ALK expression, prognostic significance, and its association with MYCNexpression in MYCN non-amplified neuroblastoma
Dinesh Babu Somasundaram' · Sheeja Aravindan2.Nandita Gupta3·Zhongxin Yu*.Ashley Baker3 .Natarajan Aravindan124D


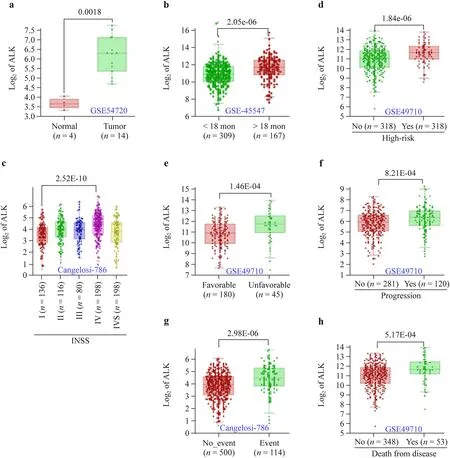
Fig. 1 Association of ALK transcription with MYCN-na-NB disease progression. Histograms from representative patient cohorts showing heightened transcription of ALK in MYCN-na-NB samples compared with normal (adrenal) controls ( a), children more than 2 years old compared with children less than 2 years old ( b), advanced disease stage (stage 4) compared with other INSS ( c), high-risk disease compared with low-risk disease ( d), unfavorable histology compared with favorable histology ( e), progressive disease compared with diagnosis ( f), in the presence of an event compared with no events ( g),and death from disease compared with no death from disease ( h). All data were obtained from R2, a web-based microarray analysis and visualization platform. The database ID is provided as an insert in each graph. n is provided in the parenthesis. Statistical significance is provided on top of the comparison bar. ALK anaplastic lymphoma kinase, NB neuroblastoma, na non-amplified, INSS International Neuroblastoma Staging System
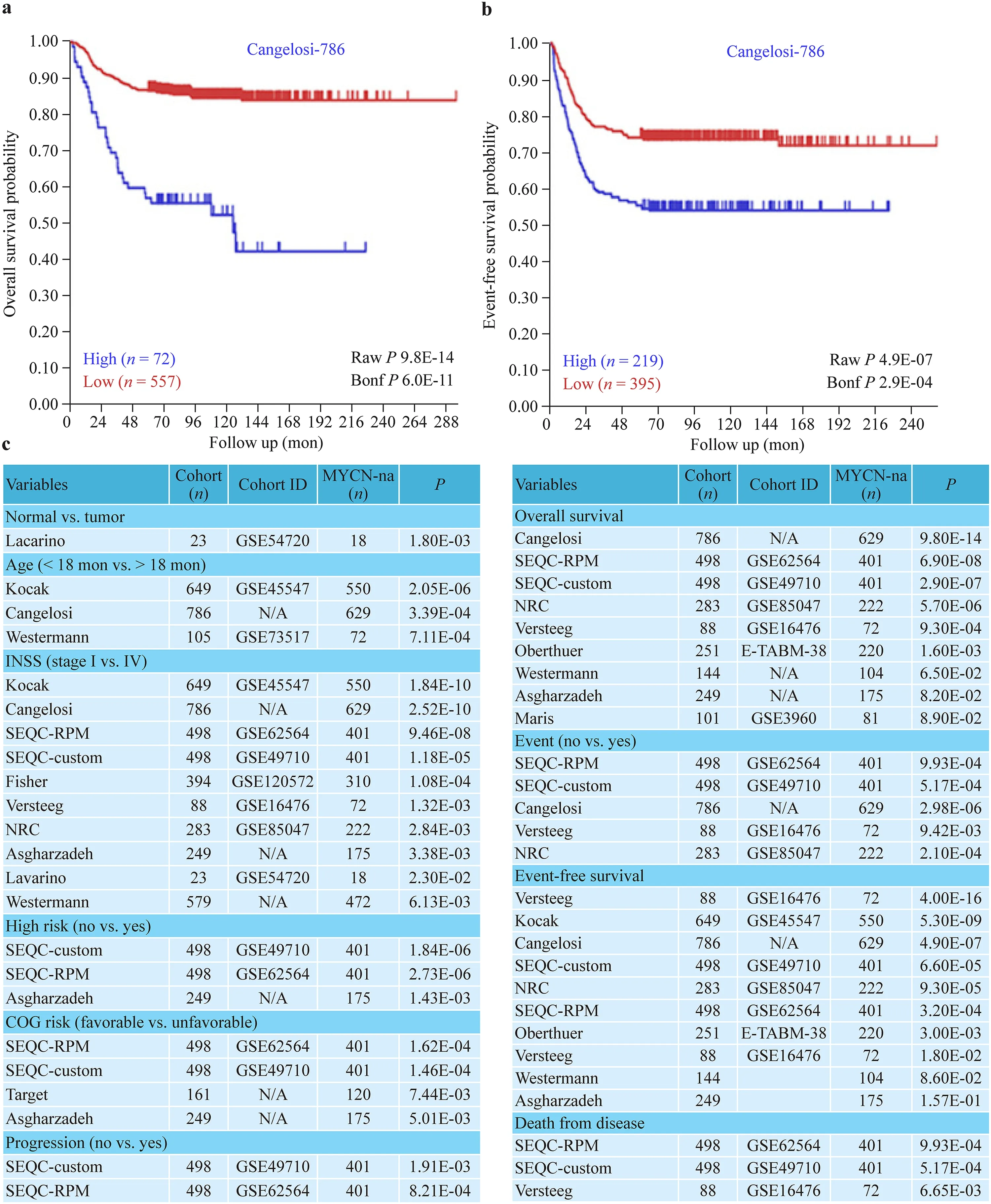
Fig. 2 Association of ALK transcription with poor clinical outcomes. Kaplan-Meier curves showing decreased overall survival ( a) and event-free survival ( b) in patients with high ALK expression compared with low ALK expression. The database ID is provided as an insert in each graph. Table showing list of patient cohorts,n in each cohort, cohort ID, n of MYCN-na patients, P values of comparison between normal vs.neuroblastomas, < 18 months vs. > 18 months, INSS stage 1 vs. stage 4, high-risk vs.low-risk, favorable vs. unfavorable, progressive disease vs. diagnosis, overall survival,presence and absence of events,event-free survival, and death from disease ( c). All data were obtained from R2, a webbased microarray analysis and visualization platform. ALK anaplastic lymphoma kinase, na non-amplified, INSS International Neuroblastoma Staging System, N/A not applicable,SEQC Sequencing Quality Control, RPM Reads Per Million,NRC Neuroblastoma Research Consortium, COG Children’s Oncology Group
