T cells promote the regeneration of neural precursor cells in the hippocampus of Alzheimer’s disease mice
Jing Liu, Yuxin Ma, Sumin Tian, Li Zhang, Mengmeng Zhao, Yaqiong Zhang, Dachuan Xu
1 Department of Human Anatomy, School of Basic Medical Sciences, Southern Medical University, Guangzhou, Guangdong Province, China
2 Department of Human Anatomy, School of Basic Courses, Guangdong Pharmaceutical University, Guangzhou, Guangdong Province, China
T cells promote the regeneration of neural precursor cells in the hippocampus of Alzheimer’s disease mice
Jing Liu1,2, Yuxin Ma2, Sumin Tian2, Li Zhang2, Mengmeng Zhao2, Yaqiong Zhang2, Dachuan Xu1
1 Department of Human Anatomy, School of Basic Medical Sciences, Southern Medical University, Guangzhou, Guangdong Province, China
2 Department of Human Anatomy, School of Basic Courses, Guangdong Pharmaceutical University, Guangzhou, Guangdong Province, China
Alzheimer’s disease is closely associated with disorders of neurogenesis in the brain, and growing evidence supports the involvement of immunological mechanisms in the development of the disease. However, at present, the role of T cells in neuronal regeneration in the brain is unknown. We injected amyloid-beta 1-42 peptide into the hippocampus of six BALB/c wild-type mice and six BALB/c-nude mice with T-cell immunode fi ciency to establish an animal model of Alzheimer’s disease. A further six mice of each genotype were injected with same volume of normal saline. Immunohistochemistry revealed that the number of regenerated neural progenitor cells in the hippocampus of BALB/c wild-type mice was signi fi cantly higher than that in BALB/c-nude mice. Quantitative fluorescence PCR assay showed that the expression levels of peripheral T cell-associated cytokines (interleukin-2, interferon-γ) and hippocampal microglia-related cytokines (interleukin-1β, tumor necrosis factor-α) correlated with the number of regenerated neural progenitor cells in the hippocampus.ese results indicate that T cells promote hippocampal neurogenesis in Alzheimer’s disease and T-cell immunode fi ciency restricts neuronal regeneration in the hippocampus. The mechanism underlying the promotion of neuronal regeneration by T cells is mediated by an increased expression of peripheral T cells and central microglial cytokines in Alzheimer’s disease mice. Our fi ndings provide an experimental basis for understanding the role of T cells in Alzheimer’s disease.
nerve regeneration; neurodegeneration; Alzheimer’s disease; beta-amyloid 1-42 peptide; neuronal precursors; mice; microglia; interleukin-2; interferon-gamma; interleukin-1β; tumor necrosis factor-α; microtubule associated protein; NSFC grant; neural regeneration
Funding:This study was supported by the National Natural Science Foundation of China, No. 30840073; the Medical Science Foundation of Guangdong Province, No. A2012298.
Liu J, Ma YX, Tian SM, Zhang L, Zhao MM, Zhang YQ, Xu DC. T cells promote the regeneration of neural precursor cells in the hippocampus of Alzheimer’s disease mice. Neural Regen Res. 2014;9(16):1541-1547.
Introduction
Alzheimer’s disease (AD), a degenerative disease of the central nervous system, is characterized pathologically by extracellular senile plaques, intracellular neurofibrillary tangles, and a reduction in the number of neurons in the cerebral cortex and hippocampus. The clinical manifestations for AD include loss of memory, and cognitive and behavioral disorders (Finder, 2010). AD is the most common type of dementia, but its etiology and pathogenesis remain unclear. At present, AD is considered a complex pathological process involving several factors. The amyloid cascade hypothesis, or amyloid-beta (Aβ) toxicity hypothesis, has dominated research for decades, and postulates that the deposition of Aβ peptide in the brain is a central event in AD (Honjo et al., 2012). Although AD is not recognized as a classic immune response-mediated disease, growing evidence highlights the immunological mechanisms closely involved in the occurrence and development of AD (Schroeter et al., 2008; Tabira, 2010). According to the immune hypothesis of AD pathogenesis, when immune dysfunction occurs, Aβ metabolism is disrupted. Subsequently, inflammatory and neurotoxic cascade reactions occur, leading to synaptic damage, and neuronal degeneration and death in the brain, ultimately inducing AD (Chopra et al., 2011).
As the mechanism underlying neuronal death in AD has been investigated, researchers have begun to focus on the opposite aspect, newborn neurons, in AD pathogenesis (Donovan et al., 2006; Zhang et al., 2007; Yu et al., 2009; Biscaro et al., 2012). The precursor cells in the subventricular zone of the brain have the ability to regenerate (Alvare-Buylla et al., 2004). In adults, neurogenesis provides a specific mechanism for plasticity of the nervous system (Lazarov et al., 2010). AD pathology studies have revealed that damage to the regions where adult neural cells form (subventricular zone and subgranular zone) leads to a dysfunction in neuronal regeneration; if the dead neurons cannot be replaced by new neurons in time, memory and cognitive disorders will inevitably occur (Demars et al., 2010). AD pathogenesis is closely associated with disorders of neuronal regeneration in the brain, and the effect of immunological mechanisms on neuronal regeneration has become a focus of current AD research. Patients with AD have a signi fi cantly higher num-ber of T cells in the brain than healthy people. Immune cells cross the blood-brain barrier and enter the brain, participating in its physiological and pathological functions (Togo et al., 2002; Cao et al., 2009; Monsonego et al., 2013). Additionally, immune cells are shown to maintain nerve cell regeneration function (Ziv et al., 2008). Central-speci fi c T cells play an important role in the maintenance of adult learning and memory capacity, and a deficiency of T cells leads to severe impairments in spatial learning and memory in adult rats (Ziv et al., 2006). We hypothesize that, in AD pathology, T cells are involved in the maintenance of nervous system plasticity, which is also related to neuron regeneration. To our knowledge, no studies have examined the correlation between T cells and neuronal regeneration in the brain. Therefore, the aim of the present study was to investigate the role of T cells in hippocampal neurogenesis in AD pathogenesis, and the underlying molecular mechanisms, in an effort to reveal the contribution of T cells in neuronal regeneration, using immunohistochemistry and quantitative PCR techniques.
