Mild hypothermia for treatment of diffuse axonal injury: a quantitative analysis of diffusion tensor imaging
Guojie Jing, Xiaoteng Yao, Yiyi Li, Yituan Xie, Wang’an Li, Kejun Liu, Yingchao Jing, Baisheng Li, Yifan Lv, Baoxin Ma
1 Department of Neurosurgery, Huizhou First People’s Hospital, Huizhou, Guangdong Province, China
2 Huizhou Neurosurgery Institute, Huizhou, Guangdong Province, China
3 Department of Neurosurgery, Huizhou Central People’s Hospital, Huizhou, Guangdong Province, China
Mild hypothermia for treatment of diffuse axonal injury: a quantitative analysis of diffusion tensor imaging
Guojie Jing1,2, Xiaoteng Yao1,2, Yiyi Li1,2, Yituan Xie1,2, Wang’an Li1,2, Kejun Liu1,2, Yingchao Jing1,2, Baisheng Li3, Yifan Lv1,2, Baoxin Ma1,2
1 Department of Neurosurgery, Huizhou First People’s Hospital, Huizhou, Guangdong Province, China
2 Huizhou Neurosurgery Institute, Huizhou, Guangdong Province, China
3 Department of Neurosurgery, Huizhou Central People’s Hospital, Huizhou, Guangdong Province, China
Fractional anisotropy values in diffusion tensor imaging can quantitatively re fl ect the consistency of nerve fi bers after brain damage, where higher values generally indicate less damage to nerve fi bers. Therefore, we hypothesized that diffusion tensor imaging could be used to evaluate the effect of mild hypothermia on diffuse axonal injury. A total of 102 patients with diffuse axonal injury were randomly divided into two groups: normothermic and mild hypothermic treatment groups. Patient’s modi fi ed Rankin scale scores 2 months after mild hypothermia were signi fi cantly lower than those for the normothermia group. The difference in average fractional anisotropy value for each region of interest before and after mild hypothermia was 1.32–1.36 times higher than the value in the normothermia group. Quantitative assessment of diffusion tensor imaging indicates that mild hypothermia therapy may be bene fi cial for patients with diffuse axonal injury.
nerve regeneration; brain injury; mild hypothermia; diffuse axonal injury; diffusion tensor imaging; region of interest; fractional anisotropy; modified Rankin scale; the Natural Science Foundation of Guangdong Province in China; neural regeneration
Funding: This study was supported by the Natural Science Foundation of Guangdong Province in China, No. 10151600101000002.
Jing GJ, Yao XT, Li YY, Xie YT, Li WA, Liu KJ, Jing YC, Li BS, Lv YF, Ma BX. Mild hypothermia for treatment of diffuse axonal injury: a quantitative analysis of diffusion tensor imaging. Neural Regen Res. 2014;9(2):190-197.
Introduction
Diffuse axonal injury accounts for 28–42% of severe brain injury, and is associated with high mortality and morbidity[1]. Currently, diffuse axonal injury is diagnosed by the presence of clinical symptoms and CT performance. However, clinical symptoms and imaging findings are often controversial, and some invasive examinations (such as pathology and intraoperative observations) are not always possible. Thus, a noninvasive, accurate, and objective technique is urgently required for quick and timely assessment of diffuse axonal injury severity and postoperative recovery. Diffusion tensor imaging detects the microstructure of the brain in vivo, and in particular, the white matter[2]. Based on diffusion-weighted imaging techniques, diffusion tensor imaging allows biopsy analysis and images molecular diffusion characteristics of the organ, thus achieving a qualitative and quantitative assessment of anatomy and function associated with white matter fi ber bundles[3]. A previous study showed that changes in fractional anisotropy values obtained by diffusion tensor imaging were positively correlated with Glasgow Coma Scale scores in the acute phase, and modi fi ed Rankin scale score at discharge, in the internal capsule and corpus callosum after diffuse axonal injury[4]. In addition, growing evidence has established the diagnostic value of diffusion tensor imaging in traumatic brain injury, including diffuse axonal injury.
Mild hypothermia (32–35°C) is a current focus of treatment of brain injury. Mild hypothermia exerted apparent neuroprotective effects in animal models of diffuse axonal injury[5-8]. However, the clinical ef fi cacy of mild hypothermia is controversial. The majority of clinical studies reported that therapeutic hypothermia improved severe brain injury prognosis[8-11], but this improvement was not observed in other studies[12-14]. Currently, there is no accurate, objective, and non-invasive means to verify the effect of mild hypothermia in diffuse axonal injury and its prognosis. In comparison with animal models, the lack of an objective assessment method hinders the assessment of clinical ef fi cacy, and clinical symptom scores often suffer from errors because such symptoms are evaluated by investigators.
In this study, we hypothesized that diffusion tensor imaging could be applied to evaluate mild hypothermia therapy in diffuse axonal injury. The efficacy of mild hypothermia and normothermia therapy in patients with diffuse axonalinjury was quantitatively assessed using diffusion tensor imaging and modified Rankin scale scores. The modified Rankin scale scores and fractional anisotropy values were compared to investigate the efficacy of mild hypothermia therapy for treatment of diffuse axonal injury.
