Acrylamide inhibits nerve sprouting induced by botulinum toxin type A
Hong Jiang, Yi Xiang, Xingyue Hu, Huaying Cai
1 Department of Neurology, Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, Hangzhou, Zhejiang Province, China
2 Second Clinical Medical College, Zhejiang Chinese Medical University, Hangzhou, Zhejiang Province, China
Acrylamide inhibits nerve sprouting induced by botulinum toxin type A
Hong Jiang1, Yi Xiang2, Xingyue Hu1, Huaying Cai1
1 Department of Neurology, Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, Hangzhou, Zhejiang Province, China
2 Second Clinical Medical College, Zhejiang Chinese Medical University, Hangzhou, Zhejiang Province, China
Botulinum toxin type A is a potent muscle relaxant that blocks the transmission and release of acetylcholine at the neuromuscular junction. Intramuscular injection of botulinum toxin type A has served as an effective and safe therapy for strabismus and focal dystonia. However, muscular weakness is temporary and after 3-4 months, muscle strength usually recovers because functional recovery is mediated by nerve sprouting and reconstruction of the neuromuscular junction. Acrylamide may produce neurotoxic substances that cause retrograde necrotizing neuropathy and inhibit nerve sprouting caused by botulinum toxin type A. This study investigated whether acrylamide inhibits nerve sprouting after intramuscular injection of botulinum toxin type A. A tibial nerve sprouting model was established through local injection of botulinum toxin type A into the right gastrocnemius muscle of Sprague-Dawley rats. Following intramuscular injection, rats were given intraperitoneal injection of 3% acrylamide every 3 days for 21 days. Nerve sprouting appeared 2 weeks after intramuscular injection of botulinum toxin type A and single-fiber electromyography revealed abnormal conduction at the neuromuscular junction 1 week after intramuscular injection of botulinum toxin type A. Following intraperitoneal injection of acrylamide, the peak muscle fi ber density decreased. Electromyography jitter value were restored to normal levels 6 weeks after injection. This indicates that the maximal decrease in fi ber density and the time at which functional conduction of neuromuscular junction was restored were delayed. Additionally, the increase in tibial nerve fi bers was reduced. Acrylamide inhibits nerve sprouting caused by botulinum toxin type A and may be used to prolong the clinical dosage of botulinum toxin type A.
nerve regeneration; peripheral nerve regeneration; botulinum toxin type A; acrylamide; nerve sprouting; electromyography; nerve fibers; neuromuscular junction; single-fiber EMG; fiber density; action potential mean consecutive difference; dysmyotonia; neural regeneration
Funding:This study was supported by TCM General Research Project of Zhejiang Province, No. 2014ZA071; Health General Research Project of Zhejiang Province, No. 2014KYA106.
Jiang H, Xiang Y, Hu XY, Cai HY. Acrylamide inhibits nerve sprouting induced by botulinum toxin type A. Neural Regen Res. 2014;9(16):1525-1531.
Introduction
Botulinum toxin type A (BTX-A), one of the exotoxins produced by Clostridium botulinum, can selectively act on the presynaptic membrane of peripheral cholinergic motor neurons. It cleaves synaptosomal associated protein 25 kDa (SNAP-25), which consequently inhibits the release of acetylcholine containing vesicles from presynaptic membranes, blocks neuromuscular transmission, and leads to flaccid paralysis in the target muscles (Blasi et al., 1993; Brin, 1997; Rossetto et al., 2006). Based on the above mechanism of action, BTX-A has been used to treat strabismus and subsequently dystonia and cluster headache, which are characterized by excessive or inappropriate muscle contractions (Allison et al., 2012; Chen, 2012; Bragg et al., 2014; Thenganatt et al., 2014). Owing to its safety and effectiveness, BTX-A has been extensively used for clinical treatment in the fi elds of neurology (Villafa?e et al., 2012), ophthalmology (Ho et al., 2014), plastic surgery (Winocour et al., 2014), rehabilitation medicine (Pimentel et al., 2014), gastroenterology (Blake et al., 2012) and urology (Rashid et al., 2013).
However, BTX-A treatment is limited by its short ef fi cacy, single administration is effective for only 3-4 months, which means that patients have to receive repeated injections. Increasing the dosage of single administration may prolong the effectiveness of the duration of the drug, but it may also aggravate the immune response and produce botulinum toxin antibodies; hence reducing the efficacy and safety of long-term treatment. The effectiveness of drug duration depends on the functional recovery of conduction at the neuromuscular junction, which is mediated by nerve sprouting and reconstruction of the neuromuscular junction (Holds et al., 1990; Juzans et al., 1996; Paiva et al., 1999; English, 2003; Harrison et al., 2007; Wright et al., 2007). BTX-A treatment blocks vesicular acetylcholine release for 3 days, nerve terminals begin to produce sprouting nerves, the number of acetylcholine receptors increases, and new neuromuscularjunctions form. Between 4 and 8 weeks after BTX-A injection, the number of neuromuscular junctions remains the same, conduction at the blocked neuromuscular junction is restored, and sprouting nerves gradually retreat. As yet, there is no method to prolong the duration of the effect of the drug, and this is worthy of further study.