Materials and Methods
Animals
Twelve BALB/c wild-type (WT) mice and 12 BALB/c-nude mice, all specific pathogen free, with T lymphocyte deficiency were provided by Guangdong Medical Laboratory Animal Center, China (license No. SCXK (Yue) 2008-0002). The mice were all male, aged 8 weeks, weighing 20-28 g, and housed for 1 week prior to experimentation. Experimental procedures were in accordance with the Guidelines of the Use of Experimental Animals, issued by the Ministry of Science in China.
Animal grouping
The mice were randomly divided into an experimental group and a control group (n= 6 per group). In experimental group I (WT + Aβ) and experimental group II (nude + Aβ), oligomeric state Aβ1-42was injected bilaterally into the hippocampal CA1 region, to establish a model of AD. In control group I (WT + NS) and control group II (nude + NS), mice received equivalent volumes of normal saline instead of Aβ1-42.
On day 7 after modeling, peripheral blood samples collected from the mice were harvested for quantitative PCR detection of interleukin-2 (IL-2) and interferon-γ (IFN-γ) expression. The mouse brain was divided symmetrically along the midline.e lehemisphere was used for immunohistochemistry of hippocampal neuronal regeneration, and the right for quantitative PCR assay of interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) expression in hippocampal tissue.
Establishment of AD models using hippocampal injection of Aβ1-42
To prepare oligomeric state Aβ1-42, freeze-dried Aβ1-42powder (500 μg; AnaSpec, San Jose, CA, USA) was dissolved in 100 μL of 1% NH4OH solution for a stock solution at a concentration of 500 μg/100 μL, which was then aliquoted (50 μg/10 μL) and stored at -20°C. At the time of experimentation, an aliquot was thawed and 15 μL normal saline was added to prepare the working solution (2 μg/μL, 50 μg/25 μL), which was incubated at 37°C for 24 hours. This allowed aggregation of Aβ1-42to toxic oligomeric Aβ (Dahlgren et al., 2002).
Mice were anesthetized by intraperitoneal injection of 0.4% sodium pentobarbital at a dose of 0.2 mL/10 g body weight. The heads were fixed onto a stereotaxic frame, and then the skull was drilled to create a hole at 2.3 mm posterior to bregma and 1.8 mm lateral to the midline, to 1.0 mm depth. A 25 μL microsyringe was inserted 2.0 mm into the brain, and 2 μL Aβ1-42working solution (experimental groups I and II) or saline (control groups I and II) was slowly (0.4 μL/minute) injected bilaterally into the hippocampal CA1viamicropipette (KDS Model 310 Plus, KD Scientific Holliston, MA, USA). The needles were maintained in place for 5 minutes and then slowly withdrawn to prevent leakage.e skin was sutured and disinfected with alcohol, followed by intramuscular injections of sodium penicillin (40,000 units) for 3 consecutive days. For the remainder of the experiment, mice were housed in speci fi c-pathogen-free cages.
Harvesting the specimens
The brain tissue was harvested 7 days after injection. In brief, mice were anesthetized with 0.4% sodium pentobarbitalviaintraperitoneal injection, and 1 mL cardiac blood was collected and placed into a tube containing the anticoagulant EDTA.e sample was stored at -20°C for gene expression analysis. Aer the blood sample was collected, the mice were quickly decapitated, and the brain was removed and cut in two along the middle.e lehemisphere was fixed in 4% paraformaldehyde and embedded in paraffin for the detection of hippocampal neuronal regeneration. The right hippocampus was removed and preserved in pre-cooling preservation tubes, then frozen in liquid nitrogen and stored at -80°C for the detection of microglial cytokine expression.
Immunohistochemistry of doublecortin (DCX) expression in hippocampal neurons
Fluorescence quantitative PCR detection of mRNA expression in peripheral blood and hippocampus of mice
Specific primers in the coding region were designed using Primer Express v2.0 software (Applied Biosystems, Foster, CA, USA) according to the target gene mRNA sequences in GenBank.e primers were synthesized using the ABI 3900 High-roughput DNA synthesizer (Applied Biosystems).
Primer sequences and product sizes are as follows:
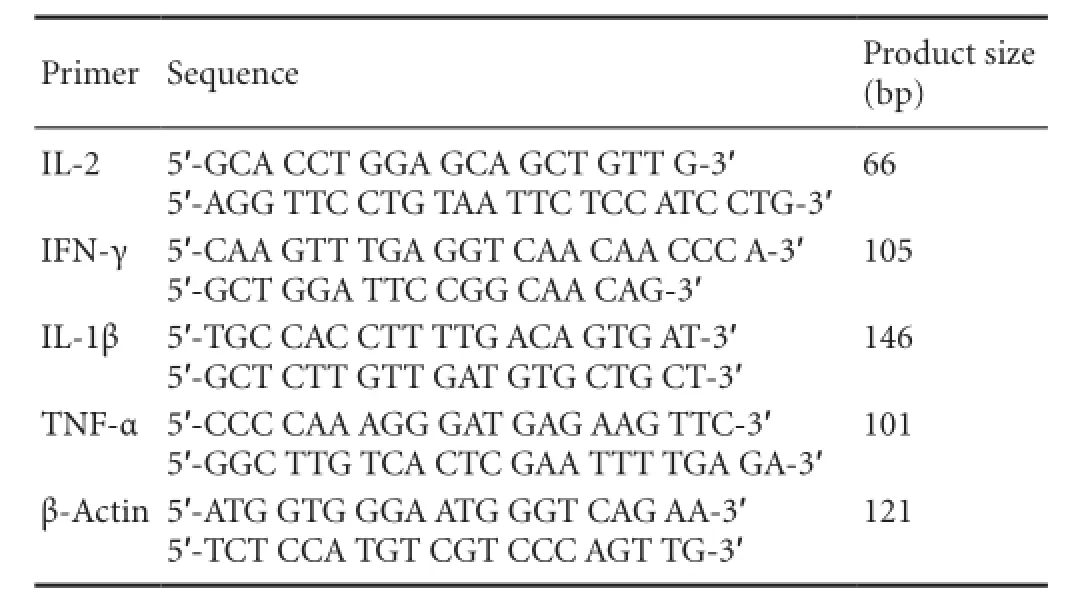
Primer Sequence Product size (bp) IL-2 5′-GCA CCT GGA GCA GCT GTT G-3′5′-AGG TTC CTG TAA TTC TCC ATC CTG-3′66 IFN-γ 5′-CAA GTT TGA GGT CAA CAA CCC A-3′5′-GCT GGA TTC CGG CAA CAG-3′105 IL-1β 5′-TGC CAC CTT TTG ACA GTG AT-3′5′-GCT CTT GTT GAT GTG CTG CT-3′146 TNF-α5′-CCC CAA AGG GAT GAG AAG TTC-3′5′-GGC TTG TCA CTC GAA TTT TGA GA-3′101 β-Actin 5′-ATG GTG GGA ATG GGT CAG AA-3′5′-TCT CCA TGT CGT CCC AGT TG-3′121
Statistical analysis
Data were analyzed using SPSS 16.0 statistical soware (SPSS, Chicago, IL, USA) and expressed as mean ± SD. Groups were compared using one-way analysis of variance followed by the least signi fi cant di ff erence tests. Statistical signi fi cance was set atP< 0.05.
