Free fatty acid receptor 2 promotes cardiomyocyte hypertrophy by activating STAT3 and GATA4
Hui Go*, Kunming Tin, Xiojun Feng, Mengqing Yn, Chen Go,Yisheng Jing, Chenho Zhu, Huzhe Zhu, Xueping Liu*, Yingfu Peng*
a Department of Pharmacology, School of Medicine, Shaoxing University, Shaoxing 312000, China
b Department of Pharmacology, School of Medicine, Jishou University, Jishou 416000, China
c Department of Environmental Toxicity, Zunyi Medical University, Zunyi 563006, China
d The First Affiliated Hospital of USTC, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei 230002, China
e Department of Pharmacology, School of Medicine, GuangXi University of science and Technology, Liuzhou 545006, China
Keywords:
FFA2
Cardiac hypertrophy
GATA4
STAT3
ERK1/2
A B S T R A C T
Free fatty acids (FFAs) play important roles in cardiovascular disease. Studies have shown that it is an important way for FAs to exert biological effects through their own receptors besides directly participating biochemical reaction in body. Free fatty acid receptor 2 (FFA2) can be activated by short-chain FAs and is involved in inflammatory reactions and lipid accumulation. Since the known pathological changes caused by FFA2 are also implicated in cardiac hypertrophy, we hypothesized that FFA2 might be pathogenic in cardiac hypertrophy. This paper showed that FFA2 expression significantly increased in cardiac hypertrophy in vivo and in vitro. FFA2 agonist 4-CMTB or TUG-1375 promoted the expression of the hypertrophy markers ANF and BNP and increased cell surface area in vitro, which was further strengthened by FFA2 overexpression,suggesting that FFA2 might contribute to cardiomyocyte hypertrophy. Furthermore, 4-CMTB treatment or FFA2 overexpression combined with 4-CMTB treatment elevated the phosphorylation and transcriptional activity of GATA4 and STAT3, which were inhibited by an ERK1/2 inhibitor, and GATA4 and STAT3 knockdown inhibited the elevation of hypertrophy biomarkers in cardiomyocytes treated with 4-CMTB. Taken together, these data indicate that FFA2 can enhance cardiomyocyte hypertrophy by activating STAT3 and GATA4 via ERK1/2, providing a potential new target for therapy.
1. Introduction
Cardiac hypertrophy describes adaptive changes in the heart caused by pressure overload, which is characterized by cardiomyocyte hypertrophy and interstitial fibrosis [1]. However,when hypertrophied, the heart is susceptible to ischemia caused by the increased ventricular wall thickness and diastolic dysfunction caused by fibrosis. Moreover, the systolic function of the heart is gradually impaired during disease progression, ultimately resulting in heart failure.
Continuous activation of the sympathetic nervous system and the renin-angiotensin system play important roles in cardiac hypertrophy [1-4], and current drugs that improve outcomes for patients with cardiac hypertrophy or heart failure usually inhibit these two systems [2-4]. However, clinical outcomes are still unsatisfactory with these current therapies, and new drugs for cardiac hypertrophy are still needed.
The role of fatty acids in cardiovascular disease such as atherosclerosis and cardiac hypertrophy has received increasing attention [5]. Interestingly, some studies have found that fatty acids not only participate in biochemical reactions in the body, but also function through their own receptors, free fatty acid (FFA) receptors [6-8].
All FFA receptors are members of G protein-coupled receptors and mainly include FFA1 (GPR40), FFA2 (GPR43), FFA3 (GPR41)and FFA4 (GPR120); their natural ligands are FFAs with different carbon chain lengths [9,10]. FFA2 is 331 amino acid residues long and can be activated by short-chain fatty acids such as acetate, butyrate,and propionate. FFA2 is mainly expressed in the gastrointestinal tract,adipose tissue, immune cells, spleen, skeletal muscle, brain, etc., and there is a moderate expression in heart [11,12]. Previous studies have shown that FFA2 is associated with inflammatory reactions [13-15]and lipid accumulation [12], which play vital roles in cardiac hypertrophy. Moreover, FFA2 can couple with Gq/11and Giproteins,both of which may be related to cardiac hypertrophy: FFA2 reduces cAMP production through Gito regulate adenylate cyclase [9,10,13-15],which might impair cardiac contraction. Furthermore, FFA2 can elevate intracellular Ca2+and activate protein kinase C (PKC) and the mitogen-activated protein kinase (MAPK) cascade by activating Gq/11[16,17], and these changes are also closely related to cardiac hypertrophy [18,19].
ERK1/2-MAPK is a downstream effector of FFA2 [16,17];furthermore, some transcription factors such as GATA4 and STAT3 can be activated by ERK1/2, which play important roles in cardiac hypertrophy [20,21]. So FFA2 might promote the transcription of hypertrophic genes and protein synthesis to induce cardiomyocyte hypertrophy by activating STAT3 and GATA4.
2. Materials and methods
2.1 Chemicals and antibodies
4-CMTB (4642) was purchased from Tocris Bioscience (Bristol,UK). TUG-1375 was purchased from Target Molecule Corp.(Boston, MA, USA). Phenylephrine (PE, P6126) was obtained from Sigma Aldrich (St. Louis, MO, USA). U0126 (9903), rabbit monoclonal anti-p-MAPK-Erk1/2 (Thr202/Tyr204) (1:2 000; 4 370),rabbit monoclonal anti-p-STAT3 (Tyr705) (1:1 000; 9145), rabbit polyclonal anti-p-STAT3 (Ser727) (1:1 000; 9134), and mouse monoclonal anti-STAT3 (1:1 000; 9139) were purchased from Cell Signaling Technology (CST, Danvers, MA, USA). Isoproterenol(sc-202188), angiotensin II (sc-363643), rabbit polyclonal anti-FFA2 (1:100; sc-32906), mouse monoclonal anti-glyceraldehyde-3-phosphate dehydrogenase (1:200; GAPDH, sc-365062), mouse monoclonal anti-GATA4 (1:100; sc-25310), and mouse monoclonal anti-phospho-GATA4 (Ser262) (1:100; sc-377543) were purchased from Santa Cruz Biotechnology (Dallas, TX, USA). Rabbit polyclonal anti-p-GATA4 (Ser105)(1:1 000; ab5245) was purchased from Abcam (Cambridge, UK).
