Several studies sought to investigate the outcomes of liver resection in youngvs.old patients. Fonget al.[26]published one of the first studies examining the effect of age on liver surgery. Their study included 133 patients older than 65 years undergoing liver resection for colorectal liver metastases, and they found that age was an independent risk factor for increased risk of morbidity. According to the authors, major hepatic resection may be safely performed and result in favorable functional outcomes on appropriately selected older patients[26]. Choet al.[2]investigated the safety of liver resection in the elderly and reported favorable outcomes in patients ≥ 70 years. Although most elderly patients were transferred to rehabilitation facilities postoperatively, there was no difference in terms of severe postoperative complications. The authors also performed a literature review and included 14 previous studies; only two (14.3%)[27,28]of them reported a statistically significant difference in severe postoperative complications and only two (14.3%)[28,29]reported a statistically significant difference in mortality between old and young patients. Additionally, a large single-center study from France showed that age ≥ 75 years is a risk factor of mortality after liver resection[30], while a multicenter study from the US showed that increasing age is associated with increased postoperative sepsis and overall mortality, but not overall morbidity[31].
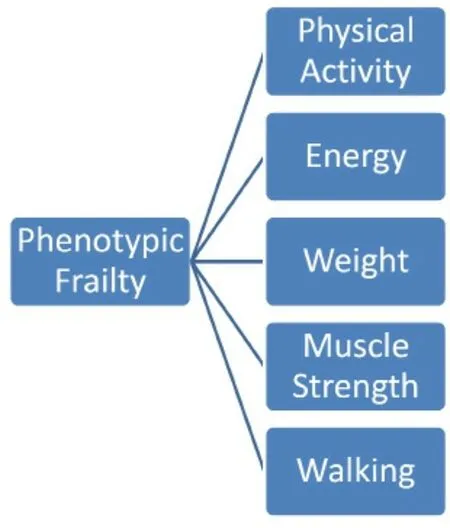
Figure 1. “Physical Frailty Phenotype” model by Fried et al.[14]
As liver resection represents the mainstay of treatment in non-metastatic HCC[32-34], several studies aimed to investigate the difference in outcomes between young and old HCC patients. Therefore, data have proven the safety and feasibility of liver resection in appropriately selected patients aged not only more than 70 years, but, in some cases, even more than 80 years[35-38]. A meta-analysis[39]reported that the morbidity and mortality rates did not differ significantly between older and younger patients undergoing hepatic resection for HCC, while the risk of mortality for younger patients was 2.7 times lower when compared to the elderly for colorectal liver metastases[39]. Interestingly, another meta-analysis reported that hepatic resection for liver malignancies is associated with a higher risk of postoperative renal failure, infection, and mortality in oldervs.younger patients, while the length of stay (LOS) in the hospital, transfusions, and disease-free survival did not differ significantly between the two groups[40]. Nevertheless, the considerable variability in patient outcomes after liver resection in the elderly underlines the inability of age alone to predict postoperative outcomes accurately. Instead, factors that reflect the overall health status of the patient, such as frailty, may serve as more accurate predictors of postoperative outcomes. On that grounds, several frailty assessment tools have been developed in order to preoperatively determine patients at risk of adverse postoperative outcomes.
FRAILTY ASSESSMENT TOOLS
Numerous frailty screening tools have been described over the years[17,18,41]. The most commonly implemented one is the “Physical Frailty Phenotype” model by Friedet al.[14], which describes frailty as the decrease in physiological reserve secondary to a multisystem functional decline. This tool assesses the following criteria to identify frail patients: (1) walking speed; (2) grip strength; (3) weight loss; (4) physical activity; and (5) exhaustion [Figure 1]. Patients meeting one or two of these criteria are deemed “pre-frail”, while those meeting at least three criteria are categorized as frail[14]. Makaryet al.[42]further validated this definition, and at the same time defined as “pre-frail” those fulfilling two or three of the above-mentioned criteria. The Phenotypic frailty tool requires only a questionnaire, a stopwatch, and a dynamometer, and thus can be completed in only 10-15 min[17]. It is also recognized by the American College of Surgeons and the American Geriatric Society for the assessment of the elderly preoperatively[43]. Nevertheless, the inherent drawback of this assessment method is the lack of psychosocial evaluation of the older patient[44].