We examined the correlation of ALK and MYCN transcription in 22 (totaln
= 4297) independent MYCN-na-NB patient cohorts (Fig. 4). All 22 studies indicated a significant, and directly proportional correlation betweenALK
andMYCN
transcription in MYCN-na-NB patient cohorts.MYCN IHC in 70 MYCN-na-NB patients revealed that expression ranged from a minimum of 0.19% to a maximum of 30.37%, with an average of 2.82% and a median of 1.29% (Fig. 5 a). Pearson correlation analysis indicated a strong and significant (P
= 0.0006) positive correlation in the expression of ALK and MYCN expression in MYCNna-NB (Fig. 5 b).NB is the heterogeneous tumor with diverse biology/evolution, from spontaneous regression to therapy-defying progression. About 50% of children presenting with HR-NB have PD and unacceptable survival. AlthoughALK
modifications and its cooperating actions withMYCN
amplifications in NB aggravation have been recognized,its prognostic relevance in MYCN-na-NB (that comprise about 70% of all HR-NB) is thus far not realized. Schulte and colleagues indicated that highALK
gene expression could contribute to a malignant phenotype in primary NB[ 33]. For the first time, our results display the landscape of ALK transcription and protein expression in MYCNna-NB and its direct association with disease progression and clinical outcomes. Our analysis in multiple large cohorts clearly portrayed a “high-is-worse” correlation with advanced disease stage, risk status, and clinical outcomes. Our TMA-IHC studies exclusively identified the association of high ALK expression with poor OS and RFS in MYCN-na-NB patients.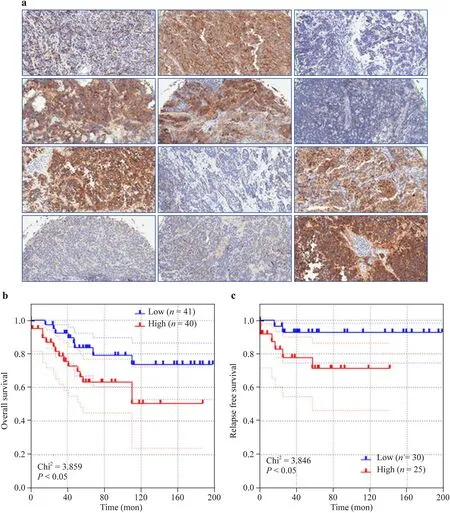
Fig. 3 ALK expression in NB clinical outcomes. Representative microphotographs showing surface expression of ALK in human MYCN-na-NB (magnification × 20) ( a); Kaplan-Meier curves showing the association of the expression of ALK with overall survival ( n = 81) ( b) and relapse-free survival in a cohort of 55 MYCN-na-NB patients ( c). All survival analysis (log rank Mantle cox test and hazard ratio) and Kaplan-Meier curves were performed in GraphPad Prism. Blue = low ALK; red = high ALK levels. ALK anaplastic lymphoma kinase, NB neuroblastoma, na non-amplified
