Synergetic effects of ciliary neurotrophic factor and olfactory ensheathing cells on optic nerve reparation (complete translation)
Dan-ping Yin, Qing-ying Chen, Lin Liu
1 Department of Disease Control and Prevention, Jinan Military General Hospital, Jinan, Shandong Province, China
2 Medical Department, Jinan Military General Hospital, Jinan, Shandong Province, China
3 Department of Ophthalmology, Renji Hospital Affiliated to Shanghai JiaoTong University School of Medicine, Shanghai, China
RESEARCH ARTICLE
Synergetic effects of ciliary neurotrophic factor and olfactory ensheathing cells on optic nerve reparation (complete translation)
Dan-ping Yin1,#, Qing-ying Chen2,#, Lin Liu3,*
1 Department of Disease Control and Prevention, Jinan Military General Hospital, Jinan, Shandong Province, China
2 Medical Department, Jinan Military General Hospital, Jinan, Shandong Province, China
3 Department of Ophthalmology, Renji Hospital Affiliated to Shanghai JiaoTong University School of Medicine, Shanghai, China
Graphical Abstract
#These authors contributed equally to this study.
orcid: 0000-0002-0649-9688 (Lin Liu)
Accepted: 2015-12-22
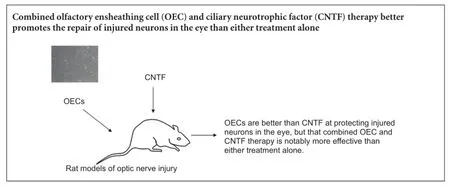
At present, there is no effective treatment for the repair of the optic nerve after injury, or improvement of its microenvironment for regeneration. Intravitreally injected ciliary neurotrophic factor (CNTF) and olfactory ensheathing cells (OECs) promote the long-distance regrowth of severed optic nerve fibers after intracranial injury. Here, we examined the efficacy of these techniques alone and in combination, in a rat model of optic nerve injury. We injected condensed OEC suspension at the site of injury, or CNTF into the vitreous body, or both simultaneously. Retrograde tracing techniques showed that 4 weeks postoperatively, the number of surviving retinal ganglion cells and their axonal density in the optic nerve were greater in rats subjected to OEC injection only than in those receiving CNTF injection only. Furthermore, combined OEC + CNTF injection achieved better results than either monotherapy. These findings confirm that OECs are better than CNTF at protecting injured neurons in the eye, but that combined OEC and CNTF therapy is notably more effective than either treatment alone.
nerve regeneration; optic nerve injury; repair; regeneration; olfactory ensheathing cells; ciliary neurotrophic factor; retinal ganglion cells; cell survival; intravitreal injection; retrograde tracing; neural regeneration
Introduction
After optic nerve injury, transplantation of nerve cells to the injury site can help retinal ganglion cells to regenerate, reach the target area via the graft, and form functional synapses (MacLaren et al., 1998). Furthermore, the local environment at the site of injury can be improved by intraocular injection of exogenous neurotrophic factors, leading to regeneration of the injured optic nerve (Lorber et al., 2015; Zhang et al., 2015). Intravitreal injection of ciliary neurotrophic factor (CNTF) has been shown to reduce or postpone optic nerve injury- or ischemia-induced apoptosis of retinal ganglion cells, and transplantation of olfactory ensheathing cells (OECs) reportedly promotes long-distance regeneration of nerve fibers after optic nerve injury (Woodhall et al., 2001).
However, to the best of our knowledge, there have been no reports of methods that completely repair the injured optic nerve. Because of a lack of timely and effective treatments, about half of all patients with optic nerve injury lose their vision (Faverani et al., 2011). Therefore, there is an urgent need to find effective methods to protect the nerve, prevent neuronal death and promote repair after damage. OECs and CNTF have been shown to promote repair and regenerationin the optic nerve (Ahmad et al., 2000). In humans, OECs in the olfactory bulb, which is responsible for the sense of smell, have lifelong regenerative ability, secrete neurotrophic substances, and thus help to promote the regrowth of peripheral nerve fibers (Sahenk et al., 2005). CNTF promotes the growth of motoneurons and prevents their denaturation and death after trauma (Hu et al., 2005).
Here, we transplanted OECs into the site of injury (an experimental treatment) and administered recombinant human CNTF intravitreally (a method that has been used in clinical practice) in rat models of optic nerve injury, to investigate whether the combined procedures were more effective than either monotherapy. The objective of this study was to investigate clinical methods of promoting optic nerve repair and providing new insight into the treatment of optic nerve injury and central nervous system damage.
Materials and Methods
Animals
Sixty healthy adult male Sprague-Dawley rats, aged 10 weeks and weighing 180—220 g, were provided by the Laboratory Animal Center, Second Military Medical University, China (license No. 2008001653258). The experimental protocol was approved by the Animal Ethics Committee, Changhai Hospital, Second Military Medical University, China. All rats were housed under controlled conditions (22°C) and a 12 hour reversed light/dark cycle, with free access to food and water. Surgery was performed under anesthesia, and all efforts were made to minimize the pain and distress of the experimental animals. All animal experiments were carried out in accordance with the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1986).