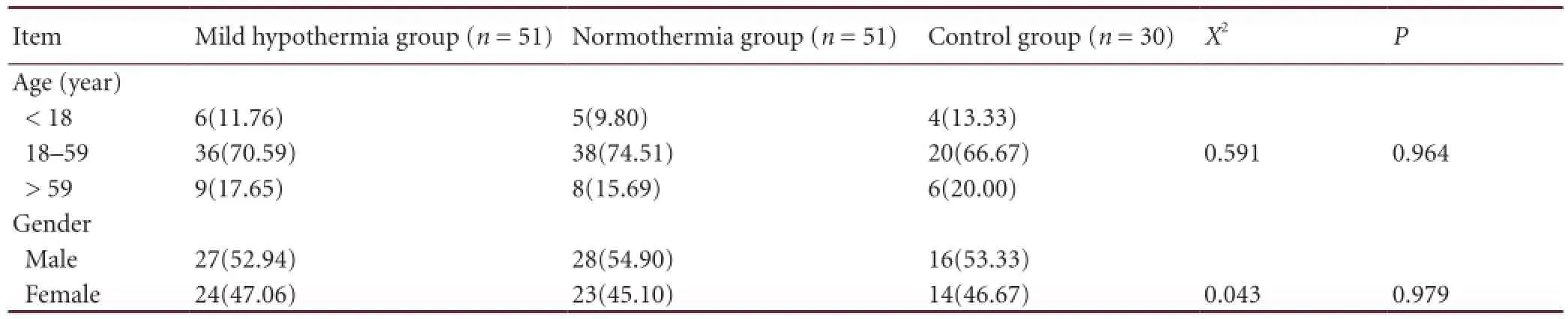
Table 1 Baseline information [n (%)] of all subjects
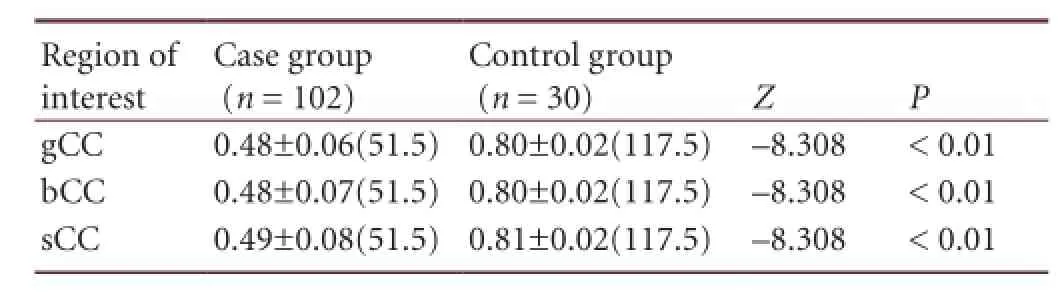
Table 2 Fractional anisotropy values in the regions of interest (genu, body, and splenium of corpus callosum) before treatment
Results
Quantitative analysis of participants
A total of 102 patients with diffuse axonal injury were randomly and equally divided into mild hypothermia and normothermia groups. Another 30 healthy subjects served as acontrol group. Finally, results from 132 subjects were analyzed, with no dropout or loss of patients.
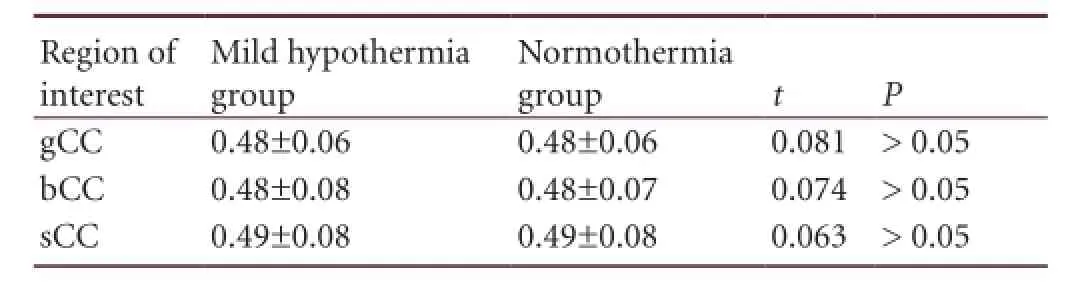
Table 3 Comparison of average fractional anisotropy values in the regions of interest (genu, body, and splenium of corpus callosum) between mild hypothermia and normothermia groups before treatment
Baseline information of participants and fractional anisotropy values in regions of interest before treatmentBaseline information of all patients is shown in Table 1. The age of patients ranged from 18–59 years old, and accident was the main injury cause. There was no signi fi cant difference in age or gender among the three groups (P > 0.05).
The difference in Glasgow Coma Scale score before treatment was not significant between mild hypothermia andnormothermia groups (average rank: 51.06 vs. 51.94; P >0.05), indicating that the clinical assessment of disease severity was similar between the two groups.