The second most commonly used frailty assessment tool is the “Deficit Accumulation Index” by Rockwoodet al.[15][Figure 2]. It defines frailty using a frailty index (FI) with the number of deficits or abnormal characteristics accumulated over several areas (i.e., physical, social, functional, and cognitive) on the numerator and the total number of characteristics assessed on the denominator[15,45,46]. Higher index values have been associated with an increased likelihood of frailty, adverse patient outcomes, disability, hospitalization, and death[45]. Although it is considered more sensitive than the Phenotypic frailty tool[16], its downsides include the fact that it is time-consuming (up to 70 characteristics assessed sometimes) and its extensive focus on comorbidities (symptoms, diagnoses, abnormal values on laboratory tests,etc.) rather than on functional decline[18]. With the aim to assess frailty in a timely fashion and in a more efficient way, several modified FIs (mFIs), which may measure as few as five factors, have been generated[47]. In fact, 11-point mFIs have already been used to evaluate patients undergoing liver resection[48,49].
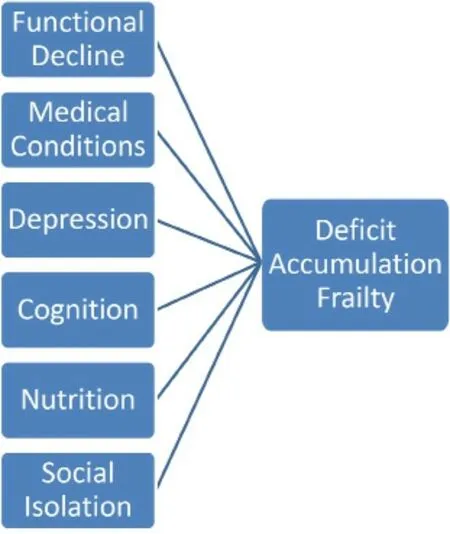
Figure 2. “Deficit Accumulation Index” by Rockwood et al.[15]
Comprehensive geriatric assessment (CGA) is another well-established approach implemented to evaluate frailty in older patients[50,51]. It utilizes assessment tools and laboratory values to assess patients from several standpoints, including physical medical comorbidities, nutritional status, mental and cognitive status, physical functioning and fitness, social networking, and environmental status[50,51]. A downside that CGA shares with FI is that it is time-consuming, mostly due to its complexity[51]. Two screening tools that can help identify elderly patients who will benefit from a more comprehensive assessment with the CGA are the Geriatric-8 (G8) and the Vulnerable Elders Survey-13 (VES-13)[52]. Those take into consideration age, self-rated health, and physical function along with other factors[53], and both of them take only 5 min to complete[52]. The first one is specifically designed to identify vulnerable older patients with cancer, while the latter is designed to detect vulnerable older patients in the community. Hence, G8 was found to be predictive of postoperative complications in elderly patients with HCC, while VES-13 was not shown to have a predictive role[53]. The abbreviated CGA is another screening tool for elderly cancer patients that incorporates only the 15 most important items of the full assessment tool and requires only 5 min to complete[54,55].