Of the 60 rats, 20 were used for OEC preparation and the remaining 40 for establishing rat models of optic nerve injury. External ophthalmopathy was not observed in any rat.
Primary culture of OECs
Twenty olfactory bulbs were harvested under sterile conditions. The pia mater was removed and the olfactory bulbs, olfactory nerve layer, and granular layer were dissociated. Olfactory bulb tissue was transferred to 0.125% trypsin and digested for 20 minutes in an incubator at 37°C. Complete culture medium (2—3 mL) was added and left for 10 minutes to neutralize the effect of trypsin. When the samples were completely digested with trypsin, they were washed twice with 2—3 mL complete culture medium, cultured with 2 mL complete culture medium, and a single cell suspension was prepared, which was incubated in a culture flask.
Purification of OECs
OECs for grafting should have high purity and good activity (Huang et al., 2001; Ahmed et al., 2005; Boruch et al., 2007; Cerveny et al., 2012). Adult rat OECs were incubated in 5% CO2at 37°C for 12 hours. The suspension, containing non-adherent cells, was inoculated into another non-coated glass flask for 12 hours. After adjustment of the cell concentration, cells were seeded into a poly-L-lysine-treated 24-well plate (1 × 105cells per well) and further cultured for 1 week. Ara-C (Sigma, St. Louis, MO, USA) at a final concentration of 1 × 10—5M was added for 48 hours. Cells were then washed, and cultured in complete culture medium containing 21 μM forskolin and 20 μg/mL BPE (both from Sigma).
Identification of OECs
OECs were identified using fluorescent stains for p75 (plasma) and Hoechst 33342 (nuclei) (both from Sigma, following the manufacturers' instructions). Under 200× magnification, 10 nonoverlapping fields were randomly selected for counting p75-positive cells at an excitation wavelength of 550 nm. Hoechst 33342-positive cells were also counted in the same visual fields, at an excitation wavelength of 405 nm. The purity of OECs was calculated as the number of p75-positive cells expressed as a percentage of Hoechst 33342-positive cells in the same visual field. Each experiment was conducted three times, and the mean value was calculated. Staining was performed at 2, 7, 14, 20 and 30 days to confirm cell purity throughout the experiment (Boruch et al., 2001). The cells were viewed under inverted phase contrast and fluorescence microscopes (Olympus, Tokyo, Japan).
Cell labeling prior to transplantation
Cells in the logarithmic growth phase were stained with 10 μg/mL Hoechst 33342 at 37°C for 30 minutes, washed twice with serum-free DF12, digested with trypsin, and collected for use.
Establishment of rat models of optic nerve injury
The left optic nerves of the rats were exposed. A small window was made on the outer membrane of the optic nerve, 2 mm from the posterior globe, using a glass micropipette (Department of Neurobiology, Second Military Medical University, China). A 0.5 mm length of optic nerve was completely removed by aspiration with this micropipette, resulting in a 0.5 mm transverse gap, but with the outer membrane and vessels of the optic nerve remaining intact. Phosphate buffered saline (PBS, 2 μL; Solomon et al., 1996) was injected into the site of disconnection of the optic nerve. The segment of optic nerve from the posterior globe to the disconnection site was designated the proximal segment, whereas the distal segment reached from the disconnection site to the optic chiasm (Figure 1).
Interventions
Forty rats were randomly divided into one model group and three intervention groups (n = 10 rats/group). In the model group, PBS (2 μL) was injected at the disconnection site immediately after optic nerve disconnection. In the OEC group, condensed OEC suspension (2 μL, 3.5 × 106/mL) was injected at the disconnection site. In the CNTF group, CNTF (2 μL, 20 mg/mL; Department of Neurobiology, Second Military Medical University, China) was injected via the vitreous body. In the OEC + CNTF group, combined injections of OEC and intravitreal recombinant human CNTF were performed.
Characterization of rat models of optic nerve injury
Fifteen hours after modeling, electroretinogram (ERG) and visual evoked potential (VEP) measurements were performed in the left (damaged) and right (control) eyes under the same conditions, to functionally confirm model establishment. For morphological identification, fluorescent cholera toxin B (CTB) staining was performed in 20 μm-thick frozen longitudinal sections of the affected optic nerve. The fluorescent density of optic nerve fibers labeled by laser-induced fluorescent CTB was analyzed using a color image analysis system (Video Pro 32; Leading Edge Pty Ltd., Adelaide, Australia). The mean fluorescent density of optic nerve fibers across two different visual fields was calculated and recorded.
Labeling with fluorescent anterograde and retrograde tracers
Four weeks after optic nerve injury, fluorescent labeling using a retrograde tracer (Fluoro-Gold; FG) and an anterograde tracer (biotinylated dextran amine; BDA) (both from Thermo Fisher, Shanghai, China) was performed in the affected eye of each rat, for quantification of surviving RGCs.