Results
Neuronal regeneration in the hippocampus of AD mice
The mice were injected with Aβ1-42peptide or normal saline for 7 days, and neuronal regeneration in the hippocampus was examined using DCX immunohistochemistry (Figure 1). Under a high magni fi cation microscope, DCX-immunopositive cells were stained brown or yellow, with unilateral radial fi lament-like projections. The results revealed signi fi cant differences in DCX expression between the groups (F= 460.707,P< 0.01). In the WT groups (Figure 1A, C), the number of positive cells was signi fi cantly higher than that in the nude groups (Figure 1B, D). DCX expression was the highest in the WT + NS group, then in the WT + Aβ group, nude + NS group, and the lowest in the nude + Aβ group. Statistical analysis showed that the WT groups had a signi fi cantly higher number of DCX-positive cells than the nude groups (P< 0.01), and that nude + Aβ mice had a higher expression of DCS than nude + NS mice (P< 0.01; Figure 2).e fi ndings con fi rm that injection of Aβ1-42peptide inhibits the regeneration of hippocampal neurons. Furthermore, they indicate that T cells promote hippocampal neuron regeneration, and that such regeneration does not notably occur without T cells.
IL-2 and IFN-γ expression in peripheral blood of AD mice
To explore the correlation between hippocampal neuronal regeneration and T cell-associated cytokine expression in AD mice, we measured the expression of cytokines in peripheral blood using quantitative PCR 7 days aer modeling. A significant difference in IL-2 gene expression was found (F= 120.109,P< 0.01). IL-2 expression in the WT mice was significantly higher than that in nude mice (P< 0.01; Figure 3A). The results of IL-2 expression were consistent with those of DCX expression in each group, suggesting that IL-2 expression might be related to neuronal regeneration. Signi fi cant di ff erences in IFN-γ expression were also found among the groups (F= 55.663,P< 0.01). IFN-γ expression was greatest in the WT + NS group, and signi fi cantly lower in the WT + Aβ and nude + NS groups (P< 0.01; Figure 3B).ese results indicate that intrahippocampal injection of Aβ1-42 inhibited the expression of IFN-γ in BALB/c-WT mice, and that the BALB/c-nude genotype was associated with lower expression of IFN-γ.
IL1-β and TNF-α gene expression in the hippocampus of AD mice
To further explore the possible mechanism of T cells on neuronal regeneration in AD mouse models, we used quantitative PCR to measure the expression of microglial cytokines in the hippocampus 7 days aer modeling (Figure 4). Significant di ff erences in IL-1β gene expression were found among the groups (F= 1217.713,P< 0.01). Expression levels of IL-1β and TNF-α in wild-type mice were higher than those in nude mice (P< 0.01).e highest level of IL-1β and TNF-α expression was found in the WT + Aβ group, suggesting that hippocampal injection of Aβ peptides may producesome toxicity, and that WT mice with normal immune function react more severely to Aβ peptide (and produced more cytokines) than nude mice with T-cell immunodeficiency, which respond weakly to Aβ peptide, producing fewer reactive cytokines.