Acrylamide monomer is moderately toxic and mainly affects the nervous system. It is also a typical neurotoxic substance that causes retrograde necrotizing neuropathy (Matthew et al., 1983; Lopachin et al., 2008; Scelsi et al., 2012). Acrylamide accumulates highly speci fi cally in the body and the degree of intoxication is closely related to the cumulative dose. Mild poisoning leads to sensorineural polyneuropathy with insidious onset; moderate poisoning is mainly an ataxia disorder caused by deep sensory disorder; severe poisoning leads to subacute onset of cerebellar dysfunction, followed by sensorimotor polyneuropathy. The symptoms may disappear after several weeks or months. The pathology of the peripheral nerve is consistent axonal degeneration. Silver-cholinesterase double staining shows early swelling of motor nerve terminals in the last node of Ranvier, and gradual deterioration at the proximal end, even at the neuromuscular junction. Large numbers of neuro fi laments aggregate in the swelling axons, with degenerating mitochondria and granules. As the poisoning intensifies, axonal degeneration exacerbates, myelin sheath dissociates and an oval cell body forms, ultimately leading to complete destruction or disintegration of the nerve fibers (Dale et al., 2002; Xiao et al., 2013). The mechanisms of poisoning include acrylamide inhibition of axonal transport, energy metabolism disorders and calcium overload (Zhu et al., 2008). Whether acrylamide inhibits nerve sprouting caused by intramuscular injection of BTX-A is poorly understood.
In this study, a nerve sprouting model was established through local injection of BTX-A into the right gastrocnemius muscle in rats (Pestronk et al., 1988), and the effect of acrylamide on nerve sprouting after intramuscular injection of BTX-A was investigated. Nerve sprouting was studied pathologically and electrophysiologically in a broader attempt to explore the possibility of acrylamide inhibiting nerve sprouting.
Materials and Methods
Animals
A total of 145 adult Sprague-Dawley rats of clean grade II, weighing 160-200 g, were provided by the Animal Center of Zhejiang Academy of Medical Sciences, China (license No. SYXK (Zhejiang) 2010-0149). Experimental protocols were approved by Animal Ethics Committee of Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, China.
Establishment of animal models and grouping
One hundred and forty-five rats were randomly divided into four groups: normal control group (n= 10), BTX-A group (n= 45), BTX-A + acrylamide group (n= 45), and acrylamide group (n= 45). Except for the normal control group, each rat in the other three groups was conventionally disinfected with PVP-I and injected with 5 U BTX-A into the right side of the gastrocnemius muscle (Lanzhou Institute of Biological Products, Ministry of Health, Lanzhou, China; license No. (97) (Lan) S-01) or saline (0.2 mL). At 3, 6, 9, 12, 15, 18, 21 days after intramuscular injection, rats were given intraperitoneal injection of 3% acrylamide (Jiangsu Yonghua Chemical Co., Ltd., Suzhou, Jiangsu Province, China) or saline 0.1 mL. Following seven injections, the cumulative acrylamide dose reached 21 mg, which was equal to 105-131 mg/kg body weight. In the BTX-A + acrylamide, BTX-A, and acrylamide groups, every fi ve rats were randomly selected at different time points (1, 2, 3, 4, 6, 8, 10, 12, 24 weeks) after BTX-A or saline injection to assess the right hindlimb muscle strength, detect electrophysiological and morphological examinations, and quantify nerve fibers. Normal control group was assayed without any treatment. A single-blind method was adopted for the determinations.
Muscle strength
The score of muscle strength in the rat right hindlimb was evaluated according to the methods of Longa et al. (1989). 0: No neurological de fi cit symptoms; 1: rats cannot fully extend right hindlimbs; 2: rats circling; 3: rats tilt to the right side when walking; 4: rats cannot spontaneously walk or are in a coma.
Single- fi ber electromyography (EMG)
Single-fiber EMG was recorded in each rat at 1, 2, 3, 4, 6, 8, 10, 12, 24 weeks after BTX-A or saline injection. Rats were intraperitoneally anesthetized using 50 mg/mL sodium thiopental and fi xed on the operation bench. The right hindlimbs were shaved to expose the popliteal fossa. The gastrocnemius branch innervated by the sciatic nerve was disinfected with PVP-I and detected with EMG for electrophysiological examination. Stimulating needle electrodes were inserted into the gastrocnemius branches and single- fi ber recording needle electrodes were inserted into the gastrocnemius muscle belly. The inserted electrodes were continuously moving to find the appropriate single-fiber action potentials (the peak-peak amplitude > 200 μV; the time of positive peak-negative peak < 300 μs; stable continuous waveform) (Lu et al., 2000). The electrical stimulation parameters were: frequency 10 Hz, intensity 4 mA, discharge 100 times. Six single-fiber EMG images photographed in different locations of gastrocnemius muscle were collected and automatically stored on computer to analyze action potential mean consecutive difference (MCD) and muscle fi ber density. Single- fi ber EMG techniques included two parameters: jitter and fi ber density. Jitter is also known as the MCD and neuromuscular dysfunction may prolong MCD. Fiber density refers to the number of single- fi ber action potentials in the range of 300 μm electrodes, and the increasing fi ber density indicates reinnervation (Lu et al., 2000; Restivo et al., 2012).
Gastrocnemius muscle weight
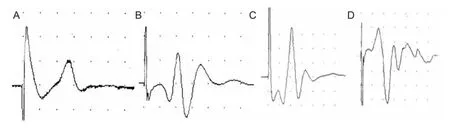
Figure 1 Single- fi ber action potential of gastrocnemius muscle of rats during nerve sprouting.