The optic nerve was fully exposed through a vertical incision made in the center of the margin of the upper eyelid in the left eye. FG (n = 5 rats) or BDA (n = 5 rats) was injected (5 μL each) approximately 0.5 cm proximal to the center of injury. CTB (5 μL, 0.25%) was injected intravitreally into the left eye to label optic nerve axons using an anterograde tracing technique. The rats were sacrificed 3 days after tracer injection.
The most distal end of the retrobulbar optic nerve was exposed and a partial outward sclera was left for labeling. The optic nerve, from the intraorbital to the intracranial segment, was removed and immediately fixed in paraformaldehyde for 24 hours. The tissue was dehydrated with 25% glucose solution until it sank, and stored at 4°C in the dark until longitudinal sectioning into 20 μm slices. The mounted sections were observed under a fluorescence microscope.
Immunofluorescence staining
Terminally anesthetized rats were placed under an operating microscope. The tissue around the eyeball and intraorbital optic nerve were dissociated. The full-length optic nerve from the intraorbital to intracranial segments containing a part scleral shell (to determine direction) was taken out and post-fixed in 4% paraformaldehyde at 4°C for 2 hours. Then the optic nerve was dehydrated in 30% sucrose at 4°C overnight. The following day, the deposited tissue blocks were embedded with tissue freezing medium (Philas, Changzhou, China), frozen, and cut into 20 μm-thick longitudinal sections using a freezing microtome (Leica CM1860, Germany). All sections were placed on gelatin-coated slides. Three or four sections from the middle part were observed under the fluorescence microscope at 250× magnification, and stored in the dark. CTB fluorescence was induced using a UV optical filter in the absorbance range of 362—437 nm.
RGCs labeled by FG and BDA were counted in fresh tissue. The fresh eyeball was dissociated from the peripheral connective tissue. The cornea was sheared along its edge, and the lens and vitreous body were removed. Under the ophthalmic microscope (Suzhou Liuliu Vision Science and Technology Co., Ltd., Jiangsu Province, China), the retina was dissociated and the nasal and temporal sides were identified. Four small incisions were made at right angles to each other, to partially divide the retina into four quadrants (designated 1—4) of similar sizes but which remained attached to each other. The retina was then peeled from the sclera starting from its edge (Figure 2), and stretched onto a gelatinized slide with its concave side upward. A few drops of paraformaldehyde were added immediately. After natural drying in the dark, the retina was mounted with 50% glycerol. Under the fluorescence microscope, labeled RGCs were counted in two visual fields (250× magnification), and the mean number per visual field calculated and recorded. The slides were frozen and stored in the dark.
Data analysis
To calculate the number of surviving RBCs 3 days after injection of the FG/BDA retrograde tracers, retinal stretching was performed. At 4 weeks postoperatively, the total number of labeled RGCs 2 mm from the optic disc in quadrants 1 and 3, and 3 mm from the optic disc in quadrants 2 and 4, were counted in each group (n = 10 rats per group) using the Metamorph image analyzer (Universal Imaging Corp, USA) under a fluorescence microscope (Olympus BX60, 250× magnification). The mean number of labeled RGCs was calculated per group. For detection of RGC axon density in the optic nerve, 3 days after injection of the CTB anterograde tracer, the full-length optic nerve from the intraorbital to intracranial segments was cut into longitudinal sections and frozen. Ten sections from 10 individual samples per group (n = 10 rats per group) were processed using a computerized image analyzer (Video Pro 32; Leading Edge Pty). Under an optical microscope (250× magnification), two visual fields, one placed at 2 mm away from the optic foramen and one 4 mm from the optic foramen, were selected to determine the mean optical density value of each section and investigate the change in optic nerve fiber density.
Statistical analysis
All data are expressed as the mean ± SD and were processed using SPSS 10.0 software (SPSS, Chicago, IL, USA). Oneway analysis of variance and Student-Newman-Keuls posthoc test were used for comparing group means. P < 0.01 was considered statistically significant.
Results
Morphology and purity of OECs
Cytochemical staining of adult rat OECs showed that after purification and 2 days of culture, 95% of cells were OECs, and they appeared spindle-shaped, triangular, and ovalshaped (Figure 3A). By 7—8 days of culture, the OECs had grown substantially and extended processes, and the spindle-shaped and triangular cells connected into a network (Figure 3B). After 7 days of culture, over 90% of cells were still positive for OEC staining and were most suitable for transplantation (Figure 4). After 12—14 days of culture, the OECs were densely arranged, a small number of cells began to degenerate and die, and a few fibroblasts were seen (Figure3). Finally, after 3 weeks of culture, fibroblasts had increased in number, and OECs had markedly degenerated and shrunk in size, and a granular substance had appeared; in addition, prominences were thin and rigid, and many short spiny processes had formed (Figure 3D).
Identification of animal models of optic nerve injury
ERG and VEP functional measurements showed a crest and a trough in the images from the control eye, but not in the damaged eye, in which only baseline measurements were observed, indicating that the affected eye had no electrical conduction function. Morphological identification using laser-induced fluorescent CTB in sections of the affected nerve showed that the nerve was disconnected, appearing as a length of vacant axoplasm with a stump on either side.
