Effects of Different Cropping Patterns of Soybean and Maize Seedlings on Soil Enzyme Activities and MBC and MBN
Yan Mu-chun, Xu Ting-ting, Song Peng-hui, and Dai Jian-jun
College of Resources and Environmental Sciences, Northeast Agricultural University, Harbin 150030, China
Introduction
Soil enzyme activity is related not only to soil conditions, but also to crop types and different planting methods in agricultural practices. Soil enzyme activity reflects the soil properties changes caused by different rotation. The study of the rhizosphere microenvironment has been paid more and more attention, and it has become the most active and sensitive research field of soil science. These researches provide theoretical support and practical basis for manual adjustment and optimization of soil ecosystem.With the development of related disciplines and the improvement of detection means, rhizosphere researches keep on expanding on the effectiveness of soil nutrients, soil enzymes and material recycling areas, and research methods are also being explored actively. However, constrained by the complexity of the rhizosphere environment and research methods, the existing results basically focused on single and inter groups as the research object, while the studies based on rhizosphere micro system of crop rotation and mixed cropping group are still very few. The results of numerous studies showed that gramineous plants cropped with the planting leguminous crops not only improved the soil enzyme activity, but also increased the soil microbial number and diversity. The researchers compared the continuous soybean cropping,soybean-maize rotation and soybeans-sorghum rotation. Under these three cropping systems, soybeanmaize crop rotation system, soil enzyme activity was greatly improved (Zhang et al., 2008). Results of Zhang et al. (2000)showed that in the soil of soybean continuous cropping, polyphenol oxidase activity was higher than that of normal cropping soil, which was due to induction effect of phenolic acids in soil increased polyphenol oxidase activity, thereby auxin oxidase activity increased and then decomposed,affecting crop growth and development. Soil phosphatase and protease activities in the soybean/ryegrass intercropping system were significantly improved (Su et al., 2010). Studies of Cha et al.(2005)showed that rhizosphere soil urease and acid phosphatase activity in maize/gram intercropping system significantly reduced compared with those of monoculture maize, and the intercropping rhizosphere microbial diversity index was significantly higher than that of monoculture. Intercropping and mixture cropping patterns of gramineae and leguminous crops could play the role of increasing soil microbes in rhizosphere of oats and vetch of different cultivations (Wang et al., 2009). In maize and soybean intercropping system, nutrient availability, rhizosphere soil microbes and enzyme activity of rhizosphere soil were significantly higher than those of the corresponding monoculture crop (Liu et al., 2007).Microbe was the main factor of directly impacting on soil enzyme activity. From the four-year studies of Plaza et al. (2004), it had been found that microbial biomass carbon between dehydrogenase, catalase, the BAA-protease and β-glucosidase showed a positive correlation, but phosphatase showed a negative correlation. Gramineous crops planted with leguminous crops are widely used and play an important role in domestic agricultural production. Rhizosphere is a dynamic micro-domain of most inf l uenced by intense activity of plant roots, and its physical, chemical and biological characteristics are different from the body soil, and it is the interaction place of plants, soil, microbial and environment. Therefore, changes in the rhizosphere soil enzyme activities and microbial biomass carbon could reflect the interaction effect between the two crops in the growing season. Two kinds of crops,soybean and maize, commonly grown in northern China were chosen as the objects, and the pot experiment was conducted. This study aimed to examine the effects of different cropping patterns of soybean and maize seedlings on rhizosphere soil enzyme activities and microbial biomass carbon and nitrogen.
Materials and Methods
Soil for pot experiment
Pot experiment was used in this study, and the soil type was black soil (Mollisol). Previous crops were soybean and maize, respectively. Topsoil (0-15 cm)was sampled from Xiangfang Test and Practice Center of Northeast Agricultural University in 2011.The Center was located in the south of Heilongjiang Province, northeast China. Basic chemical properties of the two soil sampls are shown in Table 1.