2.2 Animal model of cardiac hypertrophy
Sprague-Dawley rats (male, 180-200 g) obtained from Hunan SJA Laboratory Animal Co., Ltd. (Changsha, China) were randomly divided into two groups: sham operation group (Sham) and abdominal aortic constriction (AAC) group. AAC surgery was performed to induce pressure overload as described previously [22]. Eight weeks after the operation, all rats were anaesthetized and then sacrificed for further experiments. All experimental procedures were conducted in conformity with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996) and approved by the Animal Care and Studies Committee of Jishou University (Jishou, China).
2.3 Primary culture of neonatal rat cardiomyocytes (NRCMs)
Primary cultures of NRCMs were prepared as described previously [23]. In brief, the chopped heart tissues of 1-2-day-old Sprague-Dawley rats were repeatedly digested with 0.08% trypsin at 37 °C for 10-12 times. The cells were collected by centrifugation at 800 r/min and re-suspended in DMEM with 10% newborn calf serum (NBCS). The cells were then incubated in culture flasks for 1 h at 37 °C in a humidified atmosphere with 5% CO2to remove adherent non-cardiomyocytes. The purified cardiomyocytes were cultured in DMEM supplemented with 10% NBCS and 0.1 mmol/L 5-bromodeoxyuridine for 16-24 h in 6-well plates (1.0 × 105cells/mL).Finally, the culture medium was replaced with fresh DMEM containing 10% NBCS. Before each experiment, cells were placed in serum-free medium for 16-18 h.
2.4 RNA extraction and quantitative RT-PCR
Total RNA was extracted using Trizol reagent (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. Total RNA was reverse transcribed to first-strand cDNA using the RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific). cDNA was amplified using KOD SYBR?qPCR Mix (Toyobo, Japan) in a real-time PCR machine (LightCycler,Roche, Penzberg, Germany). The primer sequences were were listed as follows: FFA2 (F: 5’-CCCGGTGCAGTACAAGCTAT-3’,R: 5’-C T G C T C G G T T G A G T T C A G G T-3’), A N F(F: 5’-C C G T A T A C A G T G C G G T G T C C-3’, R:5’-C A G A G A G G G A G C T A A G T G C C-3’), B N P(F: 5’-A G C T G C T T T G G G C A G A A G A T-3’, R:5’-AAAACAACCTCAGCCCGTCA-3’) and GAPDH(F: 5’-A G G A G T A A G A A A C C C T G G A C-3’, R:5’-CTGGGATGGAATTGTGAG-3’). Each PCR reaction was performed in triplicate. Data are presented as fold change over control group.
2.5 RNA interference and plasmid transfection
Cardiomyocytes were transfected with 2.5 μg FFA2 plasmid using 5 μL Lipofectamine 3000 (Invitrogen, Carlsbad, CA, USA) in six-well plates according to the manufacturer’s instructions. ThepcDNA3.1-flag vector was used as a control. After transfection for 48 h, cells were harvested.
Cardiomyocytes were transfected either with GATA4 siRNA or STAT3 siRNA with non-targeting siRNA (negative control,NC) using Lipofectamine 3000 according to the manufacturer’s instructions. siRNA sequences were: GATA4 siRNA (5’-CUUC AGAGCCGACAGCACUGGAUGG-3’) [24]and STAT3 siRNA(5’-GGCAUUCGGAAAGUAUUGUTT-3’) [25].
2.6 Western blotting
Cultured cardiomyocytes or cardiac tissues were homogenized in RIPA lysis buffer (Beyotime Biotechnology, Shanghai, China)supplemented with protease inhibitor cocktail (sc-29130, Santa Cruz Biotechnology). The mixture was incubated on ice for 30 min and then centrifuged at 12 000 ×gfor 10 min at 4 °C and the total protein collected. Proteins were separated in 10% SDS–PAGE and were then transferred to PVDF membranes (Millipore, Burlington, MA,USA). Membranes were blocked with 5% bovine serum albumin and then incubated with primary antibodies overnight at 4 °C and secondary antibodies (1:5 000; Santa Cruz Biotechnology) for 1 h at room temperature. Blots were developed with an enhanced chemiluminescence substrate (Beyotime Biotechnology) and detected with the Minichemi 420 (Sage Creation Science Co., Ltd., Beijing,China). Blot intensities were quantified with the Image J software.
2.7 Measurement of the cell surface area
The cell surface area was determined by rhodamine-phalloidin staining as previously described [23]. Cardiomyocyte surface area (40 randomly chosen fields and 100 randomly chosen cells/field) was determined by Cellomics/High Content Screening (Thermo Fisher Scientific).
2.8 Dual luciferase reporter gene assay
ThepGATA4-TA-lucreporter plasmid, which contains the 2 600 bp 5’-flanking region of the ratANFgene, was constructed as described previously [26]. For the dual luciferase reporter assay,cardiomyocytes were seeded in 96-well culture plates and cotransfected with 100 ng ofpSTAT3-TA-lucreporter plasmid (Beyotime Biotechnology) orpGATA4-TA-lucreporter plasmid and 20 ng ofpRL-TKplasmid (Promega, Madison, WI, USA) using Lipofectamine 3 000 based on the manufacturer’s instructions. After 48 h, cells were lysed, and luciferase activity was determined using the dual luciferase reporter assay kit (Beyotime Biotechnology) according to the manufacturer’s recommendations. Luciferase activity was normalized to Renilla luciferase activity.
2.9 Statistical analysis
Data are expressed as mean ± S.D. Statistical analyses were performed using the unpaired Student’st-test or analysis of variance (ANOVA) followed by Bonferronipost hoctesting using SPSS 20.0 (IBM Statistics, Chicago, IL).P< 0.05 was considered statistically significant.
3. Results
3.1 FFA2 expression is elevated in cardiac hypertrophy
AAC surgery was performed to induce cardiac hypertrophy in the rats. Compared with Sham rats, AAC rats had bigger hearts, thicker left ventricular walls, larger cardiomyocyte diameters (Fig. 1A), and higher heart-to-body weight ratios (Fig. 1B), indicating successful induction of cardiac hypertrophy. As shown Figs. 1C and 1D,compared to Sham rats, cardiac FFA2mRNA and protein expression were significantly increased in AAC rats.
Next, NRCMs were treated with phenylephrine (PE), isoproterenol(ISO), or angiotensin II (Ang II) to induce cardiomyocyte hypertrophyin vitro. Gene expression of atrial natriuretic factor (ANF) and brain natriuretic peptide (BNP) (Fig. 2A) and the cell surface area(Fig. 2B) increased in cardiomyocytes treated with PE, ISO, or Ang II. ANF and BNP, which are secreted by atrial myocytes and ventricular myocytes respectively, rise in cardiac hypertrophy and are considered as important hypertrophic markers nowadays [1]. FFA2 mRNA (Fig. 2C) and protein (Fig. 2D) expression also increased after the treatments. Thus, FFA2 might play an important role in cardiac hypertrophy.