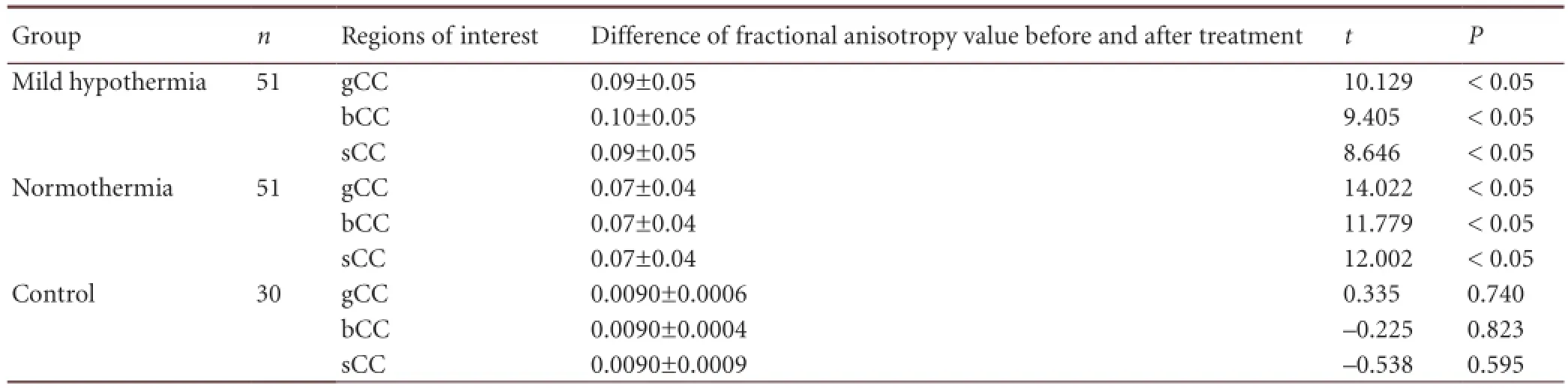
Table 4 Difference in fractional anisotropy values in the regions of interest (genu, body, and splenium of corpus callosum) among the three groups before and after treatment
Comparison of fractional anisotropy values in regions of interest (genu, body, and splenium of corpus callosum) between patients with diffuse axonal injury before treatment and healthy controls: Fractional anisotropy values in the regions of interest for this study (genu, body, and splenium of corpus callosum) in patients with diffuse axonal injury in the mild hypothermia and normothermia groups were measured before treatment, and compared with the first measurement results in the control group. Diffusion tensor imaging results showed that the average fractional anisotropy value in regions of interest was 60% of that in the control group (P < 0.01; Table 2).
Comparison of fractional anisotropy values in the regions of interest between mild hypothermia and normothermia groups before treatment: There was no signi fi cant difference in the average fractional anisotropy values in the regions of interest between the mild hypothermia group and normothermia group before treatment (P > 0.05; Table 3). This suggests that the degree of nerve fi ber damage in each region of interest assessed by fractional anisotropy values from diffusion tensor imaging was similar between the two groups before treatment.
Comparison of modi fi ed Rankin scale score between mild hypothermia and normothermia groups after treatment
The modified Rankin scale scores differed significantly between the two groups after treatment (P < 0.05). The mild hypothermia group had a lower modi fi ed Rankin scale score (mean rank: 45.49) than the normothermia group (mean rank: 57.51; P < 0.05). This suggests a beneficial effect of mild hypothermia compared with normothermia.
Comparison of fractional anisotropy values in the regions of interest among the three groups before and after treatment
After patients with diffuse axonal injury in the mild hypothermia and normothermia groups were treated for 2 months, fractional anisotropy values in regions of interest showed signi fi cant differences in the two groups before and after treatment (P < 0.05; Table 4). This evidence suggests that nerve fiber damage in regions of interest was significantly improved after mild hypothermia and normothermia therapy, compared with before treatment.
Intergroup comparison of fractional anisotropy values in the regions of interest among the three groups
There were significant differences in the average fractional anisotropy values in the regions of interest among the threegroups (P < 0.05). The difference in average fractional anisotropy values was highest in the mild hypothermia group, and was 1.32–1.36 times the values of the normothermia group (Tables 5–7).