Tracing of OECs transplanted in vivo
Immediately after optic nerve injury, OECs were injected into the optic nerve sheath at the site of disconnection. Four weeks after transplantation, survival of p75-labeled OECs was viewed under a fluorescence microscope (Figure 5). A large number of p75-labeled OECs were observed between the optic nerve sheath and the fibers. These cells appeared as small bright green circles with green-stained cell membranes and non-stained nuclei. If a cell died, the whole cell quickly disappeared by dissolution and absorption. The presence of a large number of p75-labeled OECs with regular morphology indicates that these cells are alive and play a role in the repair and protection of the optic nerve.
Survival of RGCs
The number of RGCs 2 mm from the optic disc in quadrants 1 and 3, and 3 mm from the optic disc in quadrants 2 and 4, decreased between groups (P < 0.01). No significant difference was observed between any two groups in RGC survival (P < 0.01) (Figures 6, 7, Table 1).
RGC axonal density in the optic nerve
The mean optical density of RGC axons 2 and 4 mm from the optic disc was highest in the OEC + CNTF group, followed by the OEC group, and was lowest in the CNTF group (P < 0.01) (Table 2).
Fiber fluorescence
The RGC layer showed the strongest fluorescence, and retinal cells and the optic nerve on the temporal side (and injection side) also showed strong fluorescence. In the longitudinal sections, normal optic nerve fibers were tightly arranged and injured optic nerve fibers were more sparse and disordered. The decrease in the number of nerve fibers in all three treatment groups was slower than in the model group, and the number of surviving axons was more sparse and disordered (Figures 8, 9).
Discussion
Compared with other models of optic nerve injury, the optic nerve disconnection model we used here is fairly simple to perform, and has a high reproducibility rate. The fibers 2 mm away from the optic disc were completely removed to leave a discrete, 0.5 mm long transverse gap. The method ensures consistency and accuracy of the damage to the optic nerve fiber, and avoids the variability seen with crush injury. Furthermore, unlike complete transection of the optic nerve, removal of the axoplasm alone ensures the integrity of the epineurium. The injury avoids the retinal blood vessels, thus maintaining the blood supply to the RGCs. The injury site anterior to the optic chiasm prevents interaction between the damaged and normal eyes. Furthermore, the created wound may contain peripheral nerves and nutritional factors, providing the possibility of investigating the effects of additional factors on nerve regeneration. Optic nerve injury models created by aspirating the nerve fibers are ideal for studying optic nerve injury and functional protection.
OECs have been widely used for the treatment of central and peripheral nerve system injury (Boyd et al., 2005; Huang et al., 2007; Cerveny et al., 2012; Liu et al., 2014; Ekberg and St John, 2015). There is evidence that OECs transplanted into the site of damage to the central nervous system can form a cell bridge to guide the growth of neurites and help neurites extend, contributing to repair (Goureau et al., 2004; Logan et al., 2006). Furthermore, OECs transplanted at the injury site of the optic nerve are known to promote optic nerve fiber regeneration and repair after injury (Li et al., 2003). OECs can survive for a long time in vivo and have long-lasting effects (Huang et al., 2008). However, although Cao et al. (2004) reported that OECs still secreted a large amount of glial cell-derived neurotrophic factor 4 weeks after transplantation, these cells survived no more than 8 weeks. In the present study, the survival rate of RGCs in each visual field in the OEC group was higher than in the model and CNTF groups, indicating that OECs impart considerable protection to neurons in the eye. This supports previous data showing that OECs provide a favorable internal environment for neural regeneration (Huang et al., 2008).
CNTF, a multipotent neurotrophic factor, protects RGCs and greatly promotes RGC axonal growth (Hu et al., 2005). Our results demonstrate that a single intravitreal injection of CNTF can increase the survival of RGCs and promote optic nerve regeneration, but that OECs are far superior to CNTF in promoting regeneration of RGC axons. The possible reasons for this include: (1) after optic nerve aspiration injury, only a single intravitreal administration was performed, and effective drug concentration and retention time were not evaluated; (2) many factors are involved in optic nerve repair and regeneration, and it is likely that CNTF alone does not have long-lasting effects; (3) recombinant human CNTF was used in rats, so this species difference may lead to a decreased level of CNTF. Further studies are needed to investigate the injection time and dose at which CNTF produces the optimal protective effects on the optic nerve (Fischer et al., 2012).