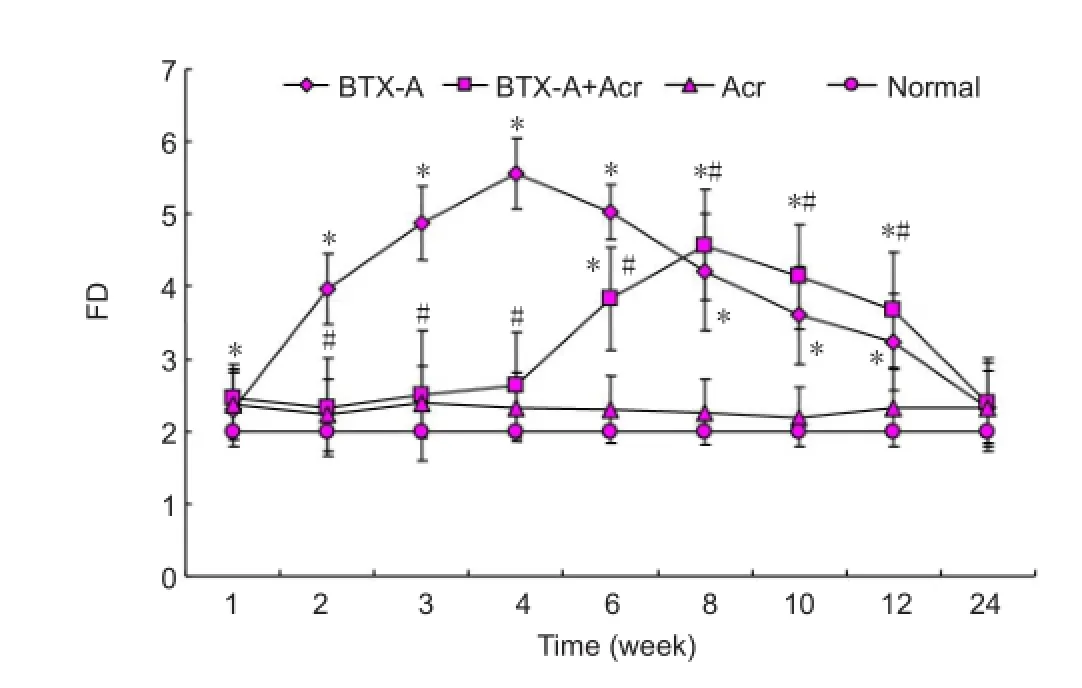
Figure 2 Fiber density of gastrocnemius muscle of rats during nerve sprouting.
After electrophysiological examination, rats were sacri fi ced by femoral artery bleeding and the bilateral gastrocnemius was quickly harvested, removing the surface fat and connective tissue. The gastrocnemius muscles were weighed using an analytical balance.
Pathological observation
At 1, 2, 3, 4, 6, 8, 10, 12, 24 weeks after BTX-A or saline injection, pathology of the gastrocnemius muscle was performed to observe nerve sprouting. The right side gastrocnemius muscle was frozen and sliced into 20 μm sections for Gros-Bielschowsky silver staining (Angaut-Petit et al., 1990). A low-power field (10 × magnification) from two slices of five rats in each group at each time point was randomly selected and observed under light microscope. The images were photographed using JVC1381 type color photography camera (Nikon, Tokyo, Japan), input into the computer, and analyzed using HPIAS-1000 high-resolution color analysis system (Tongji Medical College, Huazhong University of Science and Technology, Shanghai, China). The number of nerve fi bers per unit area was calculated. DMR microscope (Leica, Germany) was used to observe pathological fi ndings.
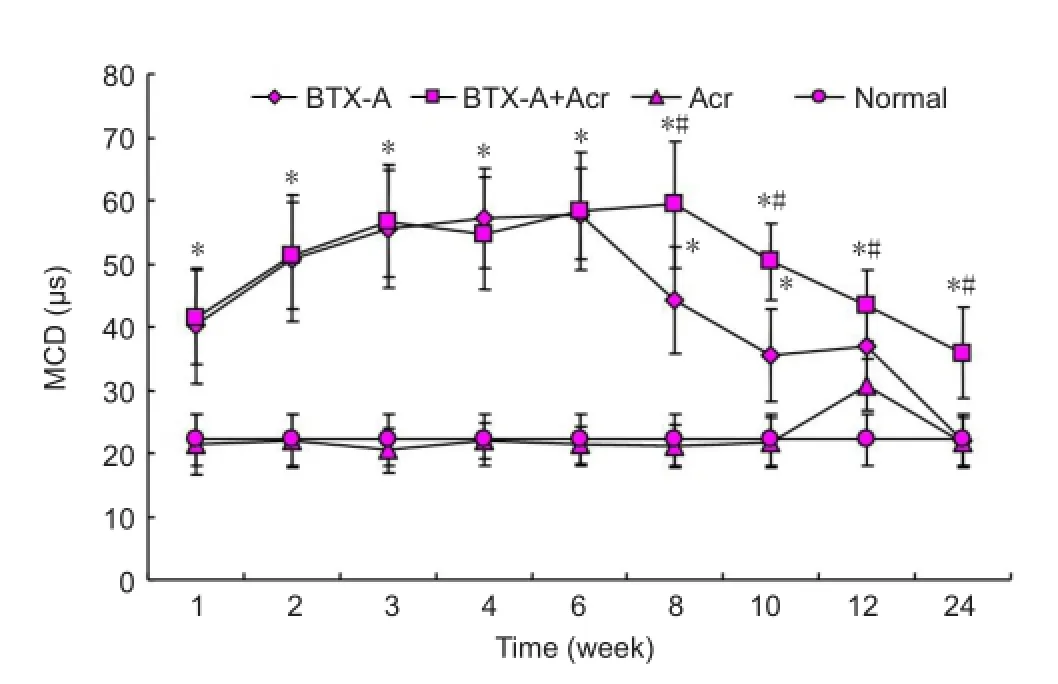
Figure 3 Changes of mean consecutive difference (MCD) of rat
Statistical analysis
Measurement data were expressed as mean ± SD, and analyzed using SPSS 21.0 software (SPSS, Chicago, IL, USA), one-way analysis of variance and the least signi fi cant difference test.P< 0.05 was considered signi fi cant.