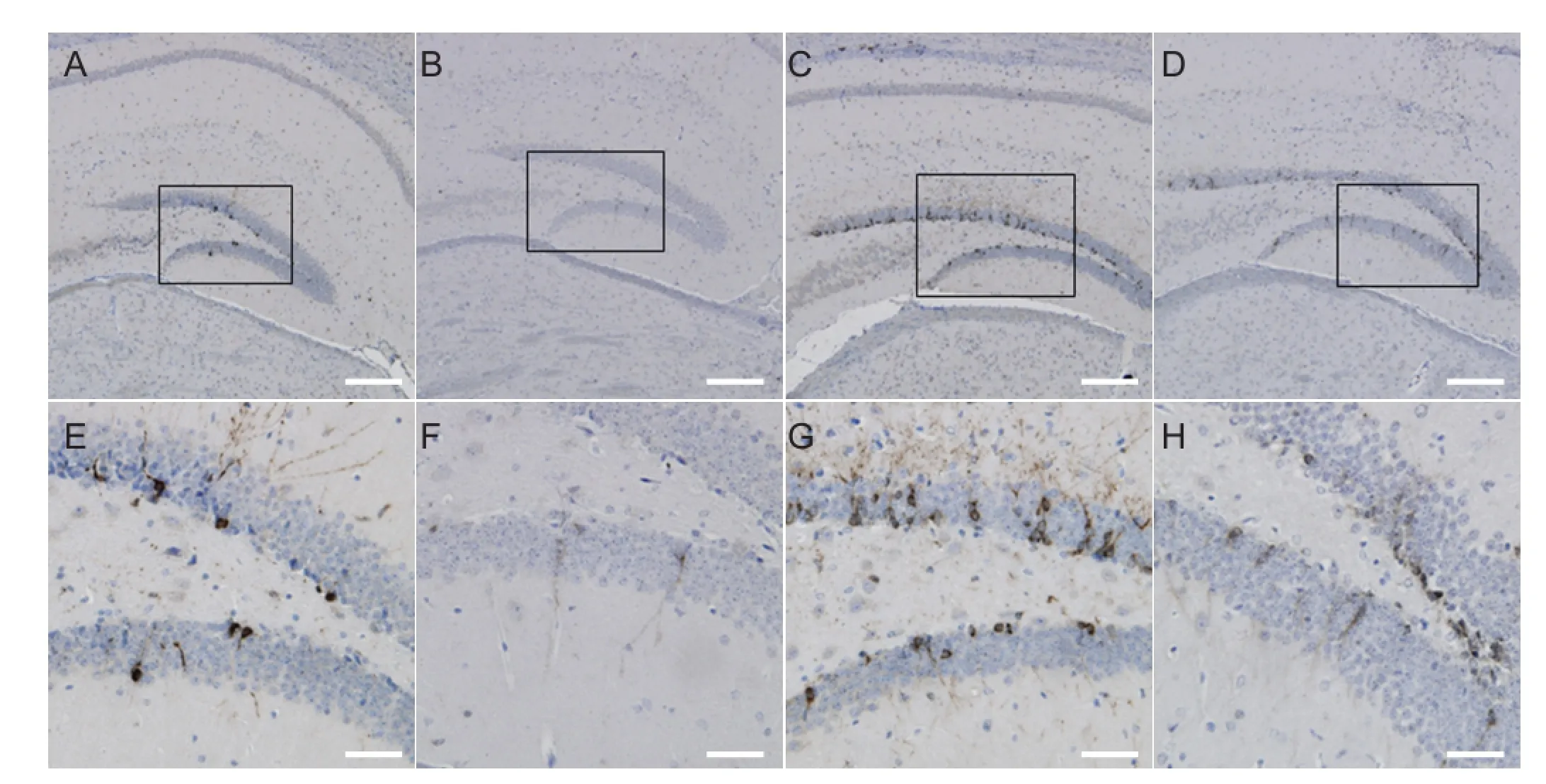
Figure 1 Immunolabeling for doublecortin-positive (newborn) neurons in the hippocampal dentate gyrus 7 days after hippocampal injection of Aβ1-42or saline.
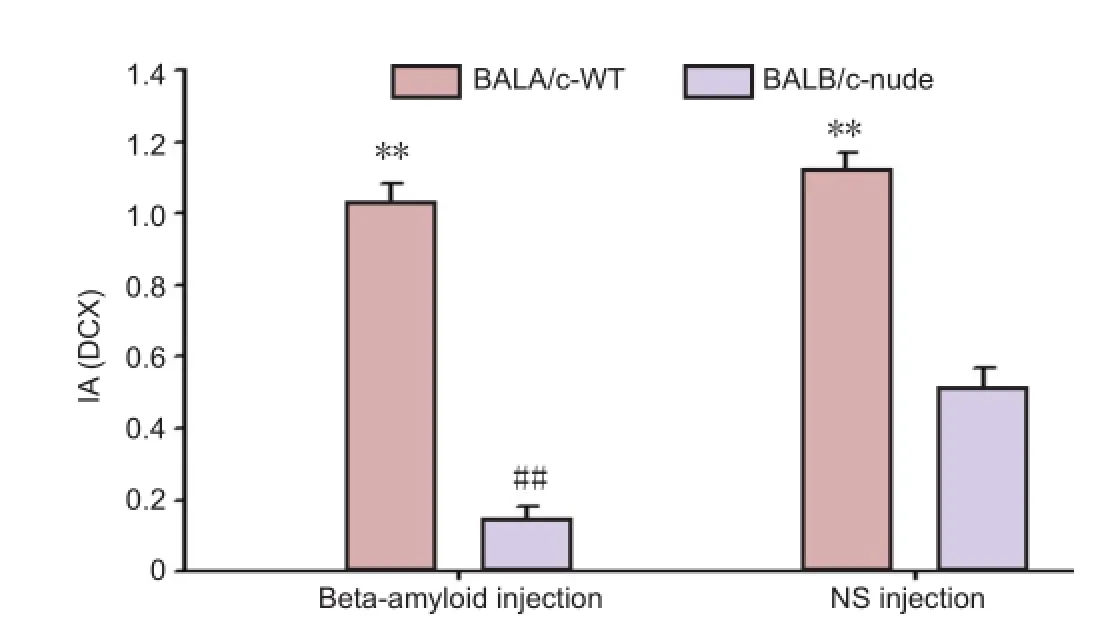
Figure 2 Quantitative analysis of doublecortin (DCX) expression in hippocampal dentate gyrus neurons 7 days after modeling.
Discussion
AD is caused by synaptic loss and neuronal death, which result from Aβ deposition-mediated chronic inflammation (Ferretti et al., 2012; Rubio-Perez et al., 2012). In the brain of patients with AD, Aβ deposition not only causes in flammation, but also allows speci fi c T cells to cross the microvascular endothelial cells of the blood-brain barrier and enter the brain parenchyma, thus exacerbating in flammatory responses (Man et al., 2007; Li et al., 2009; Fisher et al., 2011). Neuronal regeneration, in particular its induction and maintenance, is attracting increasing attention in studies of AD pathogenesis.