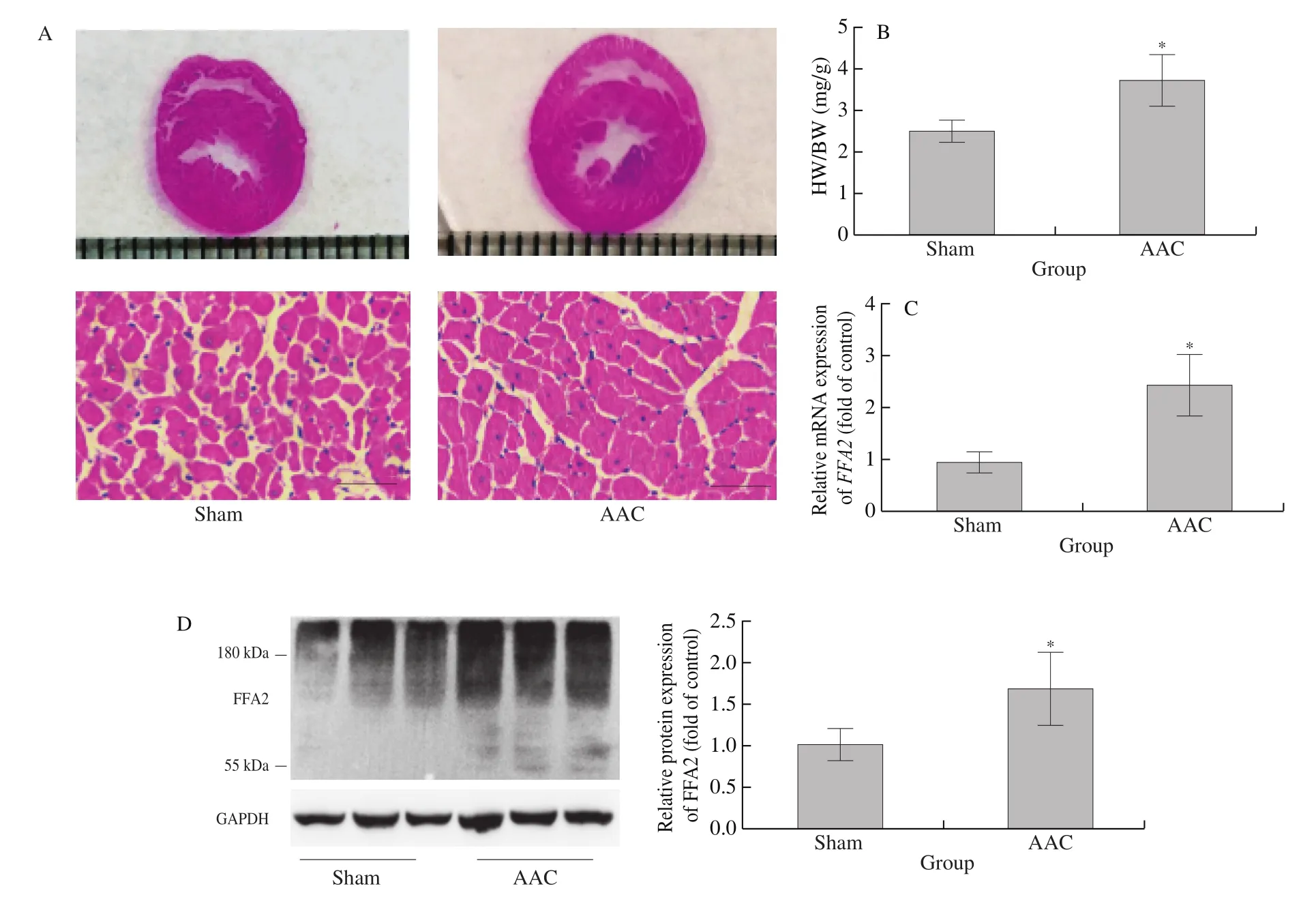
Fig. 1 FFA2 expression increases in cardiac hypertrophy induced by AAC in rats. Rats underwent AAC or Sham surgery for 8 weeks. (A) H&E staining of transverse sections of hearts; scale bar = 50 μm; (B) heart-to-body weight ratio (HW/BW); (C) mRNA and (D) protein expression of FFA2 were detected by RT-PCR or western blotting, respectively. *P < 0.05 versus Sham.
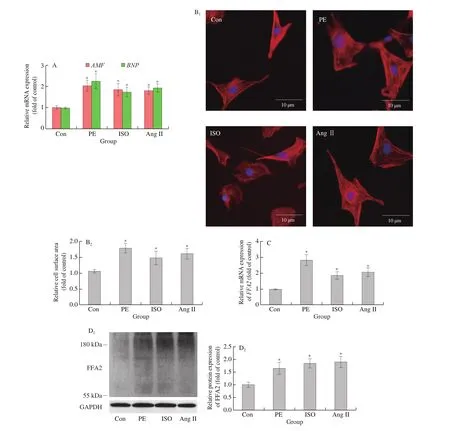
Fig. 2 FFA2 expression increases in cardiomyocyte hypertrophy induced by PE, ISO, or Ang II treatment in vitro. NRCMs were treated with or without PE(100 μmol/L), ISO (10 μmol/L), or Ang II (10 μmol/L) for 24 h. (A) mRNA expression of ANF and BNP was detected by RT-PCR (n = 4 independent experiments); (B) cell surface area was measured by phalloidin staining (n = 4 independent experiments); scale bar = 10 μm; (C) mRNA and(D) protein expression of FFA2 were detected by RT-PCR or western blotting, respectively (n = 5 independent experiments). *P < 0.05 versus Con.
3.2 FFA2 promotes cardiomyocyte hypertrophy
The increased expression of FFA2 might be associated with the development of cardiac hypertrophy. 4-CMTB, which is widely accepted as a specific FFA2 agonist that not only binds to both the orthosteric and the allosteric binding sites [27]but also activates Gqand Giproteins [17,28], or FFA2 overexpression to investigate the role of FFA2 in cardiomyocyte hypertrophy. As shown in Fig. 3, compared to control,ANFandBNPexpression and cell surface area increased in NRCMs treated with 4-CMTB. Compared to 4-CMTB treatment,these increases were even more obvious in FFA2-overexpressed NRCMs with 4-CMTB treatment (Figs. 3B and 3C). Moreover, the knockdown of FFA2 completely abolished the hypertrophic response in 4-CMTB-treated cardiomyocytes (Fig. 4), which indicated that it was a specific agonist for FFA2. To further enhance the reliability of our research, another FFA2 agonist, TUG-1375 [29]was used in this study. Similar results were obtained in cardiomyocytes treated with TUG-1375 (Figs. 3D and 3E). These results indicate that FFA2 can promote cardiomyocyte hypertrophy.