Results
Effect of BTX-A plus acrylamide on single- fi ber EMG of rat gastrocnemius muscles
The fi ber density of the normal control group was 2.2 ± 0.4. There was no significant difference in the fiber density between acrylamide group and normal control group (P> 0.05). As shown in Figures 1 and 2, fi ber density was increased in the BTX-A group and reached a peak at 4-6 weeks (5.6 ± 0.7;P< 0.05), gradually declined, and returned to normal levels at 12 weeks, suggesting neural sprouting. After BTX-A plus acrylamide injection, fiber density decreased significantly (P< 0.05) and there was a delay in peak sprouting whichwas observed at 8-10 weeks at 4.6 ± 0.8, signi fi cantly lower than the BTX-A group. These results suggest acrylamide inhibited nerve sprouting after intramuscular injection of BTX-A.
The action potential MCD of normal control group was 33 ± 4 μs. As shown in Figure 3, acrylamide intervention alone had no impact on MCD (compared with normal control group,P> 0.05). Intramuscular injection of BTX-A prolonged the action potential MCD suggesting neuromuscular junction conduction abnormalities. Prolonged MCD was most apparent in the BTX-A group at 3-6 weeks (P< 0.05), slightly improved at 8-10 weeks and returned to normal levels at 12 weeks (compared with the control group,P> 0 05). In the BTX-A + acrylamide group, MCD was significantly longer compared with the BTX-A group at 8-12 weeks (P< 0.05), indicating BTX-A plus acrylamide delayed the functional recovery of neuromuscular junction.
Effect of BTX-A plus acrylamide on nerve fi ber counts of rat gastrocnemius muscles
As shown in Figure 4, nerve sprouting occurred after intramuscular injection of BTX-A. At 2-3 weeks after injection, thin and short sprouting nerves grew from the axonal terminal of the tibial nerve and gradually extended towards adjacent muscles. In the normal control group, the number of nerve fibers at the right side of the tibial nerve was 5.52 ± 0.09 × 108/m2. There was no signi fi cant difference in fi ber density between the acrylamide group and normal control group (P> 0.05). The number of nerve fibers increased at 2-12 weeks after BTX-A interventions (P< 0.05), and the increase was the most obvious at 4-6 weeks (P< 0.05). After injection of BTX-A plus acrylamide, the increase in the number of nerve fi bers was signi fi cantly reduced (P < 0.05), the peak time of sprouting was delayed to 8-10 weeks, and the peak value was 10.65 ± 0.32 × 108/m2(P< 0.05), which was significantly lower than that in the BTX-A group (14.33 ± 0.45 × 108/m2;P< 0.05; Figure 5). These results indicate acrylamide inhibits neural sprouting after intramuscular injection of botulinum toxin.
Effect of BTX-A plus acrylamide on muscle strength of rats
The rats showed normal muscle strength in the normal control group. Acrylamide injection alone had no obvious impact on muscle strength (compared with the normal control group,P> 0.05). One week after intramuscular injection of BTX-A, hind limb dysmyotonia appeared which began to recover at 6 weeks and restored to normal levels at 12 weeks (P> 0 05). In the BTX-A + acrylamide group, the dysmyotonia began to recover at 8-10 weeks post-injection, and restored to normal levels at 24 weeks, indicating that acrylamide delayed muscle strength recovery (Figure 6). At each time point, the muscle strength on the non-injected side was normal after the injections (BTX-A group and BTX-A + acrylamide group) and in the normal control group, suggesting that BTX-A injections into the right gastrocnemius did not cause contralateral muscle paralysis and had no distant effects.
Effect of BTX-A plus acrylamide on wet weight of gastrocnemius muscle of rats
As shown in Figure 7, the wet weight of gastrocnemius muscle in BTX-A group 1 week after BTX-A injection was 67.6% of the normal control group after saline injection (P< 0.05). Four weeks post-injection, the wet weight on the side of injection decreased to its lowest level, 41.6% of the normal control group. The weight then increased but was 51.8% at 12 weeks and 88.8% at 24 weeks when compared with the normal control group. However, the wet weight was still significantly lower than the gastrocnemius wet weight after saline injection in the normal control group (P< 0.05). The gastrocnemius wet weight was similar after injection of BTX-A plus acrylamide or BTX-A alone (P> 0.05), suggesting acrylamide does not aggravate muscle atrophy. There was no significant difference in gastrocnemius wet weight on the non-injected side between the injection groups (BTX-A group, BTX-A plus acrylamide group, acrylamide group) and the normal control group (P> 0.05). BTX-A injections into the right gastrocnemius muscle may not decrease the weight of contralateral gastrocnemius and has no distant effects.