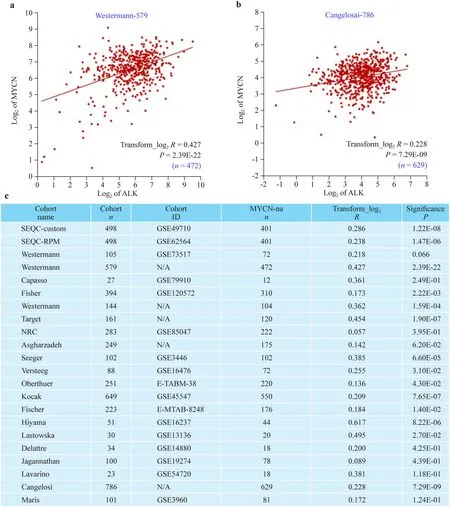
Fig. 4 ALK transcription correlates with MYCN transcription in MYCN-na-NB. XY graphs showing a linear correlation between MYCN and ALK transcripts in representative MYCN-na-NB: Westermann-579 (total n = 579, MYCN-na n = 472) ( a); and Cangelosai-786(total n = 786, MYCN-na n = 629) cohorts ( b). The database ID is provided as an insert in each graph. Axes are log 2 of ALK and MYCN.Table showing list of patient cohorts, n in each cohort, cohort ID, n of MYCN-na patients, transform log 2 R, and significance ( P values)of correlation between ALK transcription and MYCN transcription ( c).All data were obtained from R2, a web-based microarray analysis and visualization platform. All independent studies revealed a direct and linear correlation between ALK and MYCN transcription in MYCNna-NB. ALK anaplastic lymphoma kinase, NB neuroblastoma, na non-amplified
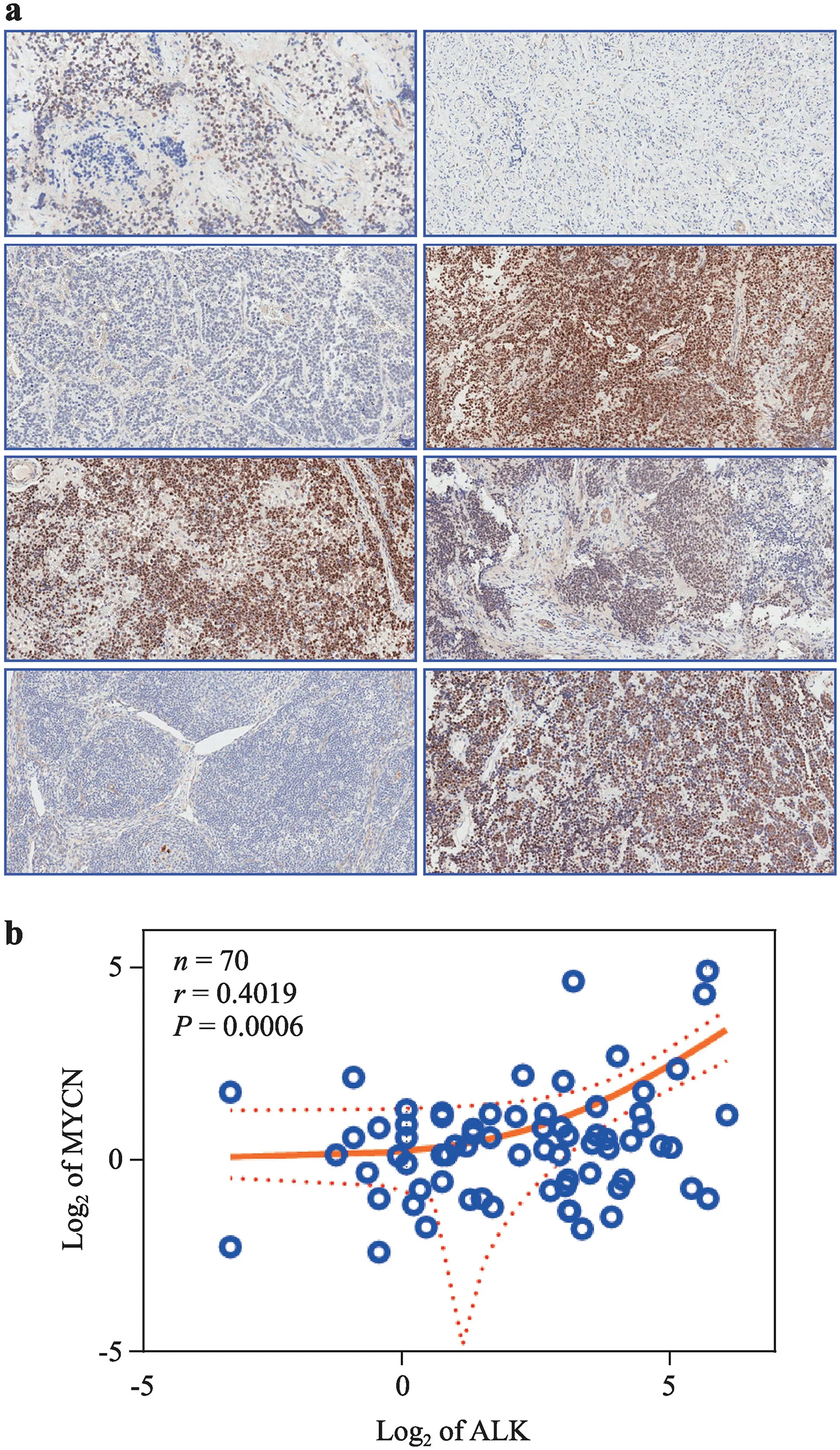
Fig. 5 ALK expression correlates with MYCN expression in MYCNna-NB. a Representative microphotographs showing nuclear expression of MYCN in human MYCN-na-NB (magnification × 20); b XY graph showing linear correlation ( r = 0.4019) between MYCN and ALK expression in MYCN-na-NB ( n = 70) patients. Axes are log 2 of ALK and MYCN expression. ALK and MYCN expression exhibit linear and direct correlation in MYCN-na-NB. ALK anaplastic lymphoma kinase, NB neuroblastoma, na non-amplified
Although NB is known to express full-length ALK [ 34,35], its prognostic relevance is still debated [ 34, 36- 38].The single-base missense mutations in the kinase domain of ALK are found in both familial and sporadic NB and promote ligand-independent signaling [ 23, 39- 41]. Three mutations(R1275, F1174, F1245) comprise about 85% of allALK
mutations in NB [ 24]. While the R1275Q mutation is found in both familial and sporadic NB, F1174 and F1245 mutants are exclusive to sporadic NB [ 41, 42].MYCN
amplification correlates withALK
mutations and cooperatively drives disease progression [ 43, 44]. Co-expression ofMYCN
andALK