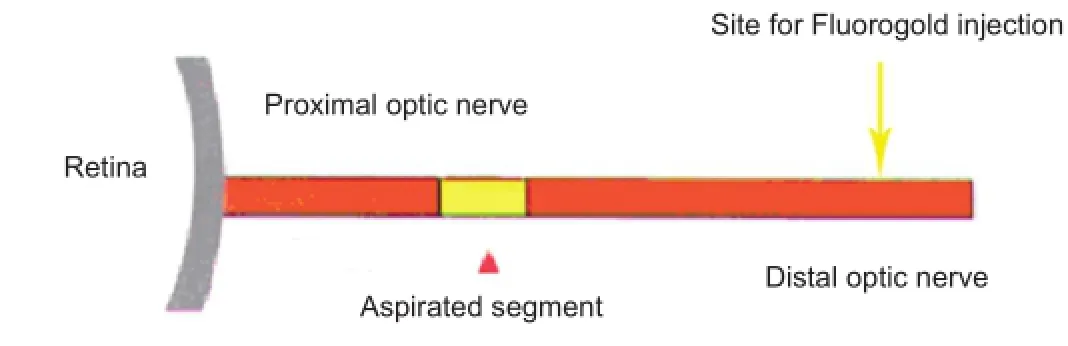
Figure 1 Schematic of optic nerve injury model.
Figure 2 Fresh retina.
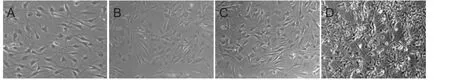
Figure 3 Morphology of olfactory ensheathing cells (OECs) after different durations in culture (inverted phase contrast microscope, × 40).
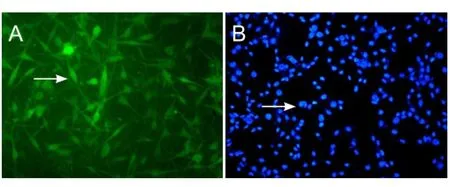
Figure 4 Immunohistochemistry of olfactory ensheathing cells (OECs) after 7 days in culture (fluorescence microscope, × 40).
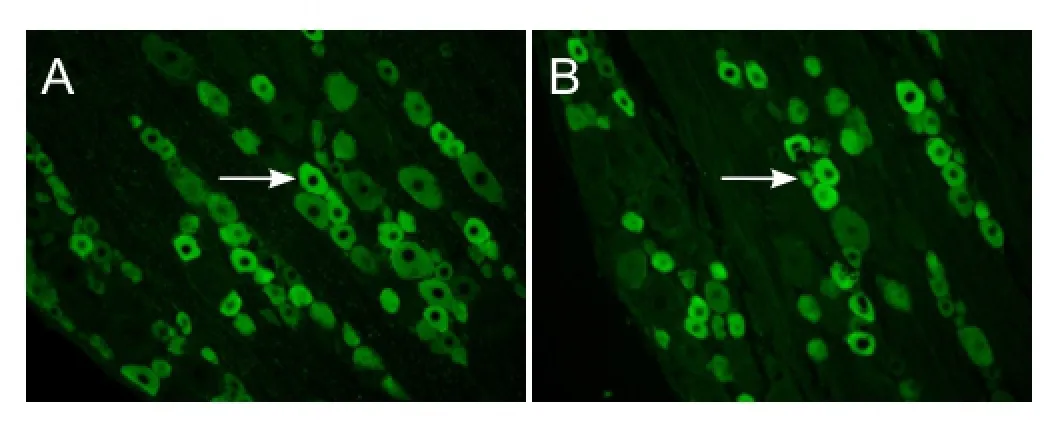
Figure 5 Survival of olfactory ensheathing cells (OECs) 4 weeks after transplantation into the optic nerve sheath of rats with optic nerve injury (immunofluorescence, × 250).
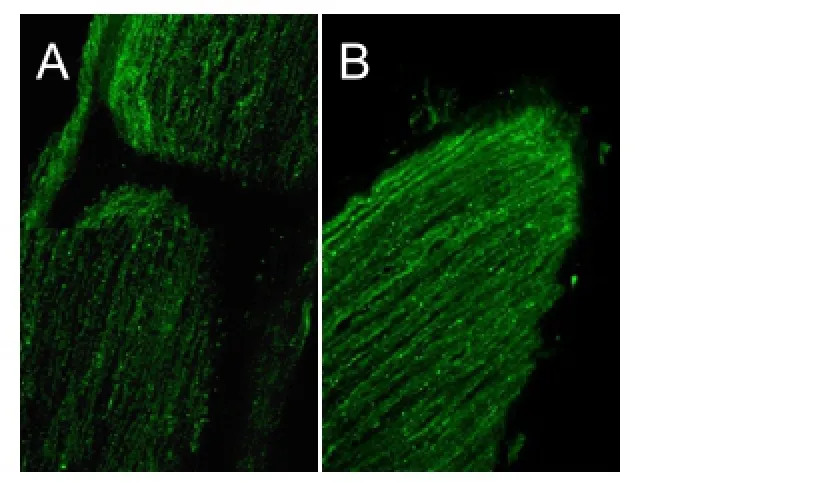
Figure 9 Morphology of nerve stumps in the OEC + CNTF group, 4 weeks after disconnection of the optic nerve (cholera toxin B subunit anterograde tracing, fluorescence microscope, × 250).