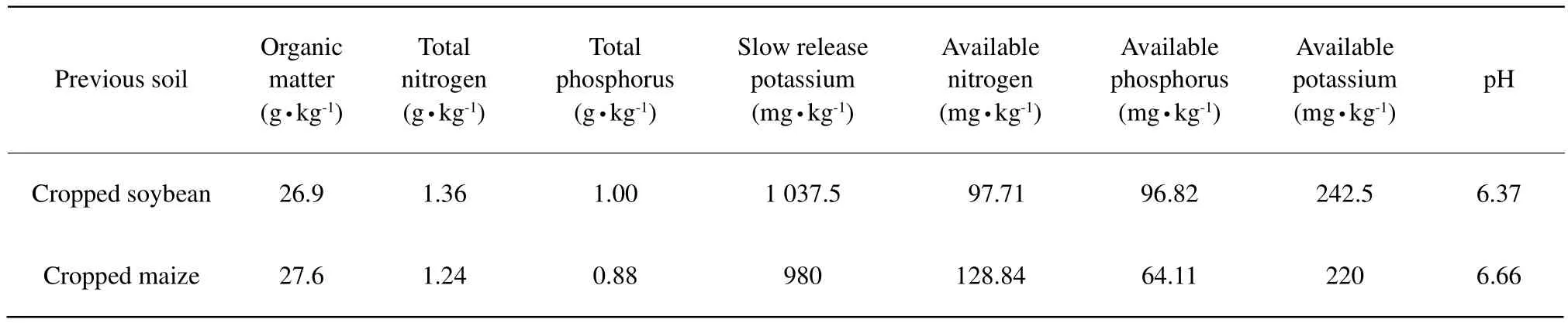
Table 1 Basic soil fertility
Pot experiment and soil sampling
Two soils of previously cropped soybean and maize were collected; air dried and then sieved out with 2 mm mesh. The soil samples were filled into each pot (250 g, 8cm×8cm), respectively, and watered to field water capacity of 60%. Chemical fertilizers,urea (N 46%), triple superphosphate (P2O546%)and potassium sulfate (K2O 50%)were applied as basal fertilizer into pot soil at the rate of N 75, P2O590 and K2O 90 kg ? hm-2, respectively. The subject crops were maize (Fengtian 6)and soybean (Suinong 14).This experiment designed the following cropping patterns: treatment 1, soybean-soybean; treatment 2,soybean-maize; treatment 3, soybean-mixed; treatment 4, maize-soybean; treatment 5, maize-maize; and treatment 6, maize-mixed (soybean-soybean was to plant soybean on previously cropped soybean soil;soybean-mixed was to use soybean-maize cropping pattern on previously cropped soybean soil; and other treatments details were all in this way), and each treatment was performed in five replicates. There were three cropping patterns and two types of soils of previously cropped soybean and maize. Crop seeds were sown in the pots on May 13, 2011, and the pots were placed in a well-ventilated and 20-25℃ room temperature. Supplied water according to the decreased the weight of each pot. After the crops grew 21 days(four leaves of soybean, fi ve leaves of maize)and the root spread entire pot soil, sampled plants together with soil from pot, and then gently shook the plant roots and dropped the attached soil on the roots, and removed root residues and mixed thoroughly, which were the samples of rhizosphere soil to be analyzed.
Measurement items and methods
Soil enzyme activities were determined as described by the method of Guan et al. (1986). Soil urease(EC 3.5.1.5)activity was measured by incubating 2 g soil with 10 mL of 10% urea solution for 24 h at 37℃. The formation of ammonium was determined calorimetrically at 578 nm using indophenol reagent and the activity was expressed as-N mg ?soil ? 24 h-1. Soil invertase (EC 3.2.1.26)activity was measured by incubating 2.5 g soil with 15 mL of 8%sucrose solution for 24 h at 37℃. The suspension reacted with 3, 5-dinitrosalicylic acid and absorbance was detected at 508 nm. Activity was expressed as glucose mg ? g-1soil ? 24 h-1. Soil catalase (EC 1.11.1.6)was measured by incubating 2 g soil with 5 mL of 0.3% H2O2for 30 min at 30℃. The suspension was titrated with 0.1 mol ? L-1KMnO4solution. Activity was expressed as 0.1000 mol ? L-1KMnO4mL ? g-1soil ? 30 min-1. Polyphenol oxidase (EC 1.10.3.1)activity was determined by incubating 5 g soil with 10 mL of 0.02 mol ? L-1catechol for 2 min at 30℃, titrated by 0.01 mol ? L-1I2-KI, and expressed as 0.01000 mol ? L-1I2mL ? g-1soil. Fresh soils were used to analyze these soil enzyme activities. All determinations of each sample were performed in triplicate, and all values reported were averages of the three determinations expressed on an oven-dried soil basis. Soil microbial biomass carbon(MBC)and microbial biomass nitrogen (MBN)were determined using fumigation-extraction methods on the basis of the difference between C or N extracted with 0.5 mol ? L-1K2SO4through chloroform-fumigation and unfumigated soil samples, using KEC(0.38)and KEN(0.54)factors, respectively (Ajit Varma and Ralf Oelmüller, 2007). The organic carbon in the extracts was measured using a dichromate oxidation procedure involving digestion at 100℃ for 30 min, and the total N in the extracts were determined through semi-micro Kjeldahl digestion method.