The FRAIL (Fatigue, Resistance, Ambulation, Illness, and weight Loss) index is another easy to complete screening tool that deems patients frail if three or more of its components are present[56]. A great advantage of this scale is that it has been validated not only in the elderly but also in middle-aged individuals[56,57]. On the other hand, the Edmonton Frail Scale (EFS) assesses the patients’ health over ten frailty domains (including cognition, general health status, mood, continence, functional performance and independence, social support, polypharmacy, and nutrition)[58]. A score below 5 indicates the “no frailty” patients, a score between 6 and 11 identifies the “apparently vulnerable” individuals, and a score between 12 and 17 distinguishes the “severe frailty” patients[59]. Studies have shown that EFS is a valid and accurate tool that can be efficiently administered by non-geriatricians to assess and preoperatively optimize geriatric patients with or without cancer[58,60,61]. The Clinical Frailty Scale (CFS) is another quick frailty assessment tool based on clinical judgment[62]. Data suggest that it can accurately identify patients who may need institutional care, as well as those less likely to survive[62]. Data have shown that CFS can reliably distinguish patients who are going to have a complicated course and prolonged LOS while in the acute medical ward[63]. A screening tool for frailty broadly used in Japan is the Kihon Checklist (KCL)[64]. KCL is a self-reporting “yes/no” survey that can help clinicians assess the status of older individuals in several frailty domains (functional, physical, psychological, and social)[65].
Moreover, there are some single item tools that are less comprehensive but can serve as quick and easy to apply screening tools. The most common tool falling into this category is the handgrip test, which utilizes a hydraulic hand dynamometer to evaluate the maximum strength of the dominant hand[52]. Its role in distinguishing “fit” from “frail” older individuals has been validated not only in the general population but also in cancer patients, as handgrip strength is highly associated with survival outcomes[52,66]. Although thehandgrip test can be applied in multiple settings, another tool particularly useful for hospitalized patients is the timed “Up & Go” test[67]. This test requires the patient to stand up and walk three meters, then to turn, walk back and sit down and can accurately assess balance and functional mobility[52]. Data suggest that it is particularly useful in identifying cancer patients at risk of postoperative complications[68]. A comprehensive list of the various tools used for the assessment of frailty is shown in Table 1.
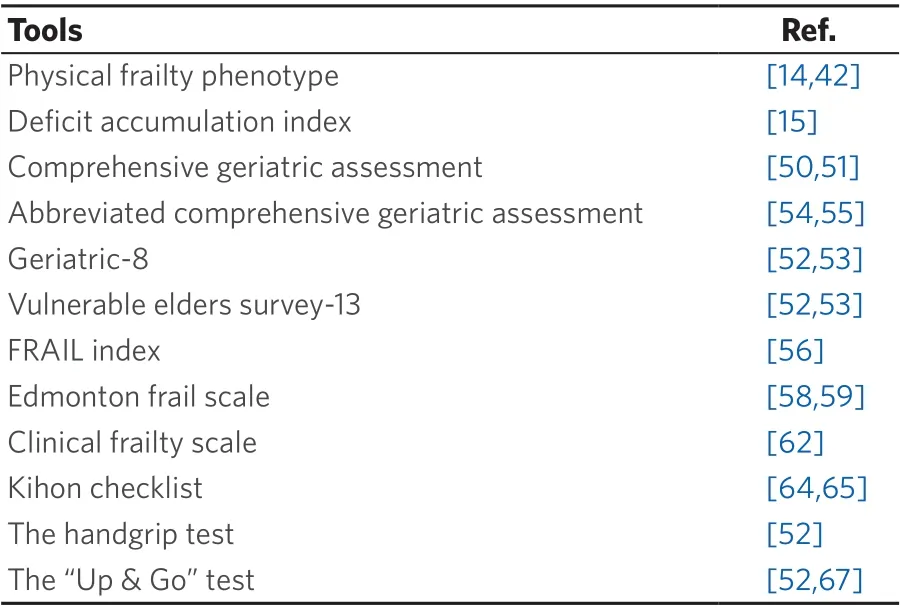
Table 1. Frailty assessment tools
LIVER RESECTION AND FRAILTY
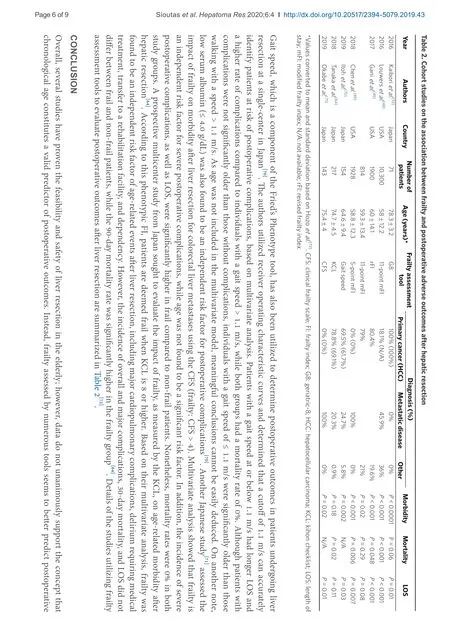