Table 5 Intergroup comparison of the difference in fractional anisotropy values in the regions of interest (genu, body, and splenium of corpus callosum) among the three groups
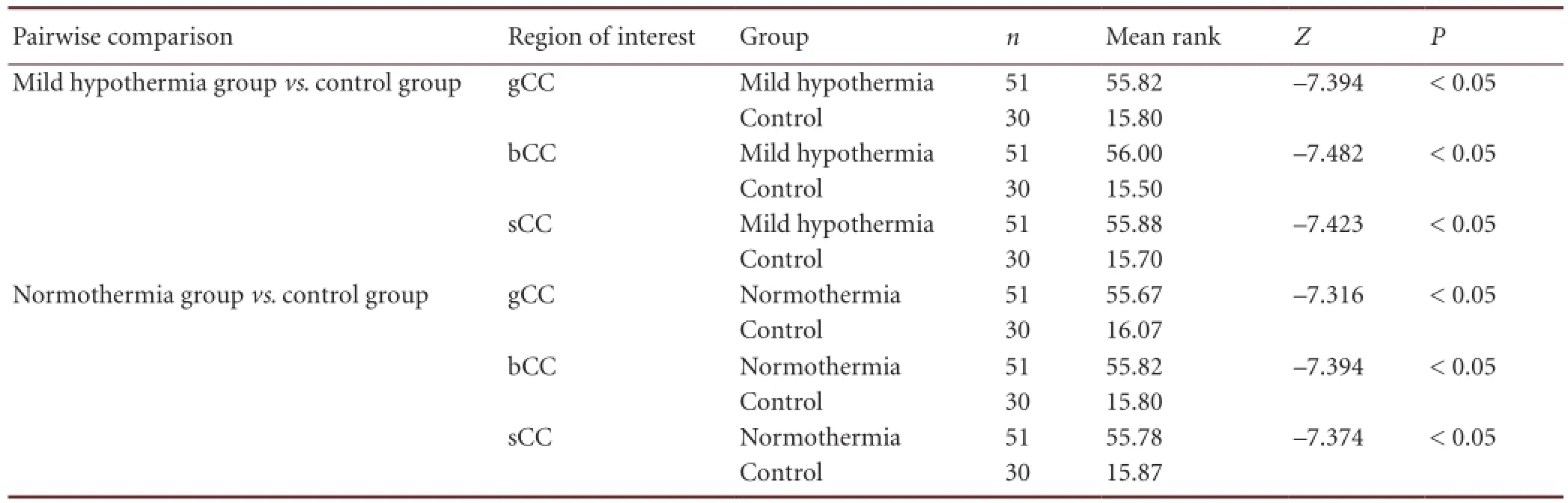
Table 6 Pairwise comparison of the difference in fractional anisotropy values in the regions of interest (genu, body, and splenium of corpus callosum) among the three groups
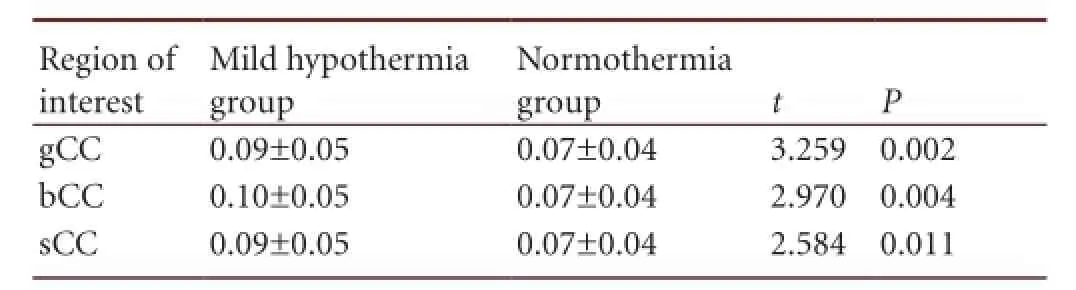
Table 7 Comparison of the average fractional anisotropy values in the regions of interest between the mild hypothermia group and normothermia group
Comparison of the fractional anisotropy image, fractional anisotropy color image, and fi ber bundle image, among the three groups
The diffusion tensor imaging images of patients with diffuse axonal injury before and after treatment, and healthy controls are shown in Figure 1. In the fractional anisotropy images of healthy controls, a homogeneous high-intensity signal was clearly visible in the genu and splenium of the corpus callosum. In the fractional anisotropy color image of healthy controls, the genu and splenium of the corpus callosum transitioned from the middle red area to the yellow-green area, and the color was relatively uniform. In the fi ber bundle image, nerve fi bers in the corpus callosum distributed in an orderly manner, presenting uniform direction and complete structure. In patients with diffuse axonal injury, the signals of white matter fiber concentrated areas (the genu, body, and splenium of the corpus callosum) were signi fi cantly reduced compared with healthy controls. In the fractional anisotropy color images of these patients, irregular dark areas were visible within the normal color area. In the fi ber bundle images, fi ber bundles at the lesion area showed partial interruption and disordered structure. After mild hypothermia and normothermia therapy, fractional anisotropy values obtained on the fractional anisotropy map were signi fi cantly improved. As shown in Figure 1B1–B4, B2–B5, C1–C4, and C2–C5, the size of lesions was reduced, and the structure and fiber bundle integrity were improved. These changes were more apparent in the mild hypothermia group.
Discussion
Diffusion tensor imaging can clearly visualize the structure of human white matter fibers[15-16]. Diffuse axonal injury mainly refers to structural damage to white matter fibers. Thus, we used diffusion tensor imaging to assess the severity and monitor the therapeutic effect of hypothermia in patients with diffuse axonal injury. This was a randomized controlled study concerning the role of diffusion tensor imaging in the diagnosis of diffuse axonal injury. It is worth noting that the experimental data were strictly selected (comparability of baseline data and consistency of lesion severity before treatment) to ensure an objective study.