Discussion
BTX-A exerts a selective effect on the presynaptic membrane of the neuromuscular junction, inhibiting the release of acetylcholine and relaxing the muscle (Simpson, 1981). It has been widely used for treatment in the fi elds of neurology, rehabilitation, and plastic surgery. However, the muscle relaxant effect is temporary and lasts only 3-4 months. Its mechanism of action is mediated by the sprouting of axon terminals and the reversal of presynaptic membrane proteins to restore the transmission effect of neuromuscular junction, thus achieving muscle strength recovery (Brin, 1997). There are many indexes for evaluating nerve sprouting and we chose two indicators for both pathological and electrophysiological evaluation. Gros-Bieschowsky sliver staining was used to display nerve fi bers and count nerve fi bers based on image analysis results. This counting method has been widely used (Brin, 1997; Pestronk et al., 1988; Meekins et al., 2007). Electrophysiology examination involved adopting a single- fi ber EMG, a sensitive indicator of the neuromuscular junction that can re flect electrical activity of different muscle fi bers and their motor end plates per motor unit. The measurement parameters include jitter and fi ber density, which are indicators of axonal regeneration activity (Bril et al., 1996). Jittering re flects neuromuscular function, which can be expressed as action potential MCD; increasing MCD represents neurological defects. Fiber density re flects the distribution of muscle fi bers per motor unit, collateral sprouting can rebuild motor unit, and increase muscle fi bers’ quantity and density. In this study, we used the pathology counting method and fiber density to observe the number of nerve fi bers, and measured muscular strength and action potential MCD to assess neuromuscular function.
Nerve sprouting appeared 3-5 days after intramuscular injection of BTX-A which gradually became longer, withextended branches and sprouts crossing through the paralyzed muscles and forming new neuromuscular junctions and ultimately achieving muscle contraction again with the process taking about 12 weeks (Santafe et al., 2000; Meunier et al., 2002; Shen et al., 2006; Harrison et al., 2011). In this study, we observed the nerve sprouting process by counting nerve fibers and detecting neuromuscular function. The number of nerve sprouts was quanti fied from sections and fi ber density was detected by single- fi ber EMG. The results showed that nerve sprouting appeared after intramuscular injection of BTX-A and declined at 6 weeks. This is inconsistent with the study by Santafe et al., who found nerve sprouting continuously increased for 12 months post-injection. The decline at 6 weeks is presumably due to the mature function of neuromuscular junction inducing a signal for the closure of sprouting. It is also the result of excessive sprout degeneration, ensuring that a muscle fiber is only innervated by one or two synapses. Neuromuscular junction function is mainly indicated by MCD. The results of this study showed that MCD increased 1 week after BTX-A injection, signi fi cantly increased at 3-6 weeks, then slightly improved at 8-10 weeks, and restored to normal levels at 12 weeks. The increasing MCD is attributed to at least two mechanisms: (1) BTX-A directly acts on neuromuscular junction; (2) new neuromuscular junction formed by nerve sprouting is immature. During the nerve sprouting process, the immature neuromuscular junction slows conduction impulse more than the originally located motor units, so MCD is increased; while mature neuromuscular function leads to normalized MCD. Muscle strength recovery observed in this study indicated the maturity of neuromuscular junction. Our experimental fi ndings were consistent with the results of Santafe and Harrison et al., who found that the neuromuscular junction became mature at 12 weeks (Santafe et al., 2000; Meunier et al., 2002; Harrison et al., 2007, 2011). In summary, intramuscular injection of BTX-A triggers nerve sprouting, and neuromuscular junction can be reconstructed at 12 weeks.
The results of this study suggest acrylamide inhibits nerve sprouting after intramuscular injection of BTX-A. Quantifi cation of nerve sprouting and nerve fi ber density showed that acrylamide delayed the peak of nerve sprouting after intramuscular injection of BTX-A and generally inhibited nerve sprouting. In BTX-A plus acrylamide group, fiber density was increased within 12 weeks, but the density was lower than BTX-A group and the increasing trend was not obvious at 2-6 weeks; in the BTX-A plus acrylamide group, fi ber density peaked at 8 weeks, which was 4 weeks later than BTX-A group; additionally, fi ber density peak value was lower than the BTX-A group. Nerve fi ber counts were generally consistent with the fiber density results. MCD detection results also demonstrated that, acrylamide inhibited the recovery of neuromuscular function. There was no signi fi cant difference between BTX-A group and BTX-A plus acrylamide group at 1-6 weeks post-injection, but the MCD was significantly abnormal in BTX-A plus acrylamide group at 8-12 weeks. Meanwhile, the muscle strength monitored was similar to the detection results of MCD. This illustrated that neuromuscular function recovery in BTX-A + acrylamide group was significantly slower than that in BTX-A group. Both the number of nerve sprouts (anatomy) and immaturity of nerve sprouting (function) suggest that acrylamide inhibited nerve sprouting after intramuscular injection of BTX-A. This effect may be related to the neurotoxicity of acrylamide. Previous studies have reported that the mechanisms associated with acrylamide-induced peripheral nerve injury include inhibition of axonal transport, inhibition of energy metabolism, and calcium overload (Matthew et al., 1983; Costa et al., 1995; Hao et al., 2000; Dale et al., 2002; Sickles et al., 2002; Lopachin et al., 2008; Zhu et al., 2008; Scelsi et al., 2012; Prasad et al., 2013; Xiao et al., 2013). We speculate that these factors lead to a de fi ciency of energy and raw materials at the axonal terminals, and accordingly inhibit nerve sprouting after intramuscular injection of BTX-A. Further studies are needed to explore the mechanisms.
Although acrylamide inhibited nerve sprouting at the gastrocnemius muscle, it had no impact on neuromuscular function in normal muscle and did not induce neurological impairment (such as hindlimb weakness, inability to walk, and muscle atrophy) and nerve regeneration after injury. This can be explained by the different doses and neurotoxicity of acrylamide. Acrylamide poisoning is determined by the cumulative dose: rats exposed to cumulative doses of 75 mg/kg have slowed axonal transport, 180 mg/kg acrylamide slowed down nerve conduction velocity, and 225 mg/kg caused neurological symptoms (such as hindlimb weakness) (Li et al., 1989; Zhang et al., 2008). In this study, exposure dose reached 105-131 mg/kg. Second, there are differences in the sensitivity of peripheral nerve to poisoning. Previous studies found that the symptoms of poisoning were most apparent in the lower extremity of experimental animals or humans because thick myelinated fi bers are more prone to damage. Among peripheral nerves, the sciatic nerve is the most sensitive and axonal degeneration fi rst appears in the gastrocnemius muscle, therefore the sciatic nerve accumulates more poison than other sites (Dong et al., 1994).