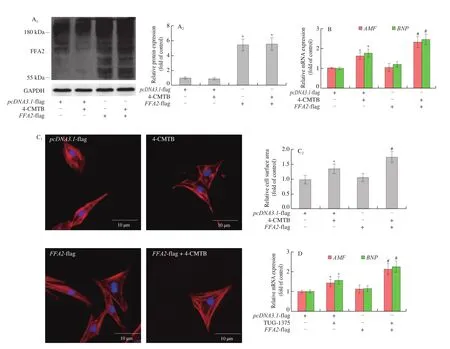
Fig. 3 FFA2 promotes cardiomyocyte hypertrophy. NRCMs were transfected with pcDNA3.1-FFA2- flag plasmid or pcDNA3.1- flag for 24 h and then stimulated with or without 4-CMTB (10 μmol/L) or TUG-1375 (10 μmol/L) for 24 h. (A) FFA2 protein expression was detected by western blotting; (B and D) mRNA levels of ANF and BNP were measured by RT-PCR; (C and E) cell surface area was measured by phalloidin staining; scale bar = 10 μm. *P < 0.05 versus pcDNA3.1,#P < 0.05 versus 4-CMTB or TUG-1375.
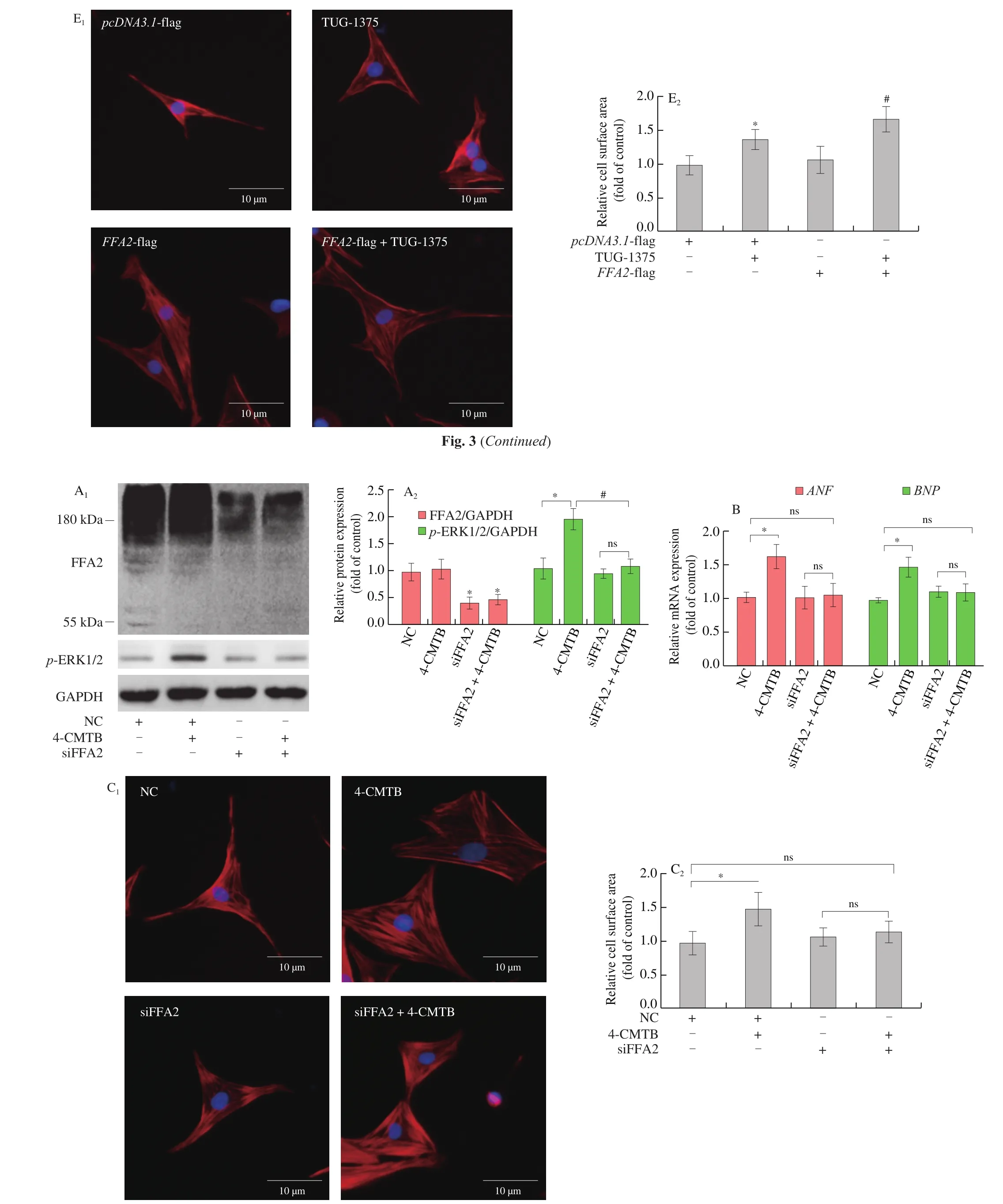
Fig. 4 FFA2 knockdown inhibits cardiomyocyte hypertrophy induced by 4-CMTB. NRCMs were transfected with siRNA targeting FFA2 or NC for 24 h and then stimulated with or without 4-CMTB (10 μmol/L) for 24 h. (A) The expressions of FFA2 and p-ERK1/2 protein were detected by western blotting; (B) mRNA levels of ANF and BNP were measured by RT-PCR; (C) cell surface area was measured by phalloidin staining; scale bar = 10 μm. *P < 0.05 versus NC, #P < 0.05 versus 4-CMTB.
3.3 GATA4 and STAT3 are involved in FFA2-induced cardiomyocyte hypertrophy
Increased transcriptional activity of GATA4 plays a key role in cardiac hypertrophy, so we next studied the effects of GATA4 in FFA2-induced cardiomyocyte hypertrophy. Compared with control,4-CMTB treatment elevated GATA4 phosphorylation at Ser105(Fig. 5A) and GATA4 transcriptional activity (Fig. 5B) by western blotting and luciferase reporter assays, respectively, which were further increased inFFA2- flag plasmid-transfected cardiomyocytes.However, GATA4 phosphorylation at Ser262 was not altered by 4-CMTB treatment (Fig. 5A). In order to investigate whether GATA4 acts as a downstream signaling molecule of FFA2 in cardiomyocyte hypertrophy, we knocked downGATA4expression in cardiomyocytes treated with 4-CMTB (Fig. 5C). Compared to 4-CMTB treatment,GATA4knockdown significantly decreasedANFandBNPmRNA levels as well as cell surface area in cardiomyocytes stimulated with 4-CMTB, which might therefore inhibit cardiomyocyte hypertrophy(Figs. 5D and 5E).