DCX is a microtubule-associated protein, which is speci fi cally expressed in neuronal precursors and is involved in the migration of immature neurons and neurite growth (Rao et al., 2004). DCX is transiently expressed for 2-3 weeks in the cytoplasm and projections of newly formed neuronal precursors, after which it begins to decline; it is not expressed in mature neurons (Brown et al., 2003).is characteristic allows the use of DCX as a specific marker of neuronal precursors.e present results indicate that T cells promote nerve cell regeneration in the subgranular zone of the hippocampus in WT mice, and a lack of T cells may impair regeneration. In addition, injection of Aβ1-42inhibits the production of hippocampal nerve cells, depending on the neurotoxicity of Aβ1-42. Previous studies compared neuronal regeneration in WT BALB/c/Ola mice and BALB/c-nude mice with a de ficiency of T cells but normal B cells.e evidence suggests that T-cells can not only promote proliferation of neural precursors in the dentate gyrus, but also a ff ect the di ff erentiation of precursor cells (Ziv et al., 2006). Immune de fi ciency in cells is considered to be the main cause of con flicting results. In the present study, we compared neuronal regenera-tion in normal BALB/c-WT mice and T-cell-de fi cient BALB/ c-nude mice; our results highlight the contribution of T cells to neuronal regeneration in the hippocampus of AD mice, consistent with previous fi ndings.us, hippocampal neuronal regeneration is presumably mediated by the activation of microglial cells.e mechanisms underlying T cell promotion of hippocampal neuronal regeneration also depend on microglial cell-secreted cytokines (Monsonego et al., 2003; Pellicanò et al., 2010; Swardfager et al., 2010).
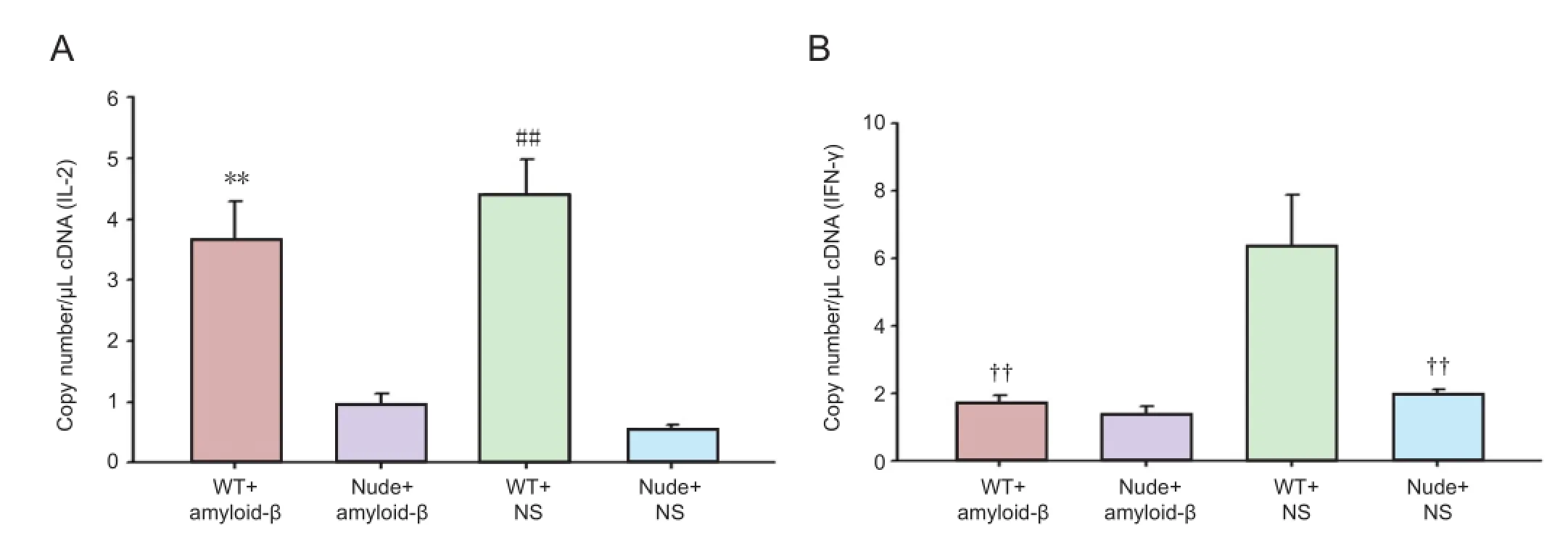
Figure 3 Quantitative fluorescence PCR for interleukin-2 (IL-2) and interferon-γ (IFN-γ) gene expression in peripheral blood of mice 7 days after modeling.
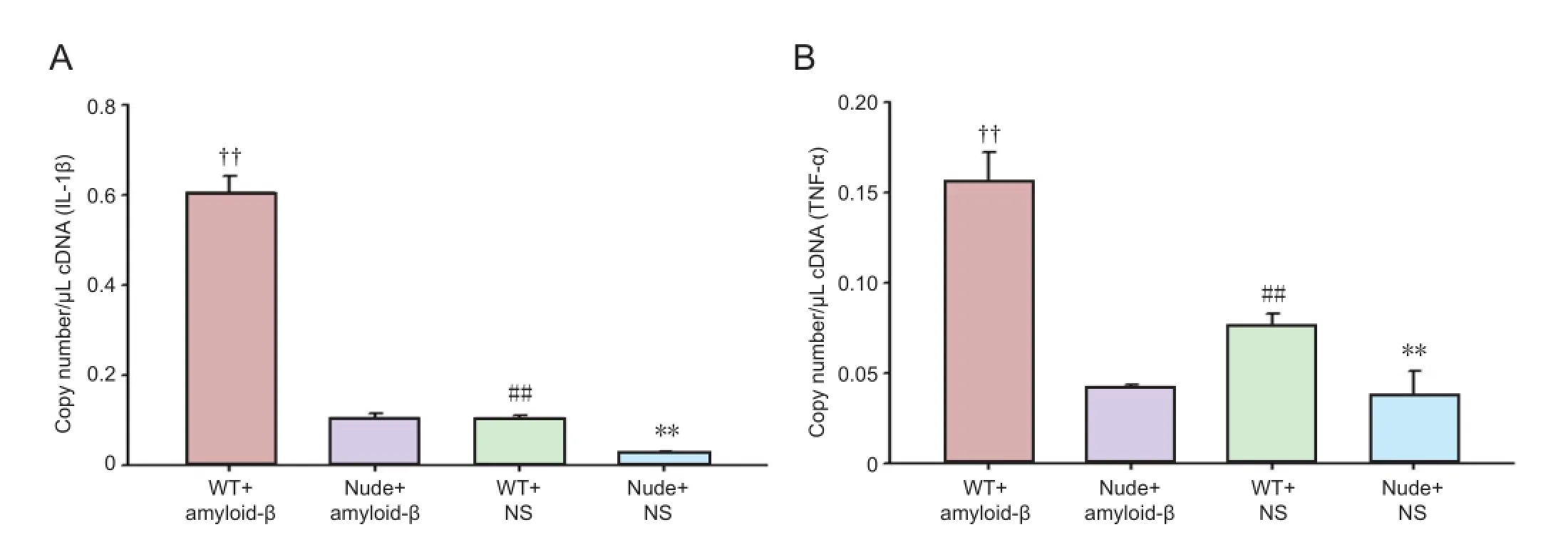
Figure 4 Interleukin-1β (IL-1β; A) and tumor necrosis factor-α (TNF-α; B) gene expression in the hippocampus of mice 7 days after modeling.