Based on the counts of surviving optic neurons and detection of RGC axons after optic nerve injury, we concluded that OECs in combination with CNTF showed better protective and reparative effects on neurons than did OECs or CNTF alone, and that the combined method produces a synergetic effect. After being transplanted to the injury site, OECs and their secreted neurotrophic factor and extracellular matrix form an environment that is conducive to neural regeneration and attenuates the inhibitory effects of the environment formed by glial cells (mainly astrocytes, oligodendrocytes, microglia and macrophages). RGC axon regeneration is co-induced by the cultured OECs and extracted CNTF. OECs promote elongation of regenerated optic nerve and guide nerve fibers to pass across the glial scar (Li et al., 1997), which facilitates CNTF promotion of RGC axon regeneration. CNTF injected into the vitreous body can circulate with the vitreous humor, rapidly dispersing overthe whole retinal surface, and is transported to the injury site of the optic nerve by axoplasmic streaming. There, it protects the RGCs and OECs and promotes their proliferation, strengthening the effects of the OECs and ultimately enhancing neural regeneration.
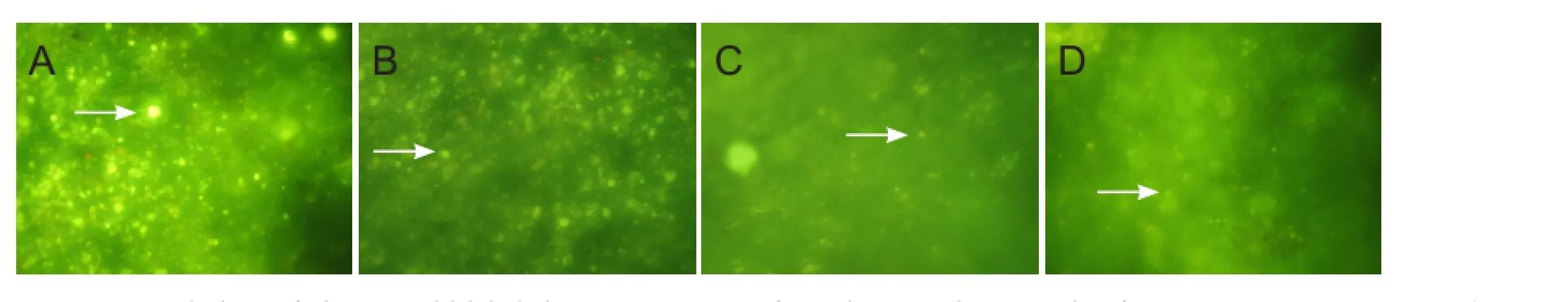
Figure 6 Morphology of Fluoro-Gold-labeled RGCs 2 mm away from the optic disc, 4 weeks after optic nerve injury in rats (retrograde tracing, fluorescence microscope, × 250).
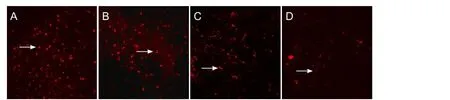
Figure 7 Morphology of biotin dextran amine-labeled OECs 2 mm from the optic disc, 4 weeks after optic nerve injury (biotin dextran amine retrograde tracing, fluorescence microscope, × 250).
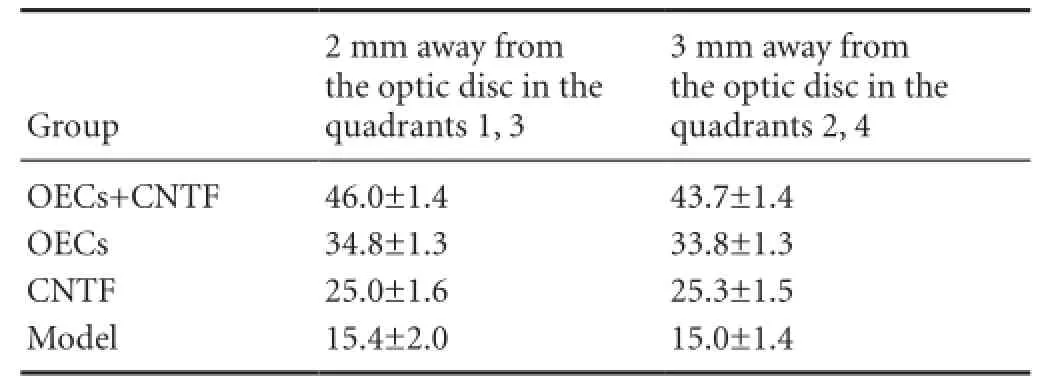
Table 1 Effects of OECs + CNTF on the number (n per ×250 visual field) of RGCs 2 mm away from the optic disc in quadrants 1 and 3, and 3 mm away from the optic disc in quadrants 2 and 4, 4 weeks after optic nerve injury
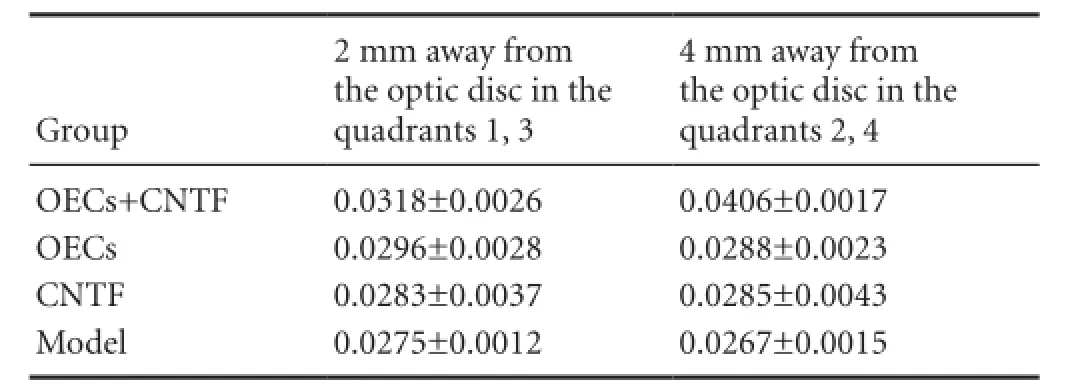
Table 2 Effects of OECs + CNTF on the mean optical density of RGC axons 2 and 4 mm away from the optic disc, 4 weeks after optic nerve injury
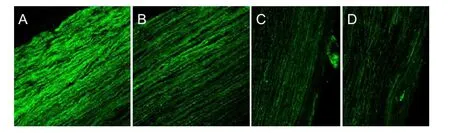
Figure 8 Retrobulbar optic nerve fibers 2 mm from the optic foramen in rats 4 weeks after optic nerve injury (cholera toxin B subunit anterograde tracing, fluorescence microscope, × 250).