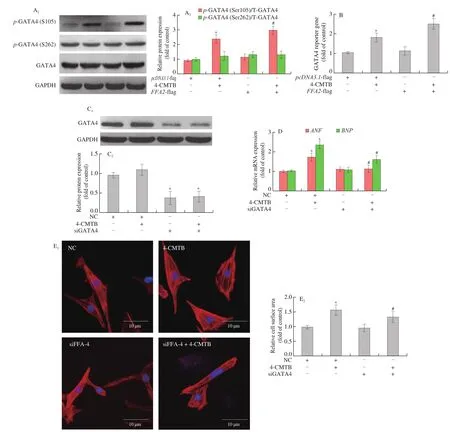
Fig. 5 GATA4 activation is involved in FFA2-induced cardiomyocyte hypertrophy. NRCMs were transfected with pcDNA3.1-FFA2- flag plasmid or pcDNA3.1- flag for 24 h and then stimulated with or without 4-CMTB (10 μmol/L) for another 24 h. (A) GATA4 phosphorylation at Ser105 and Ser262 as well as total GATA4 expression were detected by western blotting; (B) the transcriptional activity of GATA4 was measured using a luciferase reporter assay. *P < 0.05 versus pcDNA3.1, #P < 0.05 versus 4-CMTB. To investigate the role of GATA4 in FFA2-induced cardiomyocyte hypertrophy, NRCMs were transfected with siRNA targeting GATA4 or NC for 24 h and then stimulated with or without 4-CMTB (10 μmol/L) for another 24 h. (C) Western blotting was used to evaluate the silencing efficacy of siRNA targeting GATA4; (D) ANF and BNP mRNA levels as well as (E) cell surface area were measured to assess the hypertrophic responses; scale bar = 10 μm. *P < 0.05 versus NC, #P < 0.05 versus 4-CMTB.
STAT3 is another important transcription factor involved in cardiac hypertrophy. We found that STAT3 phosphorylation at Tyr705 and Ser727 (Fig. 6A) as well as its transcriptional activity(Fig. 6B) were elevated by 4-CMTB compared to control, and these changes were more obvious in 4-CMTB-treated cardiomyocytes after transfection withFFA2- flag plasmid (Figs. 6A and 6B). To investigate the role of STAT3 as a downstream signaling molecule of FFA2 in cardiomyocyte hypertrophy, we knocked downSTAT3expression in cardiomyocytes treated with 4-CMTB (Fig. 6C). Compared to 4-CMTB treatment alone,STAT3knockdown significantly decreasedANFandBNPmRNA expression as well as cell surface area in cardiomyocytes stimulated with 4-CMTB (Figs. 6D and 6E), thereby inhibiting cardiomyocyte hypertrophy.
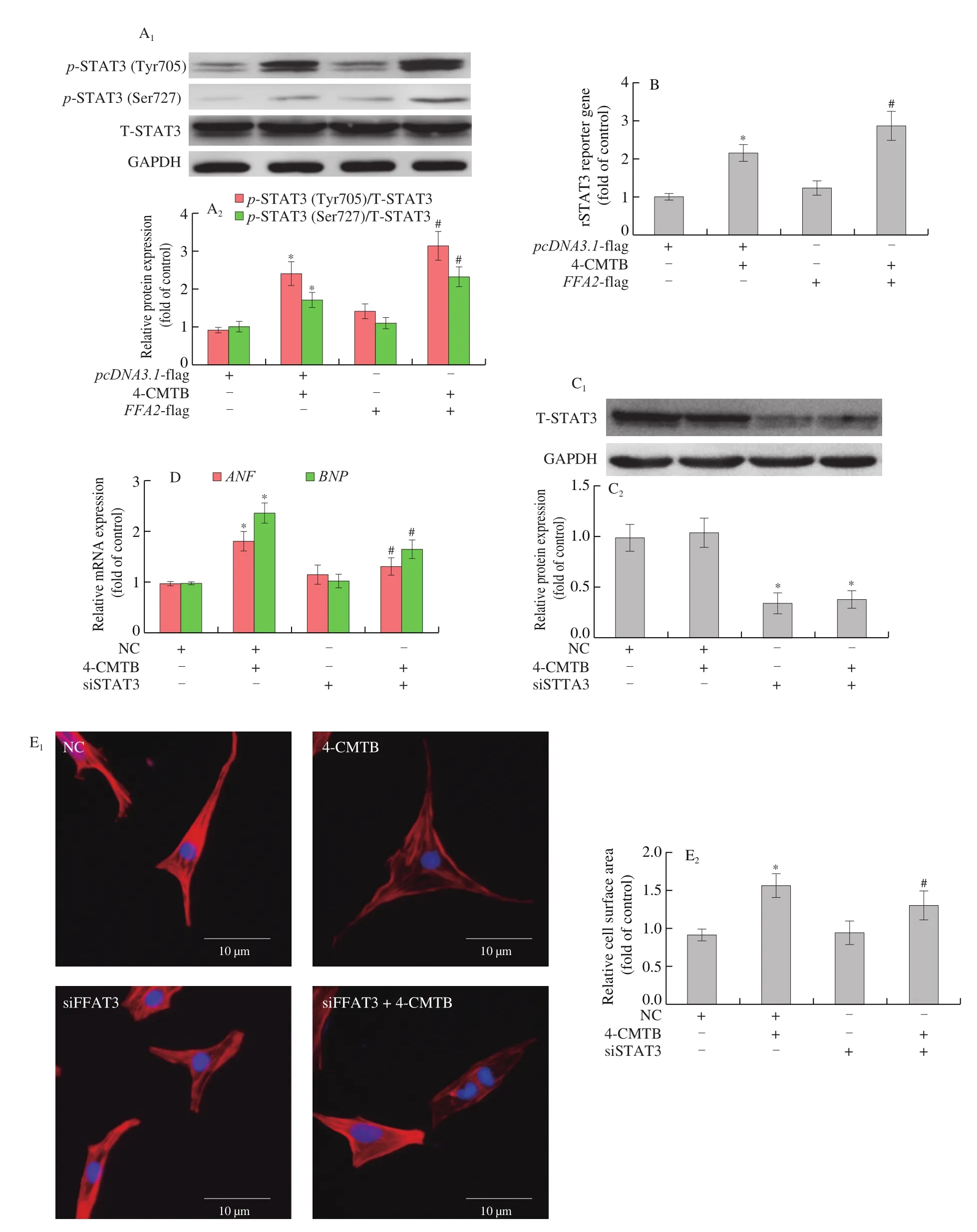
Fig. 6 STAT3 activation is involved in FFA2-induced cardiomyocyte hypertrophy. NRCMs were transfected with pcDNA3.1-FFA2- flag plasmid or pcDNA3.1-lf ag for 24 h and then stimulated with or without 4-CMTB (10 μmol/L) for another 24 h. (A) NRCMs were transfected with FFA2- flag plasmid or pcDNA3.1 for 24 h and then stimulated with or without 4-CMTB (10 μmol/L) for another 24 h. STAT3 phosphorylation at Tyr705 and Ser727 as well as total STAT3 expression were detected by western blotting; (B) the transcriptional activity of STAT3 was measured using a luciferase reporter assay. *P < 0.05 versus vector, #P < 0.05 versus 4-CMTB. To investigate the role of STAT3 in FFA2-induced cardiomyocyte hypertrophy, NRCMs were transfected with siRNA targeting STAT3 or NC for 24 h and then stimulated with or without 4-CMTB (10 μmol/L) for another 24 h. (C) Western blotting was used to evaluate the silencing efficacy of siRNA targeting STAT3; (D) ANF and BNP mRNA expression as well as (E) cell surface area were measured to assess hypertrophic responses; scale bar = 10 μm. *P < 0.05 versus NC, #P < 0.05 versus 4-CMTB.