lead to NB evolution with earlier onset, higher disease penetrance, and enhanced lethality [ 43, 45]. Correlation betweenMYCN
amplification andALK
genetic alterations [ 46, 47] and/or expression [ 48], and their coordinative prognostic roles in NB are realized. Our study is the first report to show theALKMYCN
correlation in MYCN-na-NB and support the hypothesis that linearALK
andMYCN
association drives MYCN-na-NB disease evolution. Uniquely our IHC studies exhibited the ALK-MYCN coordinating activity in MYCN-na-NB disease progression. Studies have recognized the relevance ofMYCN
signaling in adverse outcomes independent ofMYCN
amplification and risk statuses [ 49]. SinceALK
is a transcriptional target ofMYCN
[ 50] and vice versa [ 28, 51], our correlation results affirm their cooperative function in affecting survival outcomes of NB patients beyondMYCN
amplification status.The study had limitations. The cohort size was relatively small, and we were unable to perform statistically meaningful multivariate analysis. The antibody used is directed to detect total ALK protein, including fusion proteins, and may be subjective. Although it is unclear, we acknowledge thatALK
phosphorylation may have prognostic significance in MYCN-na-NB. In our study, the genetic mutation/amplification ofALK
was not included. Thus,we do not claim the relevance of any selective mode of gain of function/expression. Although we recognized the ALK/MYCN association in the MYCN-na-NB patients,elucidation ofits functional relevance is required and was not attempted here.In conclusion, our study displayed the landscape of ALK(transcription/expression) in MYCN-na-NB patients. High ALK expression predicted poor prognosis (high-is-worse)exhibiting low OS and RFS in this subset of patients. ALK and MYCN expression in MYCN-na-NB patients exhibited a linear/direct correlation. This is the first report that displaysALK
andMYCN
correlation indicating their coordinately prognostic roles in MYCN-na-NB.Supplementary Information
The online version contains supplementary material available at https:// doi. org/ 10. 1007/ s12519- 022- 00517-5.Acknowledgements