All statistical analyses were carried out with DPS 7.55 for Windows. One-way ANOVA was used to examine whether cropping patterns had significant difference, and significant difference between treatments was determined with the Duncan' test at 5% level.
Results
Rhizosphere soil enzyme activities
There was no signif i cant difference of urease activities among treatment soils (Fig.1). Catalase activity of maize-maize was the highest and signif i cantly different from that of the other treatments except maize-soybean, and catalase activities of previous soybean soil were all lower than those of previous maize soils (Fig. 2).Soil polyphenol oxidase activity of soybean-maize was the highest, and reached signif i cant level among other treatments, but there was no significant difference found among that of other treatments (Fig. 3). Invertase activities of maize-maize and maize-soybean soils were the largest and showed significant difference from those of other treatments. Invertase activities of previous soybean soil were all lower than those of previous maize soil, and were almost the same as catalase activities of the regular pattern (Fig. 4).
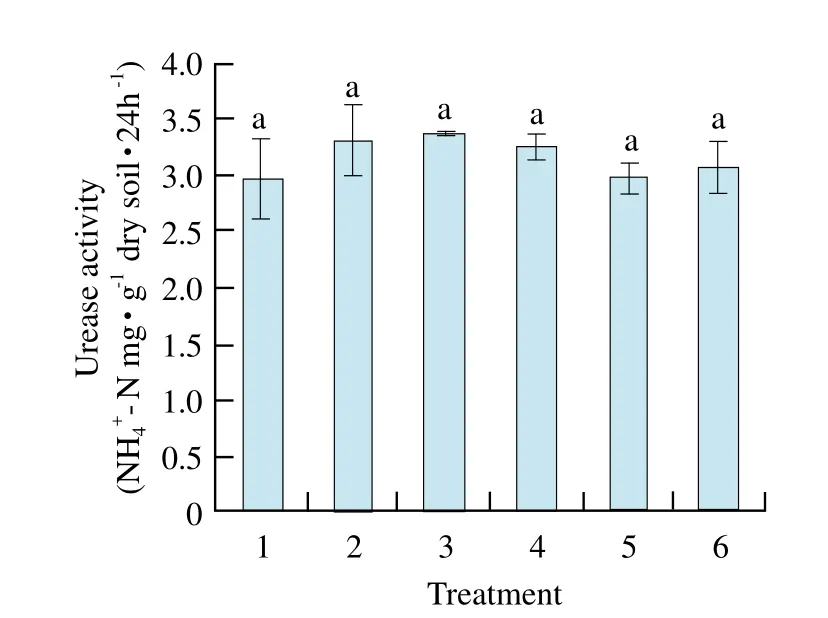
Fig. 1 Effect of different treatments on activity of urease in rhizosphere soil
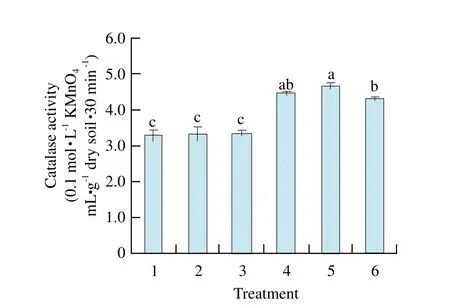
Fig. 2 Effect of different treatments on activity of catalase in rhizosphere soil

Fig. 3 Effect of different treatments on activity of polyphenol oxidase in rhizosphere soil
Microbial biomass carbon and nitrogen
MBC of maize-maize soil reached the highest and significant level compared with other treatments and that of soybean-maize and soybean-mixed was the lowest and had significant difference from other treatments. MBC of previous soybean soil was all lower than that of previous maize soil (Fig. 5). MBN of maize-mixed soil was the maximum of all cropping patterns, and had signif i cant difference from the others(Fig. 6).