ERK1/2 can promote the phosphorylation of GATA4 at Ser105 and STAT3 at Ser727 [20,21]. U0126, a MAPK-ERK1/2 pathway inhibitor [30](Fig. 7A), reduced GATA4 phosphorylation at Ser105(Fig. 7B) as well as GATA4 transcriptional activity (Fig. 7C)in cardiomyocytes treated with 4-CMTB. In addition, STAT3 phosphorylation at Ser727 (Fig. 7D) as well as its transcriptional activity (Fig. 7E) were also inhibited by U0126. Furthermore,increases inANFandBNPmRNA expression as well as cell surface area induced by 4-CMTB treatment were also reduced by U0126 treatment (Figs. 7F and 7G). Similar results were observed in cardiomyocytes treated with TUG-1375 (Fig. 8).
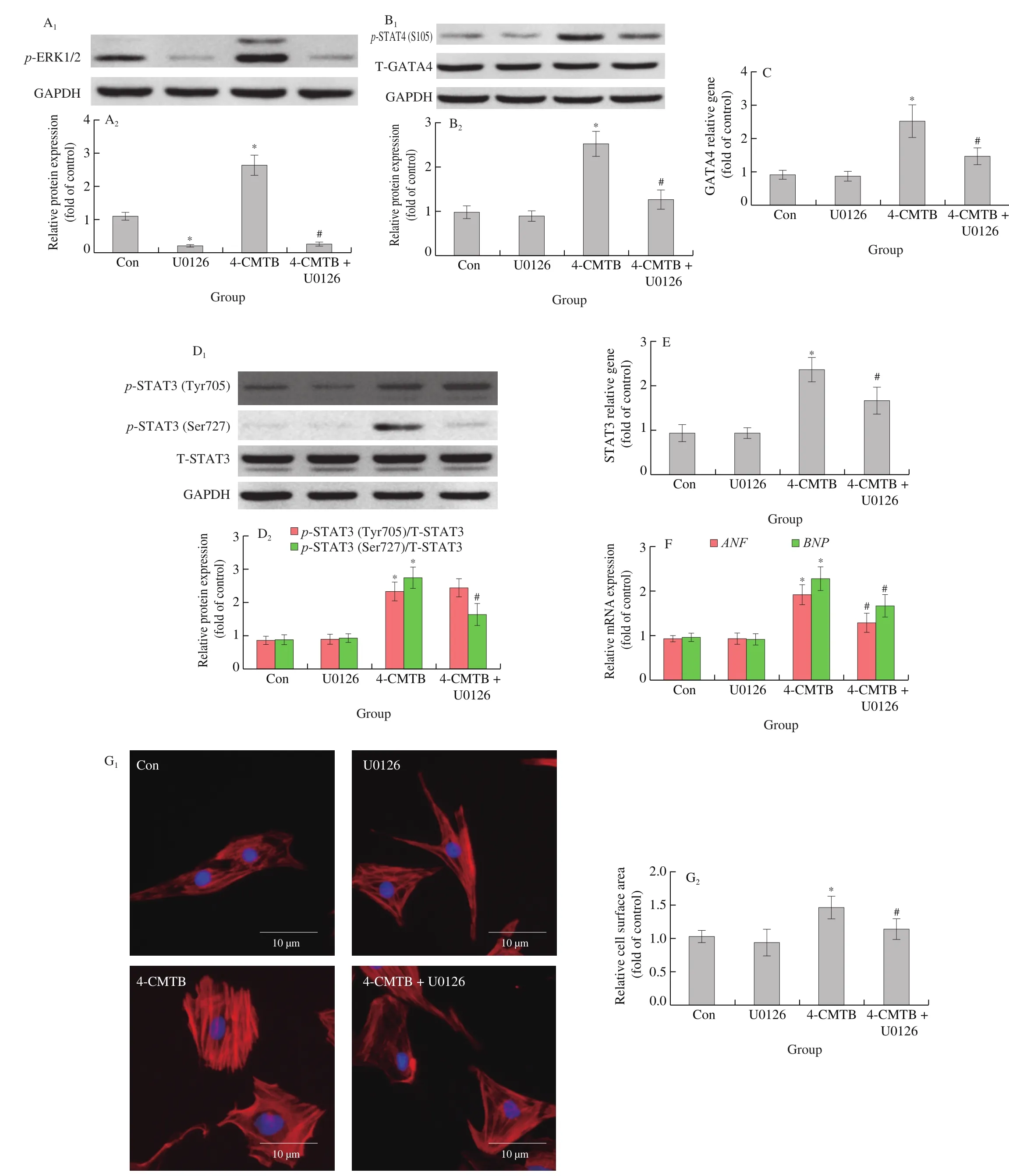
Fig. 7 The effects of 4-CMTB on GATA4 and STAT3 function as well as cardiomyocyte hypertrophy are impaired by MAPK-ERK1/2 pathway inhibition.NRCMs were stimulated with or without 4-CMTB (10 μmol/L) and simultaneously treated with or without U0126 (10 μmol/L) for 24 h. (A) p-ERK1/2,(B) p-GATA4 at Ser105, and (D) p-STAT3 at Tyr705 and Ser727 expression were detected by western blotting; the transcriptional activities of (C) GATA4 and (E) STAT3 were measured using a luciferase reporter assay; the mRNA levels of (F) ANF and BNP as well as (G) cell surface area were measured to assess hypertrophic responses; scale bar = 10 μm. *P < 0.05 versus Con, #P < 0.05 versus 4-CMTB.