Our study has a number of limitations. For example, we used low doses of OECs and CNTF; increasing the dose is likely to have better therapeutic effects. Furthermore, we did not compare the acute actions with the longer-term effects of OECs and CNTF after optic nerve injury. In addition, we did not continue VEP and ERG monitoring after treatment. All these should be investigated in future studies.
In summary, we used OEC transplantation in combination with intravitreal CNTF injection in rat models of optic nerve injury established by aspiration of a portion of the nerve. We found that the combined method exhibited better neuroprotection than OEC transplantation or intravitreal CNTF injection alone. We investigated neuronal regeneration and repair after optic nerve injury and even central nervous injury, and propose a novel therapeutic regimen with potential clinical value. Our results will inform future studies in the development of therapeutic options for optic nerve injury.
Acknowledgments: We express our thanks to Shi-heng Lu (Department of Ophthalmology, Renji Hospital Affiliated to Shanghai JiaoTong University School of Medicine, China), Qian Yu (Department of Ophthalmology, First Affiliated Hospital of Chengdu Medical College, China), Xiao-fei Dong (Department of Ophthalmology, Changhai Hospital, Second Military Medical University, China) for their help in preparation of animal models and retinal stretch. We thank Liang Yan (Department of Ophthalmology, Central Hospital of Baoshan District of Shanghai, China. We also thank all the teachers from Department of Neurobiology, Second Military Medical University, China for their guidance in experiment performance.
Author contributions: DPY conceived and designed this study, wrote the paper, provided and integrated the data. QYC analyzed the data, performed statistical analysis and provided assistance in technique or material application. LL authorized this paper. All authors read and agreed the final version of this paper for publication.
Conflicts of interest: None declared.
Author statements: This article is based on a study first reported in the Chinese Journal of Ophthalmology, 2003;49(11)∶1020-1028.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
References
Ahmad I, Tang L, Pham H (2000) Identification of neural progenitors in the adult mammalian eye. Biochem Biophys Res Commun 270:517.
Ahmed Z, Dent RG, Leadbeater WE, Smith C, Berry M, Logan A (2005) Matrix metalloproteases: degradation of the inhibitory environment of the transected optic nerve and the scar by regenerating axons. Mol Cell Neurosci 28:64-78.
Boruch AV, Conners JJ, Pipitone M, Deadwyler G, Storer PD, Devries GH, Jones KJ (2001) Neurotrophic and migratory properties of an olfactory ensheathing cell line. Glia 33:225-229.
Boyd JG, Doucette R, Kawaja MD (2005) Defining the role of olfactory ensheathing cells in facilitating axon following damage to the spinal cord. FASEB J 19:694-703.
Cao L, Liu L, Chen ZY, Wang LM, Ye JL, Qiu HY, Lu CL, He C (2004) Olfactory ensheathing cells genetically modified to secrete GDNF to promote spinal cord repair. Brain 127:535-549.
Cerveny KL, Varga M, Wilson SW (2012) Continued growth and circuit building in the anamniote visual system. Dev Neurobiol 72:328-345.
Ekberg JA, St John JA (2015) Olfactory ensheathing cells for spinal cord repair: crucial differences between subpopulations of the glia. Neural Regen Res 10:1395-1396.
Faverani LP, Capelari MM, Ramalho-Ferreira G, Gomes-Filho JC, Fabris AL, Marzola C, Toledo GL, Toledo-Filho JL (2011) Ocular reconstruction after zygomatic complex fracture with retention of a foreign body. J Craniofac Surg 22:1394-1397.