The results of this study showed that changes in fractional anisotropy values were consistent with the Glasgow Coma Scale score before treatment and modi fi ed Rankin scale score after treatment. Our fi ndings indicate that diffusion tensor imaging can be used to assess injury severity before treatment and the effect of treatment itself.
In addition, this technique simultaneously provides radiographic evidence, thus assisting the assessment of nerve fi bers at lesion locations.
Accumulating evidence has shown a positive correlation between the changes in fractional anisotropy values and the severity of damage to white matter fi ber structure in diffuse axonal injury. As a basis for clinical outcomes, the assessment of white matter fi ber structure damage helps to specify the patient’s condition, turnover, treatment, and prognosis[17-18]. Ljungqvist et al.[19]found that changes in fractional anisotropy values were highly associated with Glasgow Coma Scale score and modi fi ed Rankin scale score, suggesting a potential indicator of clinical prognosis, and especially in predicting the location of brain injury[20]. Therefore, assessing the severity of injury in patients with diffuse axonal injury should be based on the patient’s history, signs, rating scores, and diffusion tensor imaging.
The modi fi ed Rankin scale is mainly used to assess functional disability after treatment[21]and higher scores are correlated with lower functional recovery[22]. In this study, modi fi ed Rankin scale scores in the mild hypothermia group were significantly lower than those in the normothermia group, while fractional anisotropy values were signi fi cantly higher than those in the normothermia group. These fi ndings indicate that mild hypothermia is more effective than normothermia for treatment of diffuse axonal injury, and is associated with significantly lower disability score and signi fi cantly higher fractional anisotropy values after treatment.
The protective mechanisms of mild hypothermia are likley mediated by a variety of factors, such as delaying energy consumption, promoting glucose use, and reducing cell metabolic rate[23]. Moderate hypothermia (33°C) significantly decreases the oxygen delivery threshold of brain cells[24], thus improving brain cell tolerance to hypoxia and enhancing protection against secondary brain injury. Hypothermia inhibits the action of glutamate and glycine, suppresses calcium ion in fl ux, and antagonizes glutamate excitotoxicity[25]. In addition, hypothermia reduces free radical generation, regulates oxidative stress, and maintains normal permeability of the blood-brain barrier, thus reducing cerebral edema. Hypothermia also reduces matrix metalloproteinase production and inhibits activity, further enhancing blood-brain barrier permeability and reducing cerebral edema[26-27].
It is known that the prognosis of neurological functioning is highly linked with primary and secondary brain injury within 72 hours[28]. In animal experiments, the optimal window of opportunity for hypothermia is within 1 hour[29]and 32–34°C is the current optimal temperature range[30]. Therefore, mild hypothermia in this study was scheduled for 72hours, and initiated as soon as possible after injury, within a temperature range of 32–34°C.

Figure 1 Fractional anisotropy (FA) images, FA color images, and fi ber bundle images in the mild hypothermia group, normothermia group, and control group.
There are some controversies concerning the efficacy of mild hypothermia for brain injury (including diffuse axonal injury). McIntyre et al.[31]established the efficacy of hypothermia in a multicenter meta-analysis. However, no signi ficant difference was found between hypothermia and normothermia for treatment of severe brain injury in a multi-center study by Clifton et al.[14]. The results of the current study showed that mild hypothermia signi fi cantly reduced modi fi ed Rankin scale scores. Diffusion tensor imaging showed that fractional anisotropy values were signi fi cantly increased after treatment, lesions were reduced, the structure and integrity of fi ber bundles were improved, and these changes were more apparent after mild hypothermia. Thus, our fi ndings are similar to the results of McIntyre et al.[31].
In summary, mild hypothermia protected the brain, restored neurological function, and improved the prognosis ofpatients with diffuse axonal injury, and can be considered an effective treatment for diffuse axonal injury. DTI can be used to quantitatively assess the severity of diffuse axonal injury. Finally, the difference in average fractional anisotropy value for each region of interest before and after mild hypothermia was 1.32–1.36 times higher than the value in the normothermia group.