There is a growing body of evidence that frailty assessment tools are useful in identifying frail patients at higher risk of postoperative morbidity and mortality, as well as extended LOS in the hospital. In fact, Kaiboriet al.[53]evaluated the utilization of the G8 CGA tool in patients ≥ 70 years undergoing liver resection for HCC. Patients with a score lower than 14 demonstrated higher postoperative morbidity rate and extended LOS, but no difference in mortality when compared to patients with scores ≥ 14[53]. Notably, on multivariate analysis, G8 score < 14 was significantly associated with postoperative morbidity, while age ≥ 77 years was not found to be a significant risk factor[53]. It is worth mentioning that patients with HCC arising on a background of cirrhosis demonstrated a tendency towards inferior outcomes after liver resection[53]; however, further research is warranted in order to deduce meaningful conclusions.
Louwerset al.[48]investigated the impact of frailty, assessed by the 11-point mFI tool, on morbidity and mortality after open hepatectomy in 10,300 patients from the National Surgical Quality Improvement Project (NSQIP) database. As the mFI score increased, a statistically significant increase was associated with Clavien 4 complications, mortality, and extended LOS. Notably, this statistical significance was maintained in all types of hepatectomy (partial, right, left, and extended). Although this study highlighted the importance of mFI in preoperative planning and risk stratification, the authors stressed the need for simpler hepatectomy-specific frailty assessment tools[48]. Another study utilizing NSQIP hepatectomy data described the revised FI (rFI) on a “training set” of patients and compared it with the 11-point mFI (“validation set”)[49]. rFI incorporates several variables, such as preoperative serum albumin and hematocrit, American Society of Anesthesiologists score, BMI, the extent of liver resection, and underlying pathology. Higher rFI scores were significantly associated with postoperative complications, prolonged LOS, and mortality, while higher mFI scores were linked only to a higher risk of morbidity but neither mortality nor LOS[49]. Chenet al.[69]evaluated the use of a five-item mFI to assess the effect of frailty on outcomes in patients undergoing combined colorectal and liver resection for colorectal cancer and liver metastases. Patients with higher mFI scores exhibited a higher incidence of mortality, overall and severe morbidity, as well as prolonged LOS. On multivariate analysis, higher mFI scores were found to be independent risk factors for overall and severe morbidity, while age was not found to be a significant factor that affects morbidity.

DECLARATIONS
Authors’ contributions
Study concept, data analysis and interpretation, critical revision of the manuscript, final approval of the manuscript: Ziogas IA, Sioutas GS, Tsoulfas G
Data acquisition, drafting of the manuscript: Sioutas GS, Ziogas IA
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
? The Author(s) 2020.
- Hepatoma Research的其它文章
- Animal models for hepatocellular carcinoma arising from alcoholic and metabolic liver diseases
- Response rates to direct antiviral agents among hepatitis C virus infected patients who develop hepatocellular carcinoma following direct antiviral agents treatment
- The mechanism of dysbiosis in alcoholic liver disease leading to liver cancer
- Coffee protection against the development of hepatocellular carcinoma: review article