Fischer D (2012) Stimulating axonal regeneration of mature retinal ganglion cells and overcoming inhibitory signaling. Cell Tissue Res 349:79-85.
Goureau O, Rhee KD, Yang XJ (2004) Ciliary neurotrophic factor is an axoi genesis factor for retinal ganglion cells. Dev Neurosci 26:359-370.
Hu Y, Leaver SG (2005) Lentiviral-mediated transfer of CNTF to schwann cells within reconstructed peripheral nerve grafts enhances adult retinal ganglion cell survival and axonal regeneration. Mol Ther 11:906-915.
Hu Y, Leaver SG, Plant GW, Hendriks WT, Niclou SP, Verhaagen J, Harvey AR, Cui Q (2005) Lentiviral-mediated transfer of CNTF to schwann cells within reconstructed pefpheral nerve grafts enhances adult retinal ganglion cell survival and axonal regeneration. Mol Ther 11:906-915.
Huang H, Tan K, Chen L, Xue Y, Wang H, Xi H, Liu Y, Zhang F, Zhang J (2007) Short-term outcome of olfactory ensheathing ceils transplantation for treatment of amyotrophic lateral sclerosis. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 21:961-966.
Huang ZH, Wang Y, Cao L, Su ZD, Zhu YL, Chen YZ, Yuan XB, He C (2008) Migratory properties of cultured olfactory ensheathing cells by single-cell migration assay. Cell Res 18:479-490.
Li Y, Field PM, Raisman G (1997) Repair of adult rat corticospinal tract by transplant of olfactory ensheathing cell. Science 277:2000-2002.
Li Y, Sauvé Y, Li D, Lund RD, Raisman G (2003) Transplanted olfactory ensheathing cells promote regeneration of cut adult rat optic nerve axons. J Neurosci 23:7783-7788.
Liu J, Zhang L, Wang Q, Chen Y, Yu H, Ma J, Guo M, Piao M, Xiang L (2014) Meta analysis of olfactory ensheathing cell transplantation promoting functional recovery of motor nerves in rats with complete spinal cord transection. Neural Regen Res 9:1850-1858.
Logan A, Ahmed Z (2006) Neurotrophic factor synergy is required for neuronal survival and disinhibited axon regeneration after CNS injury. Brain 129:490-502.
Lorber B, Chew DJ, Hauck SM, Chong RS, Fawcett JW, Martin KR (2015) Retinal glia promote dorsal root ganglion axon regeneration. PLoS One 10:e0115996.
MacLaren RE (1998) Regeneration and transplantation of the optic nerve: developing a clinical strategy. Br J Ophthalmol 82:577-583.
Sahenk Z, Nagaraja HN, MeCracken BS (2005) Nt-3 promotes nerve regeneration and sensory improvement in CMT1A mouse models and in patients. Neurology 65:681.
Solomon AS, Lavie V, Hauben U, Monsonego A, Yoles E, Schwartz M (1996) Complete transection of rat optic nerve while sparing the meninges and the vasculature: an experimental model for optic nerve neuropathy and trauma. J Neurosci Methods 70:21-25.
Woodhall E, West AK, Chuah MI (2001) Cultured olfactory ensheathing cells express nerve growth factor, brain-derived neurotrophic factor,glia cell line-derived neurotrophic factor and their receptors. Brain Res Mol Brain Res 88:203-213.
Zhang ZJ, Li YJ, Liu XG, Huang FX, Liu TJ, Jiang DM, Lv XM, Luo M (2015) Human umbilical cord blood stem cells and brain-derived neurotrophic factor for optic nerve injury: a biomechanical evaluation. Neural Regen Res 10:1134-1138.
Copyedited by Slong-Murphy J, Raye W, Wang J, Li CH, Song LP, Zhao M
10.4103/1673-5374.184505
How to cite this article: Yin DP, Chen QY, Liu L (2016) Synergetic effects of ciliary neurotrophic factor and olfactory ensheathing cells on optic nerve reparation (complete translation). Neural Regen Res 11(6)∶1006-1012.
Funding: This study was supported by the Dean Fund of Jinan Military General Hospital in 2015, No. 2015QN02.
*Correspondence to: Lin Liu, linliu@sh163.net.
 中國(guó)神經(jīng)再生研究(英文版)2016年6期
中國(guó)神經(jīng)再生研究(英文版)2016年6期
- 中國(guó)神經(jīng)再生研究(英文版)的其它文章
- Bone marrow mesenchymal stem cell therapy in ischemic stroke: mechanisms of action and treatment optimization strategies
- Optic radiation injury in a patient with intraventricular hemorrhage: a diffusion tensor tractography study
- miR-148b-3p promotes migration of Schwann cells by targeting cullin-associated and neddylationdissociated 1
- Transplantation of human adipose tissue-derived stem cells for repair of injured spiral ganglion neurons in deaf guinea pigs
- Indirubin-3′-monoxime suppresses amyloid-betainduced apoptosis by inhibiting tau hyperphosphorylation
- ROCK inhibition enhances neurite outgrowth in neural stem cells by upregulating YAP expression in vitro