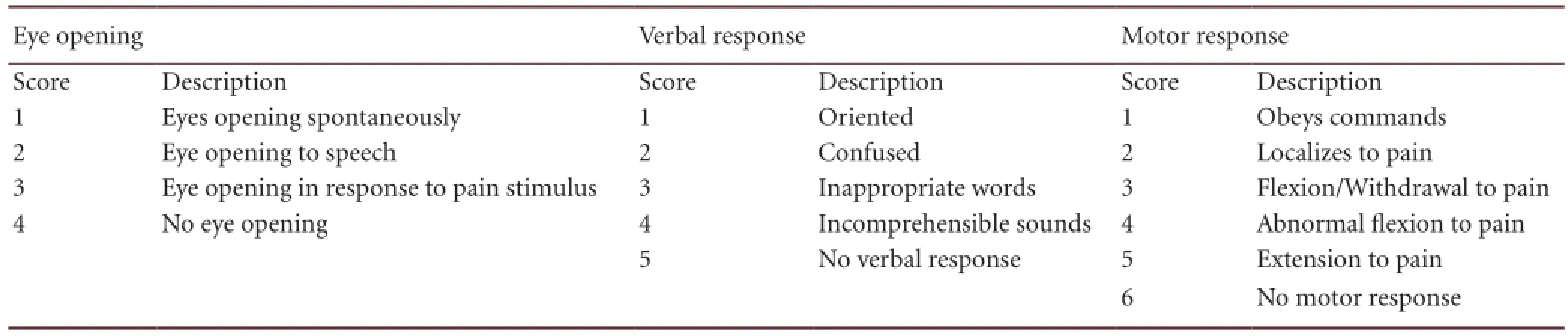
Table 8 Glasgow Coma Scale standard

Table 9 The modi fi ed Rankin scale standard
Some limitations of the study deserve to be mentioned, such as the lack of a stratified analysis of case characteristics, hypothermia time course, treatment time window, rewarming, and complications. There was also no correlation analysis conducted between the improvement of fractional anisotropy value and the modi fi ed Rankin scale score, and the follow-up period was short.
Subjects and Methods
Design
A randomized, double-blind, controlled trial.
Time and setting
The experiment was performed from October 2007 to January 2013 in Huizhou Central People’s Hospital and Huizhou First People’s Hospital, China.
Subjects
A total of 102 patients with diffuse axonal injury were recruited from Huizhou Central People’s Hospital and Huizhou First People’s Hospital, China, and were randomly divided into a mild hypothermia group and a normothermia group. Each group comprised 51 patients. At present, there are no guidelines, consensus, or final recommendations for the use of hypothermia for treatment of diffuse axonal injury, so randomization for group membership was a reasonable method in this study. In addition, 30 healthy examinees and volunteers in the same hospital formed the control group. According to Administrative Regulations on Medical Institutions issued by the State Council of China[32], all subjects were informed of the experimental outline and risks prior to experimentation, and all patients gave written informed consent.
Patients with diffuse axonal injury
Diagnostic criteria: Cases having a history of traumatic brain injury prior to admission; cases presenting consciousness disturbance immediately after injury, lasting for > 6 hours; cranial CT findings (multiple punctate or flake-shaped hemorrhage at the junction between the cerebral cortex and medulla, corpus callosum, brain stem, internal capsule, or around the ventricle) or MRI; and diffusion tensor imaging detected lesions in the white matter signal[33].
Inclusion criteria: Strict accordance with diffuse axonal injury diagnostic criteria[33]; vital signs stable at admission; and no craniotomy indications.
Exclusion criteria: Cases in a critical condition in hospital were not suitable for diffusion tensor imaging; patients or their families refused to undergo diffusion tensor imaging; cases with metallic materials in the head and face, such as dentures and cerebral aneurysm clips.
Control group
Inclusion criteria: Healthy examinees or volunteers who were willing to undergo MRI.
Exclusion criteria: Patients with central nervous system diseases or cerebral vascular diseases who could not undergo MRI, or where images obtained were unclear.
Methods
Mild hypothermia therapy
During hypothermia treatment, brain temperature was estimated using the rectal temperature[34], the target brain tem-perature was 32–34°C and was reached within 25–55 minutes (mean 45 minutes), the treatment commenced 2–10.5 hours after injury (mean 6 hours) and lasted for 30 minutes. Temperature was reduced using a mixture of pethidine hydrochloride, chlorpromazine hydrochloride, and promethazine hydrochloride (Shengyang First Pharmaceutical Technology Development Co., Ltd., Northeast Pharmaceutical Group Co., Ltd., Shenyang, Liaoning Province, China) via intravenous infusion, in combination with ice blankets and ice caps (DWK-II temperature controlled system; Beijing Dawei Genesis Medical Equipment Co., Ltd., Beijing, China). Mild hypothermia therapy was scheduled for 48–96 hours (average 72 hours); and the rewarming period was 12–24 hours. No deaths occurred after treatment.
Assessment criteria for central nervous system disease
The severity of diffuse axonal injury was evaluated using the Glasgow Coma Scale at admission[35](Table 8). A lower Glasgow Coma Scale score indicates greater severity. Two months after treatment, prognosis was evaluated using the modi fi ed Rankin scale[21](Table 9). A lower modi fi ed Rankin scale score indicates better recovery. All rating scores were determined by two experienced neurosurgery specialists who were blinded to the group.
Imaging examination
Patients were detected by routine head CT scan at emergency and by head unenhanced MR angiography and diffusion tensor imaging scans at admission. Two months after mild hypothermia or normothermia therapy, patients were checked by MR scan and diffusion tensor imaging. The healthy control group underwent diffusion tensor imaging every 2 months.