IL-2 is a T-cell growth factor, mainly produced by activated T-cells. It plays a crucial role in maintaining the growth of T-cell subsets and promoting the proliferation of activated B cells. In the present study, we used PCR to show that the secretion of IL-2 from T cells (IL-2 expression) was positively correlated with the regeneration of hippocampal neurons. IL-2 is an important pro-in flammatory cytokine, and its expression correlates closely with the degree of in flammation and neuronal loss in AD and other degenerative diseases (Meola et al., 2013).e quantity of CD4+T-cells is signi ficantly increased in the peripheral blood of AD patients, consistent with upregulated expression of IL-2, IL-6 and other in flammatory cytokines in peripheral blood, all of which are secreted by T cells (Becher et al., 2006; Wolf et al., 2009). Interestingly, IL-2 expression correlates with neuronal regeneration, indicating that the in flammatory and neuroprotective e ff ects exist simultaneously in AD, and peripheral IL-2 gene expression can re flect the activation state of T-cells.
IFN-γ is generated by a variety of immune cells including T-cells, the crucial immune regulatorsin vivo. IFN-γ can increase the expression of MHC-II molecules on the macrophage surface and promote phagocytosis. In AD model mice, IFN-γ produced by Aβ-specific T cells activates microglia to stimulate inflammation and aggravate abnormal Aβ protein deposition (Browne et al., 2013). Another study showed that Aβ-speci fi c T-cells induce immune cells to clear Aβ protein deposition in the brain through the secretion of IFN-γ (Fisher et al., 2010). Hippocampal injection of Aβ1-42downregulates the expression of IFN-γ and inhibits neuro-nal regeneration (Zheng et al., 2013). The major adaptive immune cytokine, IFN-γ, not only promotes regeneration of hippocampal neurons and improves spatial learning and memory capacity in adult WT mice, but also plays a crucial role in the regulation of brain in flammation, repair of damaged neurons, and maintenance of normal nervous system function (Baron et al., 2008; Mastrangelo et al., 2009).e present study shows that hippocampal injection of Aβ1-42peptide inhibits the expression of peripheral blood IFN-γ, and the expression level in BALB/c-nude mice is lower than that in WT mice. Changes in IFN-γ expression reflect the complexity of immunoregulation.
TNF-α is an important inflammatory factor that can kill tumor cells or cytokines that induce tumor tissue necrosis. It is mainly produced by activated microglia (Montgomery et al., 2012).e biological activity of TNF-α is diverse, and includes direct killing of cells, as well as immune regulation and the promotion of cell proliferation and differentiation (McCoy et al., 2008; Alvarez et al., 2011). Studies have shown that the loss of neurons in AD is mainly due to the activation of microglial cells by oligomerized Aβ protein, altering the cell cycle via the TNF-α and c-Jun kinase signaling pathways, affecting normal neuronal differentiation and ultimately leading to apoptosis (Bhaskar et al., 2014). The present results suggest that TNF-α expression correlates positively with neuronal regeneration in each group.
As AD progresses, the Aβ-activated microglia produce inflammatory cytokines (such as IL-1α, IL-1β and TNF-α), inducing neuronal death and memory impairment (Fang et al., 2010). Neurotrophic factors (including NGF, BDNF and GDNF) are also produced, to maintain neuronal regeneration and learning and memory functions (Scharfman et al., 2005; Ji et al., 2011; Lilja et al., 2013). Changes in the balance of inflammatory cytokines and neurotrophic factors will accelerate or delay the AD process. Here, we have examined the regeneration of hippocampal neurons and the expression of pro-inflammatory cytokines (IL-2, IFN-γ, IL-1β, TNF-α) in AD mice at 7 days, and found a positive correlation, that is, signi fi cant neuronal regeneration and high expression levels of cytokines are found in the same group.e present results suggest that both nerve inflammation and neuroprotective effects might be concomitant in the AD pathological state, and they may maintain a dynamic balance under normal immune function; when an immune imbalance or defect occurs, neuroprotective e ff ects are decreased, a ff ecting nerve regeneration. Our experimental results showed that neuronal regeneration in BALB/ c-WT mice was notably better than in T-cell-de fi cient BALB/ c-nude mice.e contribution of T cells to neuronal regeneration in AD mice is closely related to immune status, microglial activation, and the secretion of cytokines.
Author contributions:Liu J was responsible for the study design, implementation and writing the manuscript. Ma YX and Tian SM participated in the experimental implementation and data analysis. Zhang L, Zhao MM and Zhang YQ prepared animals models and implemented the experiments. Xu DC supervised the study and modified the manuscript. All authors approved the final version of the manuscript.
Con flicts of interest:None declared.
Alvare-Buylla A, Lim DA (2004) For the long run: maintaining germinal niches in the adult brain. Neuron 41:683-686.
Alvarez S, Blanco A, Fresno M, Mu?oz-Fernández Má (2011) TNF-α contributes to caspase-3 independent apoptosis in neuroblastoma cells: role of NFAT. PLoS One 6:e16100.