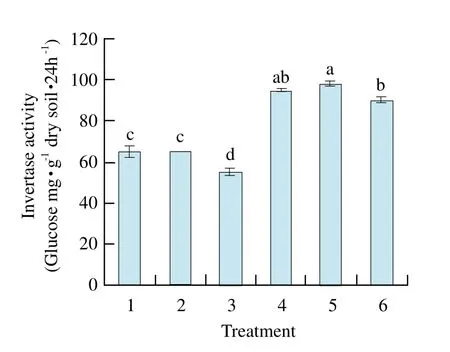
Fig. 4 Effect of different treatments on activity of invertase in rhizosphere soil
Correlation analysis between soil enzyme activities, MBC and MBN
MBC and C/N ratio had positive and very signif i cant correlations with soil catalase activity and invertase activity, respectively, but significant correlations were not found among other soil enzyme activities(Table 2). Different cultivation patterns not only affected catalase, invertase enzyme activities, MBC,MBN and C/N ratio, but also the transformation of soil carbon and nitrogen.
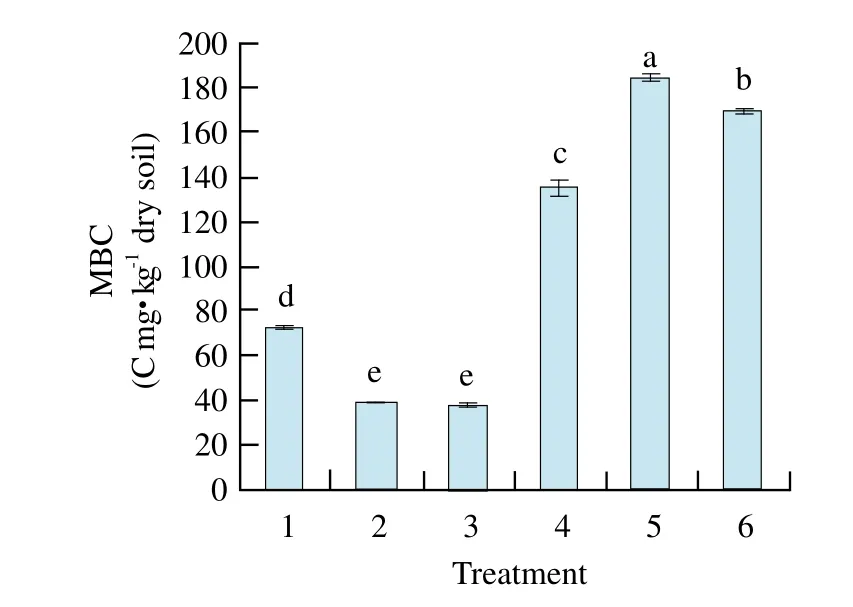
Fig. 5 Changes of soil MBC in different treatments
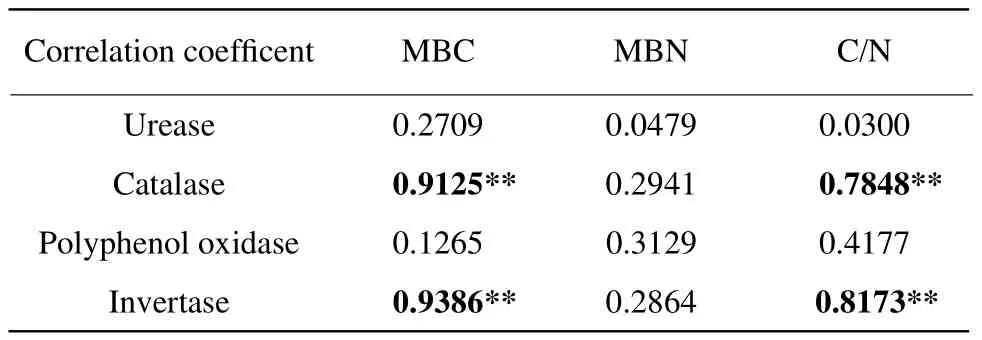
Table 2 Correlation analysis among soil enzyme activities,MBC and MBN
Discussion
A number of crops have continuous cropping obstacles, but soybean has been concerned by even more people. Organic matter and nutrient content in the rhizosphere soil of continuous soybean cropping were lower than that of normal cropping soil (Sun,2008), and a significant change of rhizosphere soil microbial community in continuous cropping system could also been found (Li et al., 2006). Number of beneficial bacteria increased in gramineae and leguminous rotation system (Liu et al., 2007; Liu et al.,2009), which alleviated barriers of continuous cropping, and crop yield and quality were improved. Crop rotation could effectively regulate the microbial fl ora,which was conducive to the improvement of microbial community diversity and stability, and the soil microecological environment was improved consequently(Dong et al., 2010).