Image acquisition: LightSpeed 64-slice spiral CT (GE Healthcare, Fairfield, Connecticut, USA) parameters: layer thickness = 5 mm, layer space = 5 mm, tube voltage = 120 kV, tube current = 250 mA, fi eld of view (FOV) = 220 mm × 220 mm, matrix = 512 × 512. 1.5T MR scanner (Philips, The Netherlands) parameters: standard 8-channel coil; T1WI sequence parameters: repetition time (TR) = 488 ms, echo time (TE) = 15 ms, slice thickness = 5 mm, FOV = 230 mm × 230 mm; T2WI sequence parameters: TR = 3,600 ms, TE = 100 ms, thickness = 5 mm, FOV = 230 mm × 230 mm; diffusion tensor imaging sequence parameters: TR = 11,000 ms, TE = 71 ms, slice thickness = 5 mm, FOV = 224 mm × 224 mm, 15 diffuse gradient directions, b value = 1,000; positioning line: parallel to the link between anterior and posterior lines.
Data collection: After data were processed using Extended MR WorkSpace (Philips), fractional anisotropy maps, fractional anisotropy color maps, and diffusion tensor imaging fiber bundle maps were obtained. Regions of interest for diffusion tensor imaging scanning included the genu, body and splenium of the corpus callosum, a 50-mm2area in each region of interest was measured by radiologists, to obtain average fractional anisotropy values. To avoid subjective bias, the double-blind objects were patients or their families and physicians who were responsible for the collected data.
Quantitative outcome measure: The difference in fractional anisotropy values before and after treatment was considered the indicator of efficacy assessment in each group, as previously described[4].
Statistical analysis
Data were analyzed using SPSS 13.0 software (SPSS, Chicago, IL, USA). Measurement data are expressed as mean ± SD, count data are expressed as the constituent ratio, and rank data are expressed as the mean rank. Measurement data between groups were compared using a two-sample t-test. Count data between groups were compared using a Pearson chi-square test, and rank data between groups were compared using the Mann-Whitney U test or Kruskal-Wallis H test. A value of P < 0.05 was considered statistically signi fi cant.
Author contributions:Jing GJ was responsible for the study concept and design. Jing GJ, Liu KJ, Yao XT, Jing YC, Li WA, Li YY, Xie YT and Li BS provided and integrated experimental data. Liu KJ, Yao XT, Jing YC, Lv YF, Ma BX and Li WA were responsible for data analysis, writing the manuscript, supervising the paper, and performing statistical analysis. Jing GJ was the head of the funds. All authors approved the final version of the paper.
Con fl icts of interest:None declared.
Peer review:This study is a logic and prospective study addressing two issues: whether hypothermia is effective in treatment of diffuse axonal injury; whether diffusion tensor imaging can be applied for quantitative evaluation of diffuse axonal injury. The limitations of this study is the absence of stratified research and short follow-up periods.
[1] Smith DH, Hicks R, Povlishock JT. Therapy development for diffuse axonal injury. J Neurotrauma. 2013;30(5):307-323.
[2] Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994;66(1):259-267.
[3] Gulani V, Sundgren PC. Diffusion tensor magnetic resonance imaging. J Neuroophthalmol. 2006;26(1):51-60.
[4] Huisman TA, Schwamm LH, Schaefer PW, et al. Diffusion tensor imaging as potential biomarker of white matter injury in diffuse axonal injury. AJNR Am J Neuroradiol. 2004;25(3):370-376.
[5] Geeraerts T, Vigue B. Cellular metabolism, temperature and brain injury. Ann Fr Anesth Reanim. 2009;28(4):339-344.
[6] Kobbe P, Lichte P, Wellmann M, et al. Impact of hypothermia on the severely injured patient. Unfallchirurg. 2009;112(12):1055-1061.
[7] Kovacs E, Jenei Z, Horvath A, et al. Physiologic effects of hypothermia. Orv Hetil. 2011;152(5):171-181.
[8] Polderman KH, Tjong TJ, Peerdeman SM, et al. Effects of therapeutic hypothermia on intracranial pressure and outcome in patients with severe head injury. Intensive Care Med. 2002;28(11):1563-1573.
[9] Qiu W, Zhang Y, Sheng H, et al. Effects of therapeutic mild hypothermia on patients with severe traumatic brain injury after craniotomy. J Crit Care. 2007;22(3):229-235.
[10] Jiang JY, Xu W, Li WP, et al. Effect of long-term mild hypothermia or short-term mild hypothermia on outcome of patients with severe traumatic brain injury. J Cereb Blood Flow Metab. 2006;26(6):771-776.
[11] Zhi D, Zhang S, Lin X. Study on therapeutic mechanism and clinical effect of mild hypothermia in patients with severe head injury. Surg Neurol. 2003;59(5):381-385.
[12] Gal R, Cundrle I, Zimova I, et al. Mild hypothermia therapy for patients with severe brain injury. Clin Neurol Neurosurg. 2002;104(4):318-321.
[13] Shiozaki T, Nakajima Y, Taneda M, et al. Efficacy of moderate hypothermia in patients with severe head injury and intracranial hypertension refractory to mild hypothermia. J Neurosurg. 2003;99(1):47-51.