Baron R, Nemirovsky A, Harpaz I, Cohen H, Owens T, Monsonego A (2008) IFN-gamma enhances neurogenesis in wild-type mice and in a mouse model of Alzheimer’s disease. FASEB J 22:2843-2852.
Becher B, Bechmann I, Greter M (2006) Antigen presentation in autoimmunity and CNS in flammation: how T lymphocytes recognize the brain. J Mol Med 84:532-543.
Bhaskar K, Maphis N, Xu G, Varvel NH, Kokiko-Cochran ON, Weick JP, Staugaitis SM, Cardona A, Ransoho ff RM, Herrup K, Lamb BT (2014) Microglial derived tumor necrosis factor-α drives Alzheimer’s disease-related neuronal cell cycle events. Neurobiol Dis 62:273-285.
Biscaro B, Lindvall O, Tesco G, Ekdahl CT, Nitsch RM (2012) Inhibition of microglial activation protects hippocampal neurogenesis and improves cognitive de fi cits in a transgenic mouse model for Alzheimer’s disease. Neurodegener Dis 9:187-198.
Brown JP, Couillard-Despres S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG (2003) Transient expression of doublecortin during adult neurogenesis. J Comp Neurol 467:1-10.
Browne TC, McQuillan K, McManus RM, O’Reilly JA, Mills KH, Lynch MA (2013) IFN-γ Production by amyloid β-specific Th1 cells promotes microglial activation and increases plaque burden in a mouse model of Alzheimer’s disease. J Immunol 190:2241-2251.
Cao C, Arendash GW, Dickson A, Mamcarz MB, Lin X, Ethell DW (2009) Abeta-speci fi c2 cells provide cognitive and pathological bene fi ts to Alzheimer’s mice without in fi ltrating the CNS. Neurobiol Dis 34:63-70.
Chopra K, Misra S, Kuhad A (2011) Neurobiological aspects of Alzheimer’s disease. Expert Opiner Targets 15:535-555.
Dahlgren KN, Manelli AM, Stine WB Jr, Baker LK, KraGA, LaDu MJ (2002) Oligomeric and fi brillar species of amyloid-beta peptides di ff erentially a ff ect neuronal viability. J Biol Chem 277:32046-32053.
Demars M, Hu YS, Gadadhar A, Lazarov O (2010) Impaired neurogenesis is an early event in the etiology of familial Alzheimer’s disease in transgenic mice. J Neurosci Res 88:2103-2107.
Donovan MH, Yazdani U, Norris RD, Games D, German DC, Eisch AJ (2006) Decreased adult hippocampal neurogenesis in the PDAPP mouse model of Alzheimer’s disease. J Comp Neurol 495:70-83.
Fang F, Lue LF, Yan S, Xu H, Luddy JS, Chen D, Walker DG, Stern DM, Yan S, Schmidt AM, Chen JX, Yan SS (2010) RAGE-dependent signaling in microglia contributes to neuroinflammation, Abeta accumulation, and impaired learning/memory in a mouse model of Alzheimer’s disease. FASEB J 24:1043-1055.
Farfara D, Lifshitz V, Frenkel D (2008) Neuroprotective and neurotoxic properties of glial cells in the pathogenesis of Alzheimer’s disease. J Cell Mol Med 12:762-780.
Ferretti MT, Bruno MA, Ducatenzeiler A, Klein WL, Cuello AC (2012) Intracellular Aβ-oligomers and early inflammation in a model of Alzheimer’s disease. Neurobiol Aging 33:1329-1342.
Finder VH (2010) Alzheimer’s disease: a general introduction and pathomechanism. J Alzheimers Dis 22:5-19.
Fisher Y, Nemirovsky A, Baron R, Monsonego A (2010) T cells speci fi cally targeted to amyloid plaques enhance plaque clearance in a mouse model of Alzheimer’s disease. PLoS One 5:e10830.
Fisher Y, Nemirovsky A, Baron R, Monsonego A (2011) Dendritic cells regulate amyloid-β-specific T-cell entry into the brain: the role of perivascular amyloid-β. J Alzheimers Dis 27:99-111.
Graber JJ, Dhib-Jalbut S (2009) Protective autoimmunity in the nervous system. Pharmacoler 121:147-159.
Honjo K, Black SE, Verhoe ff NP (2012) Alzheimer’s disease, cerebrovascular disease, and the β-amyloid cascade. Can J Neurol Sci 39:712-728.
Lazarov O, Marr RA (2010) Neurogenesis and Alzheimer’s disease: at the crossroads. Exp Neurol 223:267-281.
Li M, Shang SD, Zhao WD, Tian L, Li B, Fang WG, Zhu L, Man SM, Chen YH (2009) Amyloid β interaction with receptor for advanced glycation end products up-regulates brain endothelial CCR5 expression and promotes T cells crossing the blood-brain barrier. J Immunol 182:5778-5788.
Lilja AM, R?jdner J, Mustafiz T, Thomé CM, Storelli E, Gonzalez D, Unger-Lithner C, Greig NH, Nordberg A, Marutle A (2013) Age-dependent neuroplasticity mechanisms in Alzheimer Tg2576 mice following modulation of brain amyloid-β levels. PLoS One 8:e58752.
Man SM, Ma YR, Shang DS, Zhao WD, Li B, Guo DW, Fang WG, Zhu L, Chen YH (2007) Peripheral T cells overexpress MIP-1α to enhance its transendothelial migration in Alzheimer’s disease. Neurobiol Aging 28:485-496.
Mastrangelo MA, Sudol KL, Narrow WC. Bowers WJ (2009) Interferon-gamma di ff erentially a ff ects Alzheimer’s disease pathologies and induces neurogenesis in triple transgenic-AD mice. Am J Pathol 175:2076-2088.
McCoy MK, Tansey MG (2008) TNF signaling inhibition in the CNS: implications for normal brain function and neurodegenerative disease. J Neuroin flammation 5:45.