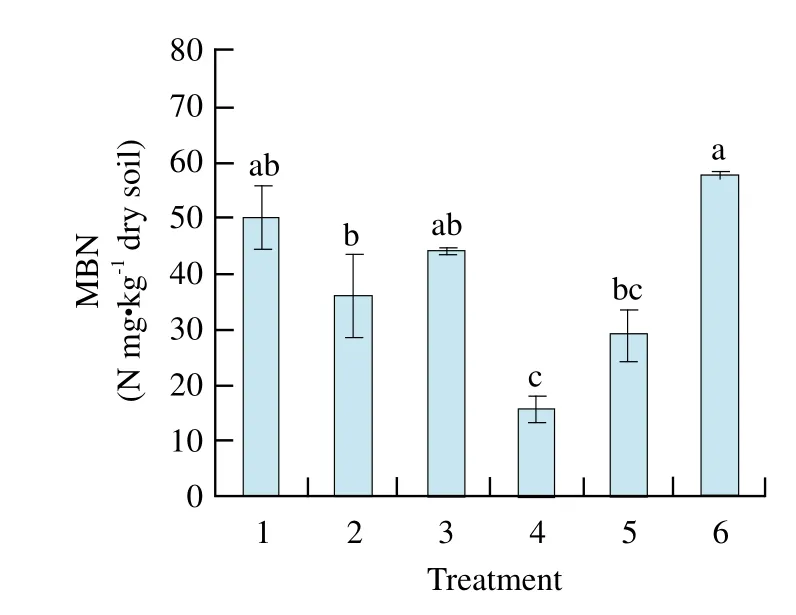
Fig. 6 Changes of soil MBN in different treatments
Soil enzymes and soil microbes are both the executors of soil organic matter transformation and the pools of available nutrients. Soil enzymes are derived from plant roots and their debris, soil animals and their remains and microorganisms, which are capable of catalyzing the transformation of complex organic matter to simple inorganic compounds in soil, and participating in the natural material cycle including soil biochemical processes (Pan et al., 2011; Yao et al.,2010; Lei et al., 2010). Soil MBC is a sensitive indicator of soil organic carbon, and MBN is an important part of soil nitrogen mineralization (Shen et al., 1999). In recent years, MBC and MBN are being gradually recognized as the important role in nutrient cycling, and which are used as the indicators to evaluate soil changes by more and more researchers,but there are many factors inf l uencing MBC and MBN transformation, and which should be studied further.
Plant root exudates are important sources of soil enzymes. A variety of plants continue to self-improve the nutrient availability in rhizosphere and secrete specif i c soil enzymes (Radersma and Grierson, 2004),and rhizosphere soil enzymes occupy a very important position in material exchange process. Thus, to study rhizosphere soil enzyme activity of different cropping patterns is of great signif i cance to explore the process and mechanism of the interaction among different crop root exudates. Results of this study were obtained from the pot experiment; however, there might be more differences from that of field experient. Rhizosphere soil enzyme activity of different cropping patterns in fi eld soil should be studied further.
Conclusions
By pot experiment, our study showed that catalase activity and invertase activity of maize-maize were the highest and significantly different from those of other treatments except maize-soybean. Soil polyphenol oxidase activity of soybean-maize was the highest, and reached significant level among other treatments, but there was no significant difference of urease activity among treatment soils; MBC of maize-maize soil and MBN of maize-mixed soil reached the highest and significant levels compared with other treatments; MBC and C/N ratio had positive and very significant correlations with soil catalase activity and invertase activity, respectively.
Varma A, Oelmüller R. 2007. Advanced techniques in soil microbiology. Soil Biology, 11: 202-207.