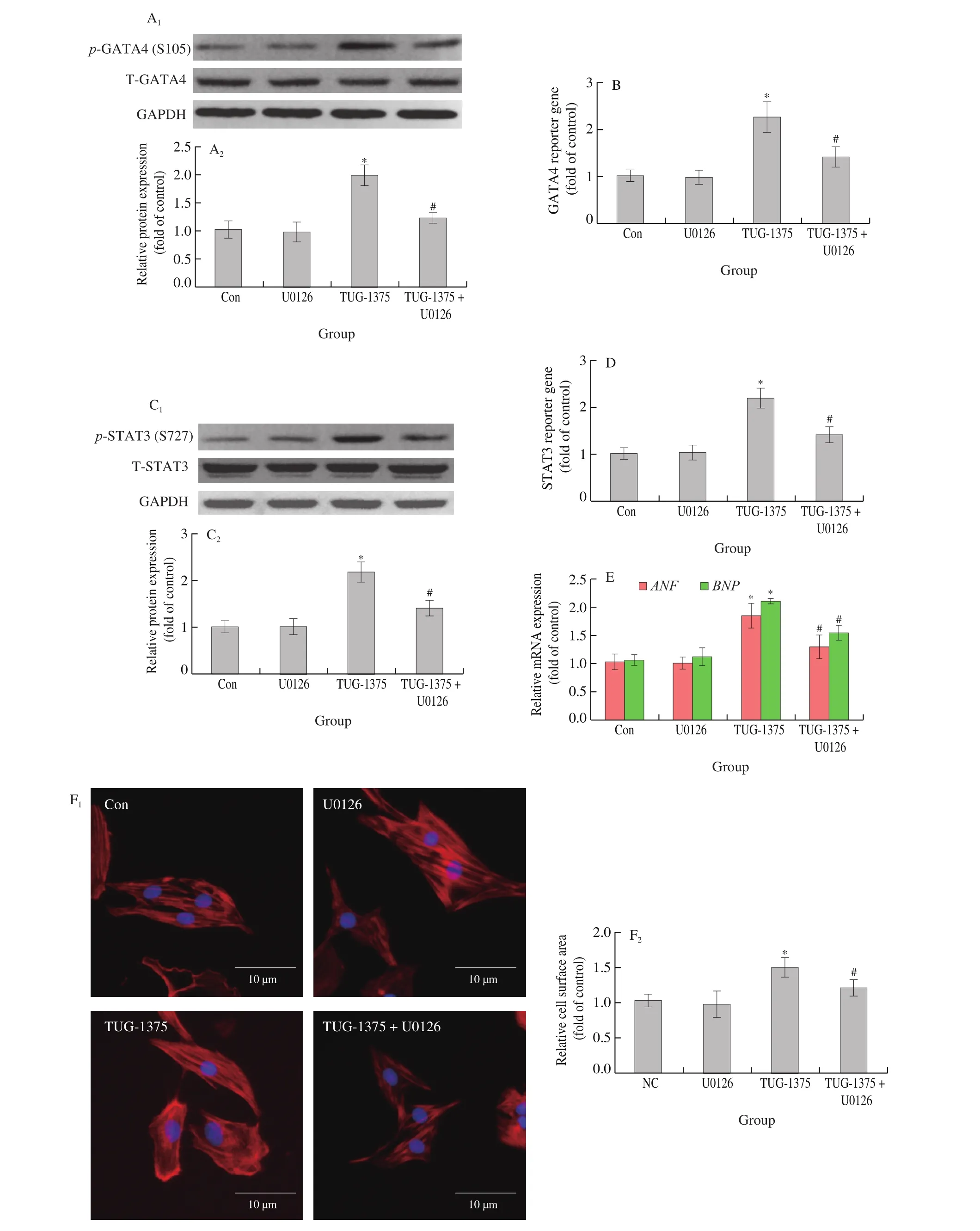
Fig. 8 The effects of TUG-1375 on GATA4 and STAT3 function as well as cardiomyocyte hypertrophy are impaired by MAPK-ERK1/2 pathway inhibition.NRCMs were stimulated with or without TUG-1375 (10 μmol/L) and simultaneously treated with or without U0126 (10 μmol/L) for 24 h. (A) p-GATA4 at Ser105, and (C) p-STAT3 at Ser727 expression were detected by western blotting; the transcriptional activities of (B) GATA4 and (D) STAT3 were measured using a luciferase reporter assay; the mRNA levels of (E) ANF and BNP as well as (F) cell surface area were measured to assess hypertrophic responses;scale bar = 10 μm. *P < 0.05 versus Con, #P < 0.05 versus 4-CMTB.
4. Discussion
Heart failure remains a serious public health challenge, and inhibiting the development or reducing the severity of cardiac hypertrophy is important for the improved prevention and treatment of heart failure. FFA2 is a physiological protein involved in immune responses, insulin secretion, and energy metabolism [10].Additionally, FFA2 has been associated with inflammation [13-15]and lipid accumulation [12], although its exact role in these pathological processes remains uncertain and sometimes contradictory [31,32]. It should be noted that chronic inflammation and disorders of lipid metabolism are often observed during cardiac hypertrophy [1,33]. Therefore, whether FFA2 mediates cardiac hypertrophy aroused our interest. Here we found that FFA2 expression increasedin vitroandin vivoin a cardiomyocyte hypertrophy model (Figs.1 and 2). Specific agonist or FFA2 overexpression with agonist elevatedANFandBNPexpression as well as the cell surface area, ultimately inducing cardiomyocyte hypertrophyin vitro(Fig. 3). Simultaneously,FFA2 overexpression alone could not induce cardiomyocyte hypertrophyin vitro, which was probably due to the lack of natural ligands in culture medium. In addition, we found that the band of FFA2 was obviously shifted up and has a large molecular weight span from 55 kDa to over 180 kDa in western blot analysis, which might be associated with the heavy glycosylation of FFA2 [34]. The glycosylation modification is ubiquitous in GPCRs [35]and can cause the bands to move up significantly in SDS-PAGE electrophoresis [36,37].