In summary, acrylamide could inhibit nerve sprouting after intramuscular injection of BTX-A, which explores a novel way for prolonging the ef fi cacy of BTX-A and solving nerve sprouting following intramuscular injection of BTX-A. Any clinical signi fi cance needs further study. Acrylamide has potential neurotoxicity, genotoxicity and carcinogenicity, which needs further investigation at the injection way, optimal dose and systemic toxicity. The mechanism of action associated with BTX-A triggering nerve sprouting remains unclear, but at least four cytokines are involved in the process of nerve sprouting, including insulin-like growth factor, nerve interleukin, ciliary neurotrophic factor, and cell adhesion molecules (Caroni et al., 1994a, b; English, 2003; Carli et al., 2009). Further studies are needed to investigate the molecular mechanisms of neural sprouting, to fi nd a feasible method to inhibit neuronal sprouting, and to prolong the ef fi cacy and duration of BTX-A (Harrison et al., 2011; Guo et al., 2013).
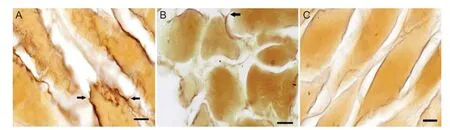
Figure 4 Nerve sprouting after intramuscular injection of botulinum toxin type A in the right side of rat gastrocnemius muscles (Gros-Bielschowsky silver staining, × 200).
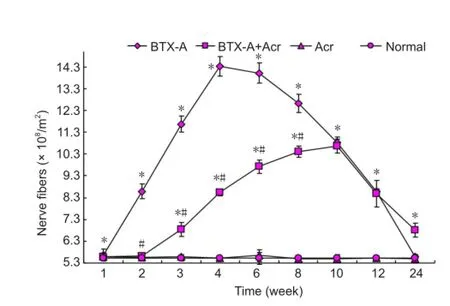
Figure 5 Effect of botulinum toxin type A (BTX-A) and acrylamide (Acr) on tibial nerve fi bers count in rats.
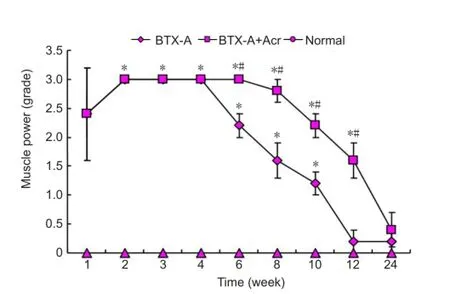
Figure 6 Effect of botulinum toxin type A (BTX-A) plus acrylamide (Acr) on muscle strength of rats.
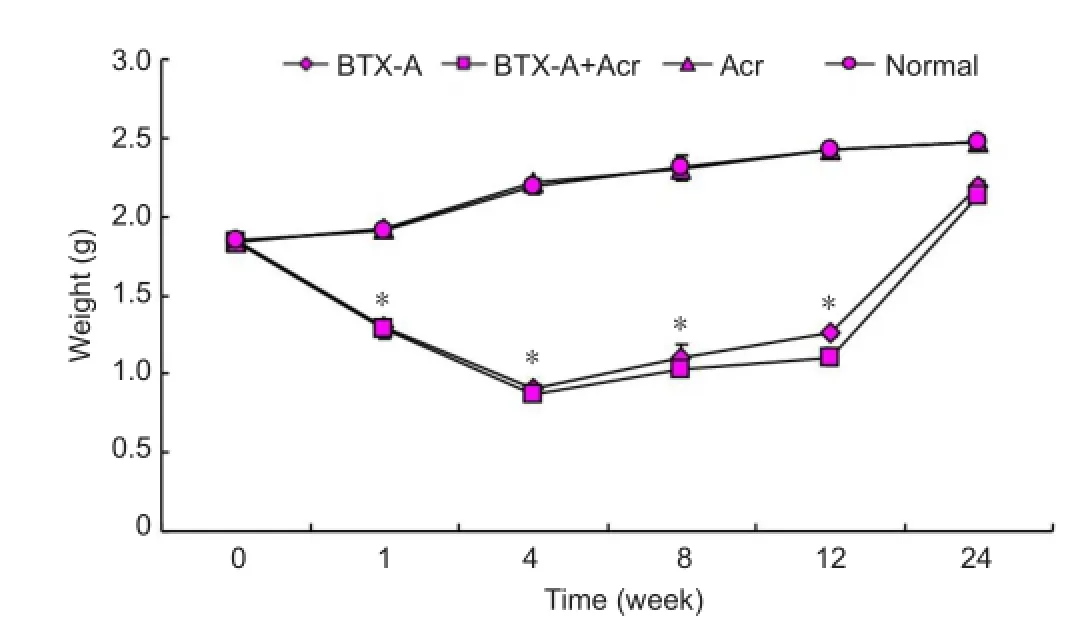
Figure 7 The wet weight of right gastrocnemius muscle of rats after BTX-A injection.
Author contributions:Jiang H was responsible for electro-physiological examination, performing statistical analysis, and writing the manuscript. Cai HY conducted pathological exam-ination, designed the study and implemented the experiment. Hu XY and Xiang Y instructed the experiments. All authors approved the final version of the manuscript.