The authors acknowledge the Stephenson Cancer Center (SCC) Cancer Tissue pathology core and the SCC Cancer Functional Genomics core for all the TMA, IHC, and scanning services. The authors also acknowledge the OUHSC Staff Editor (Ms. Kathy Kyler)for the help in critically reviewing this manuscript.Author contributions
SDB contributed to performing experiments,acquisition of the data, data analysis and interpretation. AS contributed to performing experiments, acquisition of the data, data analysis and interpretation, and helped in critically revising the manuscript.GN contributed to the acquisition of in silico data from R2 portal.YZ and BA contributed to biospecimen and clinical data collection,histopathological grading, and data interpretation. AN contributed to the conception and design of the experiments, performing experiments,data acquisition, analysis and interpretation, and drafted the manuscript. All authors read and approved the final manuscript.Funding
This work was partially or in full funded by the Oklahoma Center for the Advancement of Science and Technology (No. OCASTHR19-045), National Institutes of Health (No. NIH-P20GM103639),and NIH-NCI Cancer Center Support Grant (No. P30CA225520).Data availability
All data generated or analyzed during this study are included in this published article.Declarations
Ethical approval
All protocols were approved by the Institutional Review Board (IRB) University of Oklahoma Health Sciences Center,with permission for the research use of de-identified specimens.Conflict of interest
All authors have no conflict of interest to declare. World Journal of Pediatrics2022年4期
World Journal of Pediatrics2022年4期
- World Journal of Pediatrics的其它文章
- Role of Omicron variant of SARS-CoV-2 in children in Germany
- Continuing interventions in a quality improvement bundle to reduce bronchopulmonary dysplasia
- Catheter-related bloodstream infections in children with intestinal failure: a 6-year review from an intestinal rehabilitation center in China
- Different approaches for patent ductus arteriosus in premature infants using acetaminophen
- Therapeutic benefits of zinc sulfate on neonatal hyperbilirubinemia
- A novel homozygous RAG1 mutation in a girl presenting with granulomas and alopecia capitis totalis