It is intriguing how FFA2 induces cardiac hypertrophy. GATA4 and STAT3 are two key transcription factors implicated in cardiac hypertrophy, and phosphorylation plays important roles in their activation. GATA4 is essential in heart development becauseGATA4knockout caused the death of mice at early embryonic stages [38,39].On the other hand,GATA4transgenic mice showed significant cardiac hypertrophy [40]. Furthermore GATA4 promotes the transcription of hypertrophic genes such asβ-myosin heavy chain,ANF, BNP and so on in response to hypertrophic stimulation [21].Two key phosphorylation sites are responsible for GATA4 activation.The phosphorylation at S105 promotes its DNA binding activity and increases the expression of its target genes; moreover, the phosphorylation at S262 induces interaction with p300 and then result in GATA4 acetylation and activation [21]. We found that 4-CMTB significantly induced phosphorylation of GATA4 at Ser105 as well as its transcriptional activity, which were further reinforced by FFA2 overexpression (Figs. 5A and 5B). Moreover,GATA4knockdown rescued 4-CMTB-induced cardiomyocyte hypertrophy(Figs. 5D and 5E). Similarly, STAT3 is involved in models of cardiac hypertrophy such as pressure overload [41,42]and in Ang II type 1 receptor transgenic mice [43]. Furthermore, cardiomyocytespecificSTAT3transgenic mice showed spontaneous cardiac hypertrophy [44]; conversely, inhibition of STAT3 alleviated cardiac hypertrophy [41,45,46]. It has also been shown that activation of FFA2 increases the phosphorylation of STAT3 at Tyr705 and expression of its target genes [47-49]. The phosphorylation at Tyr705 facilitates its homo/hetero-dimerization and is essential for DNA binding; in addition, STAT3 at Ser727 can be also phosphorylated for obtaining maximal transcriptional activity [20]. In the present study,we found that STAT3 phosphorylation at Tyr705 and Ser727 as well as STAT3 transcriptional activity increased after treatment with 4-CMTB (Figs. 6A and 6B). Moreover, cardiomyocyte hypertrophy induced by 4-CMTB treatment was attenuated bySTAT3knockdown(Figs. 6D and 6E). Hence, abnormal activation of GATA4 and STAT3 appear to participate in FFA2-meditated cardiomyocyte hypertrophy.
Both GATA4 at Ser105 and STAT3 at Ser727 are phosphorylated by MAPK-ERK1/2 [20,21]. ERK1/2 is a downstream target of FFA2 that can be used to evaluate FFA2 activity [16,50]. We found that U0126, a MAPK-ERK1/2 pathway inhibitor, reduced phosphorylation levels of GATA4 at Ser105 and STAT3 at Tyr705 in 4-CMTB-treated cardiomyocytes (Figs. 7A and 7B). These results indicate that ERK1/2 is likely to mediate the increase in GATA4 and STAT3 transcriptional activity by FFA2. In addition, STAT3 phosphorylation at Tyr705 could not be inhibited by U0126, which indicates this site cannot be phosphorylated by ERK1/2. This post-translational modification of STAT3 might be related to FFA2-mediated release of inflammatory factors such as IL-6 [14,51]and TNF-α [52].
It is generally considered that the ERK1/2 signaling pathway promotes cardiac hypertrophy. For instance, constitutively ERK1/2 activation in the heart induced cardiac hypertrophy in MEK1(upstream of ERK1/2) transgenic mice [53]. On the contrary, the inhibition of ERK1/2 signaling ameliorated cardiac hypertrophy[30,33]. FFA2 can induce the activation of ERK1/2 through both Giand Gqsignaling, but Gqpathway might be predominant [50].Furthermore, ERK1/2 autophosphorylation at Thr188 site via Gqsignaling triggered its nuclear localization and phosphorylation of target proteins and then promoted cardiac hypertrophy. Therefore, it is a possible way for the activation of GATA4 and STAT3 induced by FFA2 via ERK1/2. However, the inhibition of ERK1/2 by U0126 could not completely abolish the hypertrophy induced by FFA2, as did the knockdown of GATA4 or STAT3, which indicated that other pathways such as Ca2+-mediated signaling and PKC [54]might were involved in this pathological process.
It should be noted that although short-chain fatty acids (SCFAs)such as acetate, butyrate, and propionate are natural ligands for FFA2, SCFAs can exert their functions via both FFA2-dependent and independent mechanisms. For example, SCFAs can activate FFA3 or enter the cytoplasm to inhibit histone deacetylase [55,56]and SIRT3 [57], which might lead to inconsistent results; so high selective agonists are needed in the study of FFA2. It may be worthy to explore the selective agonists in the natural food.
In summary, FFA2 might promote cardiomyocyte hypertrophy through activation of STAT3 and GATA4 via ERK1/2. Our results provide new insights into the role of FFA2 in cardiac biology and suggest that further studies of it as a therapeutic target in cardiac hypertrophy and heart failure are warranted. Since FFA2 contains two ligand-binding sites and can couple to different G proteins, and FFA2 is widely involved in the regulation of physiological functions and pathological processes, so further investigation of the specificity of downstream signaling events in different diseases and under different inhibitory conditions are required.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81760058, 81560059, 81660042,31800891), the Scientific Research Project of Health and Family Planning Commission of Hunan Province (No. C2017025), the Project of Medical and Health Science and Technology of Shaoxing City(No. 2020A13063) and the Startup Fund for Research of Shaoxing University (No. 20205021).
 食品科學(xué)與人類(lèi)健康(英文)2022年2期
食品科學(xué)與人類(lèi)健康(英文)2022年2期
- 食品科學(xué)與人類(lèi)健康(英文)的其它文章
- Effects of different processing methods on the lipid composition of hazelnut oil: a lipidomics analysis
- Deletion of the waaf gene affects O antigen synthesis and pathogenicity in Vibrio parahaemolyticus from shell fish
- Comprehensive evaluation of Actinidia arguta fruit based on the nutrition and taste: 67 germplasm native to Northeast China
- Transcriptome-based insights into the calcium transport mechanism of chick chorioallantoic membrane
- Flavonoids of Rosa rugosa Thunb. inhibit tumor proliferation and metastasis in human hepatocellular carcinoma HepG2 cells
- Effects of different drying methods on phenolic components and in vitro hypoglycemic activities of pulp extracts from two Chinese bayberry(Myrica rubra Sieb. et Zucc.) cultivars