Plaza C, Hernández D, García-Gil J C, et al. 2004. Microbial activity in pig slurry-amended soils under semiarid conditions. Soil Biology and Biochemistry, 36(10): 1577-1585.
Cha Q, Huang P, Huang G B. 2005. Effect of intercropping on soil microbial and enzyme activity in the rhizosphere. Acta Prataculturae Sinica, 14(5): 105-110.
Dong Y, Lu Y, Dong K, et al. 2010. Effect of rotation patterns on soil microbial community and enzyme activities under protected cultivation. Chinese Journal of Soil Science, 41(1): 53-55.
Guan S Y, Zhang D S. 1986. Soil enzymology and research methods.Agricultural Press, Beijing. pp. 247-340.
Lei T, Dell E, Wei S. 2010. Chemical composition of dissolved organic matter in agroecosystems: correlations with soil enzyme activity and carbon and nitrogen mineralization. Applied Soil Ecology, 46:426-435.
Li C G, Li X M, Wang J G. 2006. Effect of soybean continuous cropping on bulk and rhizosphere soil microbial community function.Acta Ecologica Sinica, 26(4): 1144-1150.
Liu J B, Xu Y L, Lv G Z, et al. 2009. Black soil region fusarium population structure and quantity in soybean rhizosphere of different rotation system. Soybean Science, 28(1): 97-102.
Liu J X, Lu Y G, Yuan H W, et al. 2007. Effects of intercrop maize and soybean on rhizosphere soil microbes and enzyme activity. Guizhou Agricultural Sciences, 35(2): 60-61.
Liu X J, Xu Y L, Li C J, et al. 2007. Effect of soybean rotation system on the bacterial physiological groups. Soybean Science, 26(5):723-727.
Pan X J, Zhang W E, Luo G H, et al. 2011. Dynamic change of soil enzyme activities under different soil managements in citrus root zone. Chinese Journal of Soil Science, 42(5): 1116-1119.
Radersma S, Grierson P F. 2004. Phosphorus mobilization in agroforestry: organic anions, phosphatase activity and phosphorus fractions in the rhizosphere. Plant and Soil, 259(1): 209-219.
Shen H, Cao Z H, Xu B S. 1999. Dynamics of soil microbial biomass and soil enzyme activity and their relationships during maize growth.Chinese Journal of Applied Ecology, 10(4): 471-474.
Su J X, Zhuo S, Li H S, et al. 2010. Effects of rhizospheric microbes and soil enzymes on the biodegradation of PCB in the soil within intercropping systems. Journal of Agro-Environment Science, 29(11):2114-2120.
Sun L. 2008. Effect of soybean continuous cropping on the rhizosphere soil nutrition. Chinese Agricultural Science Bulletin, 24(12):266-269.Wan Z M, Song C C. 2009. Advance on response of soil enzyme activity to ecological environment. Chinese Journal of Soil Science,40(4): 951-956.
Yao J H, Niu D K, Li Z J, et al. 2010. Effects of antibiotics oxytetracycline on soil enzyme activities and microbial biomass in wheat rhizosphere. Scientia Agricultura Sinica, 43(4): 721-728.
Zhang G N, Chen L J, Chen Z H, et al. 2008. Effects of different cropping systems of soybean on chernozem enzyme activities and kinetic parameters. Soybean Science, 27(5): 795-800.
Zhang S X, Gao Z Q, Liu H L. 2000. Continuous cropping obstacle and rhizospheric microecology Ⅲ. Soil phenolic acids and their biological effect. Chinese Journal of Applied Ecology, 11(5): 741-744.
 Journal of Northeast Agricultural University(English Edition)2012年4期
Journal of Northeast Agricultural University(English Edition)2012年4期
- Journal of Northeast Agricultural University(English Edition)的其它文章
- Effects of Row-Spacing on Canopy Structure and Yield in Different Plant Type Rice Cultivars
- Effects of Subchronic Aluminum Exposure on Amino Acids Neurotransmitters in Chicken Brain
- Study on Classification and Genetic Diversity of Kentucky Bluegrasses by Using RAPD Markers
- Effects of Phosphate Fertilizer on Cold Tolerance and Its Related Physiological Parameters in Rice Under Low Temperature Stress
- Study on Improving Development Strategies of New Rural Social Pension Insurance System in Heilongjiang Province
- Storage System Design Scheme in Virtualization Construction