Acknowledgments:We would like to thank Dr. Shao YQ from Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, China for guiding neuropathological staining.
Con flicts of interest:None declared.
Allison R, Knapp KM (2012) Spasticity management with botulinum toxin: development and evaluation of a tool for audit. J Rehabil Med 44:558-561.
Angaut-Petit D, Molgo J, Comella JX, Faille L, Tabti N (1990) Terminal sprouting in mouse neuromuscular junctions poisoned with botulinum type A toxin: morphological and electrophysiological features. Neuroscience 37:799-808.
Bertorini TE, Stalberg E, Yuson CP, Engel WK (1994) Single- fi ber electromyography in neuromuscular disorders: correlation of muscle histochemistry, single- fi ber electromyography, and clinical fi ndings. Muscle Nerve 17:345-353.
Blake KD, MacCuspie J, Corsten G (2012) Botulinum toxin injections into salivary glands to decrease oral secretions in CHARGE syndrome: prospective case study. Am J Med Genet A 158:828-831.
Blasi J, Chapman ER, Link E, Binz T, Yamasaki S, De Camilli P, Sudhof T, Niemann H, Jahn R (1993) Botulinum neurotoxin A selectively cleaves the synaptic protein SNAP-25. Nature 365:160-163.
Bragg DC, Sharma N (2014) Update on treatments for dystonia. Curr Neurol Neurosci Rep 14:454.
Bril V1, Werb MR, Greene DA, Sima AA (1996) Single- fi ber electromyography in diabetic peripheral polyneuropathy. Muscle Nerve 19:2-9.
Brin MF (1997) Botulinum toxin: chemistry, pharmacology, toxicity, and immunology. Muscle Nerve 6:S146-168, 20:146-168.
Carli L, Montecucco C, Rossetto O (2009) Assay of diffusion of different botulinum neurotoxin type A formulations injected in the mouse leg. Muscle Nerve 40:374-380.
Caroni P, Schneider C (1994a) Signaling by insulin-like growth factors in paralyzed skeletal muscle: rapid induction of IGFI expression in muscle fi bers and prevention of interstitial cell proliferation by IGFBP5 and IGF-BP4. J Neurosci 4:3378-3388.
Caroni P, Schneider C, Kiefer MC, Zapf J (1994b) Role of muscle insulin like growth factors in nerve sprouting: suppression of terminal sprouting in paralyzed muscle by IGF-binding protein 4. J Cell boil 125:893-902.
Chen S (2012) Clinical uses of botulinum neurotoxins: current indications,limitations and future developments. Toxins (Basel) 4:913-939.
Costa LG, Deng H, Calleman CJ, Bergmark E (1995) Evaluation of the neurotoxicity of glycidamide, an epoxide metabolite of acrylamide: behavioral neurochemical and morphological studies. Toxicology 98:151-161.
Dale WS, Derek S, Marvin AF (2002) Fast axonal transport: a site of Acrylamide: a rebuttal. Neurotoxicology 23:265-270.
De Paiva A, Meunier FA, Molgo J, Aoki KR, Dolly JO (1999) Functional repair of motor endplates after botulinum neurotoxin type A poisoning: biphasic switch of synaptic activity between nerve sprouts and their parent terminals. Proc Natl Acad Sci U S A 96:3200-3205.
Dong XM, Jiao XY, He FS (1989) Research of nerve pathology in rats poisoned with acrylamide. Weisheng Yanjiu 18:44-48.
English AW (2003) Cytokines, growth factors and sprouting at the neuromuscular junction. J Neurocytol 32:943-960.
Guo Y, Jin LJ, Liu WC, Zheng YG, Guan Q, Pan LZ, Nie ZY (2013) The impact of polyclonal neural cell adhesion molecule antibody on the potency of botulinum toxi. Yixue yu Kangfu Zazhi 35:833-838.
Hao QY, Han MF, Rao ML (2000) Effect of acrylamide on the ions in nerve tissue of mouse. Zhongguo Gongye Yixue Zazhi 13:265-267.
Harrison AR, Anderson BC, Thompson LV, McLoon LK (2007) Myofi ber length and three-dimensional localization of NMJs in normal and botulinum toxin treated adult extraocular muscles. Invest Ophthalmol Vis Sci 48:3594-3601.
Harrison AR, Berbos Z, Zaldivar RA, Anderson BC, Semmer M, Lee MS, McLoon LK (2011) Modulating neuromuscular junction density changes in botulinum toxin-treated orbicularis oculi muscle. Invest Ophthalmol Vis Sci 52:982-986.
Ho MC, Hsu WC, Hsieh YT (2014) Botulinum toxin type a injection for lateral canthal rhytids: effect on tear fi lm stability and tear production. JAMA Ophthalmol 132:332-337.
Holds JB, Alderson K, Fogg SG, Anderson RL (1990) Motor nerve sprouting in human orbicularis muscle after botulinum A injection. Invest Ophthalmol Vis Sci 31:964-967.
Juzans P, Comella JX, Molgo J, Faille L, Angaut-Petit D (1996) Nerve terminal sprouting in botulinum type-A treated mouse levator auris longus muscle. Neuromuscul Disord 6:177-185.
Li G, Zhang SL, He FS (1989) Research of nerve electrophysiology in rats poisoned with acrylamide. Weisheng Yanjiu 18:4-6.
Longa EZ, Weinstein PR, Carlson S, Cummins R (1989) Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20:84-91.
Lopachin RM, Gavin T (2008) Acrylamide-induced nerve terminal damage: relevance to neurotoxic and neurodegenerative mechanisms. J Aqric Food Chem 56:5994-6003.