Meola D, Huang Z, Ha GK, Petitto JM (2013) Loss of neuronal phenotype and neurodegeneration: e ff ects of T lymphocytes and brain interleukin-2. J Alzheimers Dis Parkinsonism Suppl 10:3.
Monsonego A, Imitola J, Zota V, Oida T, Weiner HL (2003) Microglia-mediated nitric oxide cytotoxicity of T cells following amyloid beta-peptide presentation to1 cells. J Immunol 171:2216-2224.
Monsonego A, Nemirovsky A, Harpaz I (2013) CD4 T cells in immunity and immunotherapy of Alzheimer’s disease. Immunology 139:438-446.
Montgomery SL, Bowers WJ (2012) Tumor necrosis factor-alpha and the roles it plays in homeostatic and degenerative processes within the central nervous system. J Neuroimmune Pharmacol 7:42-59.
Pellicanò M, Bulati M, Bu ff a S, Barbagallo M, Di Prima A, Misiano G, Picone P, Di Carlo M, Nuzzo D, Candore G, Vasto S, Lio D, Caruso C, Colonna-Romano G (2010) Systemic immune responses in Alzheimer’s disease: in vitro mononuclear cell activation and cytokine production. J Alzheimers Dis 21:181-192.
Rao MS, Shetty AK (2004) E ffi cacy of doublecortin as a marker to analyse the absolute number and dendritic growth of newly generated neurons in the adult dentate gyrus. Eur J Neurosci 19:234-246.
Rubio-Perez JM, Morillas-Ruiz JM (2012) A review: inflammatory process in Alzheimer’s disease, role of cytokines. Scientific World Journal 2012:756357.
Scharfman H, Goodman J, Macleod A, Phani S, Antonelli C, Croll S (2005) Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp Neurol 192:348-356.
Schroeter S, Khan K, Barbour R, Doan M, Chen M, Guido T, Gill D, Basi G, Schenk D, Seubert P, Games D (2008) Immunotherapy reduces vascular amyloid-beta in PDAPP mice. J Neurosci 28:6787-6793.
Song C, Zhang Y, Dong Y (2013) Acute and subacute IL-1β administrations differentially modulate neuroimmune and neurotrophic systems: possible implications for neuroprotection and neurodegeneration. J Neuroin flammation 10:59.
Swardfager W, Lanct?t K, Rothenburg L, Wong A, Cappell J, Herrmann N (2010) A meta-analysis of cytokines in Alzheimer’s disease. Biol Psychiatry 68:930-941.
Tabira T (2010) Immunization therapy for Alzheimer disease: a comprehensive review of active immunization strategies. Tohoku J Exp Med 220:95-106.
Tachida Y, Nakagawa K, Saito T, Saido TC, Honda T, Saito Y, Murayama S, Endo T, Sakaguchi G, Kato A, Kitazume S, Hashimoto Y (2008) Interleukin-1 beta up-regulates TACE to enhance alpha-cleavage of APP in neurons: resulting decrease in Abeta production. J Neurochem 104:1387-1393.
Togo T, Akiyama H, Iseki E, Kondo H, Ikeda K, Kato M, Oda T, Tsuchiya K, Kosaka K (2002) Occurrence of T cells in the brain of Alzheimer’s disease and other neurological diseases. J Neuroimmunol 124: 83-92.
Wolf SA, Steiner B, Akpinarli A, Kammertoens T, Nassenstein C, Braun A, Blankenstein T, Kempermann G (2009) CD4-positive T lymphocytes provide a neuroimmunological link in the control of adult hippocampal neurogenesis. J Immunol 182:3979-3984.
Yu Y, He J, Zhang Y, Luo H, Zhu S, Yang Y, Zhao T, Wu J, Huang Y, Kong J, Tan Q, Li XM (2009) Increased hippocampal neurogenesis in the progressive stage of Alzheimer’s disease phenotype in an APP/ PS1 double transgenic mouse model. Hippocampus 19:1247-1253.
Zhang C, McNeil E, Dressler L, Siman R (2007) Long-lasting impairment in hippocampal neurogenesis associated with amyloid deposition in a knock-in mouse model of familial Alzheimer’s disease. Exp Neurol 204:77-87.
Zheng M, Liu J, Ruan Z, Tian S, Ma Y, Zhu J, Li G (2013) Intrahippocampal injection of Aβ1-42 inhibits neurogenesis and down-regulates IFN-γ and NF-κB expression in hippocampus of adult mouse brain. Amyloid 20:13-20.
Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, Cohen H, Kipnis J, Schwartz M (2006) Immune cells contribute to the maintenance of neurogenesis and spatial learening abilities in adulthood. Nat Neurosci 9:268-275.
Ziv Y, Schwartz M (2008) Orchestrating brain-cell renewal: the role of immune cells in adult neurogenesis in health and disease. Trends Mol Med 14:471-478.
Copyedited by Murphy S, Norman C, Wang J, Yang Y, Li CH, Song LP, Zhao M
Dachuan Xu, M.D., Department of Human Anatomy, School of Basic Medical Sciences, Southern Medical University, Guangzhou 510515, Guangdong Province, China, chjcana@126.com.
10.4103/1673-5374.139481
http://www.nrronline.org/
Accepted: 2014-07-05
 中國(guó)神經(jīng)再生研究(英文版)2014年16期
中國(guó)神經(jīng)再生研究(英文版)2014年16期
- 中國(guó)神經(jīng)再生研究(英文版)的其它文章
- Neuroprotective effects of berry fruits on neurodegenerative diseases
- Brain structural changes and their correlation with vascular disease in type 2 diabetes mellitus patients: a voxel-based morphometric study
- Cutaneous sensory nerve as a substitute for auditory nerve in solving deaf-mutes’ hearing problem: an innovation in multi-channel-array skin-hearing technology
- Acrylamide inhibits nerve sprouting induced by botulinum toxin type A
- Transplantation of neurotrophin-3-transfected bone marrow mesenchymal stem cells for the repair of spinal cord injury
- Shifting balance from neurodegeneration to regeneration of the brain: a novel therapeutic approach to Alzheimer’s disease and related neurodegenerative conditions