[14] Clifton GL, Miller ER, Choi SC, et al. Lack of effect of induction of hypothermia after acute brain injury. N Engl J Med. 2001;344(8): 556-563.
[15] Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magn Reson Med. 1996;36(6):893-906.
[16] Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15(7-8):435-455.
[17] Li J, Li XY, Feng DF, et al. Quantitative evaluation of microscopic injury with diffusion tensor imaging in a rat model of diffuse axonal injury. Eur J Neurosci. 2011;33(5):933-945.
[18] Jang SH. Prediction of motor outcome for hemiparetic stroke patients using diffusion tensor imaging: a review. NeuroRehabilitation. 2010;27(4):367-372.
[19] Ljungqvist J, Nilsson D, Ljungberg M, et al. Longitudinal study of the diffusion tensor imaging properties of the corpus callosum in acute and chronic diffuse axonal injury. Brain Inj. 2011;25(4):370-378.
[20] Sidaros A, Engberg AW, Sidaros K, et al. Diffusion tensor imaging during recovery from severe traumatic brain injury and relation to clinical outcome: a longitudinal study. Brain. 2008;131(Pt 2):559-572.
[21] Burn JP. Reliability of the modified Rankin Scale. Stroke. 1992; 23(3):438.
[22] Ghandehari K, Ghandehari K, Saffarian-Toosi G, et al. Comparative interrater reliability of Asian Stroke Disability Scale, modi fi ed Rankin Scale and Barthel Index in patients with brain infarction. ARYA Atheroscler. 2012;8(3):153-157.
[23] Sasaki T, Boni L, Riemer RK, et al. Cerebral oxygen metabolism during total body fl ow and antegrade cerebral perfusion at deep and moderate hypothermia. Artif Organs. 2010;34(11):980-986.
[24] Sinard JM, Vyas D, Hultquist K, et al. Effects of moderate hypothermia on O2consumption at various O2deliveries in a sheep model. J Appl Physiol. 1992;72(6):2428-2434.
[25] Globus MY, Alonso O, Dietrich WD, et al. Glutamate release and free radical production following brain injury: effects of posttraumatic hypothermia. J Neurochem. 1995;65(4):1704-1711.
[26] Lee JE, Yoon YJ, Moseley ME, et al. Reduction in levels of matrix metalloproteinases and increased expression of tissue inhibitor of metalloproteinase-2 in response to mild hypothermia therapy in experimental stroke. J Neurosurg. 2005;103(2):289-297.
[27] Gancia P, Pomero G. Brain cooling therapy. Minerva Pediatr. 2010;62(3 Suppl 1):173-175.
[28] Edwards P, Arango M, Balica L, et al. Final results of MRC CRASH, a randomised placebo-controlled trial of intravenous corticosteroid in adults with head injury-outcomes at 6 months. Lancet. 2005;365(9475):1957-1959.
[29] Markgraf CG, Clifton GL, Moody MR. Treatment window for hypothermia in brain injury. J Neurosurg. 2001;95(6):979-983.
[30] Colbourne F, Sutherland G, Corbett D. Postischemic hypothermia. A critical appraisal with implications for clinical treatment. Mol Neurobiol. 1997;14(3):171-201.
[31] McIntyre LA, Fergusson DA, Hebert PC, et al. Prolonged therapeutic hypothermia after traumatic brain injury in adults: a systematic review. JAMA. 2003;289(22):2992-2999.
[32] State Council of the People’s Republic of China. Administrative Regulations on Medical Institution. 1994-09-01.
[33] Hilton G. Diffuse axonal injury. J Trauma Nurs. 1995;2(1):7-12.
[34] Jiang JY. Clinical study of mild hypothermia treatment for severe traumatic brain injury. J Neurotrauma. 2009;26(3):399-406.
[35] Jennett B, Teasdale G. Aspects of coma after severe head injury. Lancet. 1977;1(8017):878-881.
Copyedited by Phillips A, Li KY, Wang X, Wang J, Yang Y, Li CH, Song LP, Liu WJ, Zhao M
10.4103/1673-5374.125348
Guojie Jing, Department of Neurosurgery, Huizhou First People’s Hospital, Huizh ou, Guangdong Province, China, Huizhou Neurosurgery Institute, Huizhou, Guangdong Province, China, jingguojie888@163. com.
http://www.nrronline.org/
Accepted: 2013-11-25
- 中國神經(jīng)再生研究(英文版)的其它文章
- Acupuncture/electroacupuncture enhances antidepressant effect of Seroxat: the Symptom Checklist-90 scores
- Effects of Wen Dan Tang on insomnia-related anxiety and levels of the brain-gut peptide Ghrelin
- Role of the nucleus tractus solitarii in the protection of pre-moxibustion on gastric mucosal lesions
- Optimal duration of percutaneous microballoon compression for treatment of trigeminal nerve injury
- The optimal distance between two electrode tips during recording of compound nerve action potentials in the rat median nerve
- Acupuncture at Baihui and Dazhui reduces brain cell apoptosis in heroin readdicts