Lu ZN, Wang ZZ (2000) Practical Electromyography. Beijing: People’s Health Publishing House 1:579-585.
Matthew SM, Mary JM, Thomas FB (1983) Altered retrograde axonal transport of nerve growth factor after single and repeated dose of Acrylamide in the rat. Toxicol Appl Pharmacol 69:96-101.
Meekins GD, Carter GT, Emery MJ, Weiss MD (2007) Axonal degeneration in the Trembler-j mouse demonstrated by stimulated single- fiber electromyography. Muscle Nerve 36:81-86.
Meunier FA, Schiavo G, Molgo J (2002) Botulinum neurotoxins: from paralysis to recovery of functional neuromuscular transmission. J Physiol Paris 96:105-113.
Pestronk A, Drachman DB (1988) Motor nerve outgrowth: reduced capacity for sprouting in the terminals of longer axons. Brain Res 463:218-222.
Pimentel LH, Alencar FJ, Rodrigues LR, de Sousa FC, Mendes JB (2014) Effects of botulinum toxin type A for spastic foot in post-stroke patients enrolled in a rehabilitation program. Arq Neuropsiquiatr 72:28-32.
Prasad SN, Muralidhara (2013) Neuroprotective efficacy of eugenol and isoeugenol in acrylamide-induced neuropathy in rats: behavioral and biochemical evidence. Neurochem Res 38:330-345.
Rashid TG, Ockrim JL (2013) Male incontinence: onabotulinum toxin A and sacral nerve stimulation. Curr Opin Urol 23:545-551.
Restivo DA, Pavone V, Nicotra A (2012) Single- fi ber electromyography in hyper C Kemia: the value of fi ber density. Neurol Sci 33:819-824.
Rossetto O, Morbiato L, Caccin P, Rigoni M, Montecucco C (2006) Presynaptic enzymatic neurotoxins. J Neurochem 97:1534-1545.
Santafe MM, Urbano FJ, Lanuza MA, Uchitel OD (2000) Multiple types of calcium channels mediate transmitter release during functional recovery of botulinum toxin type A-poisoned mouse motor nerve terminals. Neuroscience 95:227-234.
Scelsi R, Candura SM (2012) Occupational toxic neuropathies: morphology in peripheral nerve biopsies. G Ital Med Lav Ergon 3:410-419.
Shen J, Ma J, Lee C, Smith BP, Smith TL, Tan KH, Koman LA (2006) How muscles recover from paresis and atrophy after intramuscular injection of botulinum toxin A: study in juvenile rats. J Orthop Res 24:1128-1135.
Sickles DW, Stone JD, Friedman MA (2002) Fast axonal transport a site of acrylamide neurotoxicity. Neurotoxicology 23:223-251.
Simpson LL (1981) The origin, structure, and pharmacological activity of botulinum toxin. Pharmacol Rev 33:155-188.
Thenganatt MA, Jankovic J (2014) Treatment of dystonia. Neurotherapeutics 11:139-152.
Villafa?e JH, Fernandez-de-Las-Pe?as C, Pillastrini P (2012) Botulinum toxin type A combined with cervical spine manual therapy for masseteric hypertrophy in a patient with Alzheimer-type dementia: a case report. J Chiropr Med 11:280-285.
Winocour S, Murad MH, Bidgoli-Moghaddam M, Jacobsona S, Bitea U, Saint-Cyra M, Trana N, Lemainea V (2014) A systematic review of the use of Botulinum toxin type A with subpectoral breast implants. J Plast Reconstr Aesthet Surg 67:34-41.
Wright MC, Cho WJ, Son YJ (2007) Distinct patterns of motor nerve terminal sprouting induced by ciliary neurotrophic factor vs botulinum toxin. J Comp Neurol 504:1-16.
Xiao JW, Niu Y, Zhang ZR, Yu CY, Li ZS (2013) Changes of ATPase in dorsal root ganglion induced by acrylamide. J Toxicol 27:235-238.
Zhang YQ,Yao L, Wang GZ, Zhang YS (2008) Changes of neurobehavior function and electrophysiology in rats exposed to acrylamide. Chin Occup Med 35:278-280.
Zhu YJ, Zeng T, Zhu YB, Yu SF, Wang QS, Zhang LP, Guo X, Xie KQ (2008) Effects of acrylamide the nervous tissue antioxidant system and sciatic nerve electrophysiology in the rat. Neurochem Res 33:2310-2317.
Copyedited by Paul P, Norman C, Yang Y, Wang J, Li CH, Song LP, Zhao M
Huaying Cai, M.D., Ph.D., Department of Neurology, Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, Hangzhou 310016, Zhejiang Province, China, caihuaying2004@163.com.
10.4103/1673-5374.139479
http://www.nrronline.org/
Accepted: 2014-07-03
- 中國神經(jīng)再生研究(英文版)的其它文章
- Neuroprotective effects of berry fruits on neurodegenerative diseases
- Brain structural changes and their correlation with vascular disease in type 2 diabetes mellitus patients: a voxel-based morphometric study
- T cells promote the regeneration of neural precursor cells in the hippocampus of Alzheimer’s disease mice
- Cutaneous sensory nerve as a substitute for auditory nerve in solving deaf-mutes’ hearing problem: an innovation in multi-channel-array skin-hearing technology
- Transplantation of neurotrophin-3-transfected bone marrow mesenchymal stem cells for the repair of spinal cord injury
- Shifting balance from neurodegeneration to regeneration of the brain: a novel therapeutic approach to Alzheimer’s disease and related neurodegenerative conditions

