Fenton-like oxidation of azo dye in aqueous solution using magnetic Fe3O4-MnO2nanocomposites as catalysts
Zhen-dong Fang,Kai Zhang,Jie Liu,Jun-yu Fan,Zhi-wei Zhao*
Department of National Defense Architecture Planning and Environmental Engineering,Logistical Engineering University,Chongqing 401311,China
1.Introduction
Refractory organic matter in natural water may cause a deficiency of oxygen,generate aquatic toxins,and have other harmful effects on living organisms(Soon and Hameed,2011).However,the traditional water treatment process cannot degrade refractory organic matter efficiently(Nidheesh,2015;Xing et al.,2011).It is necessary to develop efficient,practical,and low-cost water treatment processes for removal of refractory organic matter.
As an advanced oxidation process,the heterogeneous Fenton process can not only produce hydroxyl radicals to oxidize organic matters rapidly(He et al.,2016;Liu et al.,2016;Zhang et al.,2009;Ramirez et al.,2007),but also overcome the drawbacks of the homogeneous Fenton process,including the production of nondisposable sludge and the narrow range of operative pH values(Hou et al.,2014;Wang et al.,2017;Munoz et al.,2015).Fe3O4(Costa et al.,2008),Fe2O3(Zhang et al.,2010),FeS2(Liu et al.,2015b),Fe0(Segura et al.,2012),CuO(Pan et al.,2015),CeO2(Gogoi et al.,2017),and MnO2(Cui et al.,2011)have all been used as heterogeneous Fenton-like catalysts.MnO2has received intense attention due to its large surface area,low cost,environmental friendliness,and high level of stability in neutral media.Moreover,MnO2of various crystal phases,including the α,β,γ,and δ types,has shown significant catalytic ability in Fenton-like reactions under neutral conditions.Zhang et al.(2006)used β-MnO2nanorods as Fenton-like catalysts to oxidize the methylene blue dye in aqueous solutions,and found that the degree of decoloration could reach 95%in only 15 min,demonstrating the high catalytic ability of β-MnO2.Kim et al.(2017)reported that the catalytic ability of MnO2was strongly related to the crystal structures,and the catalytic abilities associated with different crystal structures could be ranked in the following descending order:γ-MnO2,β-MnO2,α-MnO2,and δ-MnO2.However,due to the low apparent density of MnO2,ultrafine particles are usually formed in the water,resulting in difficulty in achieving solid-liquid separation after use.
Fe3O4is sensitive to external magneticfields,and its structure is uniform and stable.Moreover,the high activity for activation of H2O2(Gao et al.,2007),persulfate(Yan et al.,2011),peroxymonosulfate(PMS)(Liu et al.,2017),and O3(Yin et al.,2016)due to the existence of Fe2+in the crystal has been demonstrated.Thus,Fe3O4has usually been used as supporter in composites for catalysis reaction.In our previous work,Fe3O4-MnO2core-shell nanocomposites were synthesized as PMS activators for oxidative removal of 4-chlorophenol(Liu et al.,2015a).The results showed that the core-shell nanocomposites not only activated the PMS to generate sulfate radicals efficiently,but can also be separated rapidly with external magneticfields.However,the application of Fe3O4-MnO2nanocomposites as heterogeneous Fenton catalysts has not been reported.
In this study,Fe3O4-MnO2core-shell nanocomposites were used for thefirst time as Fenton-like catalysts for degradation of acid orange 7(AO7)in an aqueous solution.The effects of different initial conditions on the removal efficiency of AO7,including pH,catalyst dosage,oxidant dosage,and temperature,were investigated,and the catalytic mechanism was also analyzed based on the results.
2.Experiment setup
2.1.Materials
All chemicals used in the experiments were analytical grade.Ferrous sulfate(FeSO4·7H2O),potassium permanganate(KMnO4),hydrochloric acid(HCl),boric acid(H3BO3),sodium tetraborate(Na2B4O7·10H2O),and sodium hydroxide(NaOH)were obtained from Xilong Chemicals,Co.,Ltd.(Shantou,China).Polyvinylpyrrolidone(PVP)K-30,tert-butyl alcohol(TBA),chloroform(CHCl3),and AO7 were purchased from Sinopharm Chemical Reagent Co.,Ltd.(Shanghai,China).
2.2.Synthesis of catalysts
1.668 g of FeSO4·7H2O and 1 g of PVP were dissolved in 100 mL of water and heated to 353 K.Then,6 mL of NaOH solution(2 mol/L)were added to the solution,while it was stirred.Five minutes after the addition of 10 mL of KMnO4solution(0.1 mol/L),the solution of precipitation changed from dark green to dark brown.After stirring at 353 K for 2 h,the mixture was cooled to room temperature,and then washed with ultrapure water and ethanol three times alternately.The solids were dried under a vacuum to a constant weight.
2.3.Character of catalysts
Scanning electron microscopy(SEM)images were obtained fromascanningelectronmicroscope(FEINovaNanoSEM450,USA).Transmission electron microscopy(TEM)images were acquiredusingatransmissionelectronmicroscope(TecnaiF20,USA)operated at 200 kV.Brunauer-Emmett-Teller(BET)surface area measurements of the samples were collected at 77 K by dinitrogen with an accelerated surface area and porosimetry system(Micrometrics ASAP 2020,USA).A vibrating sample magnetometer(VSM JDM-13,China)was used to determine the magnetic properties of the resultant samples.
2.4.Experimental procedure
The experiment was carried out in a 250-mL conicalflask.First,both the 25 mL of AO7 stock solution(200 mg/L)and the 9 mL of H2O2solution(with a mass fraction of 30%)were added into 50 mL of deionized water.The pH value of the solution was adjusted to 5.5 with 25-mmol/L tetraborate buffer.Then,a certain amount of catalyst with ultrasonic dispersion was added into the mixture and diluted to 100 mL.Finally,the conicalflask was placed in an air bath shaker under 303 K.A certain amount of solution was taken by a disposable syringe at afixed time during the reaction.Afterfiltration with a 0.22-μm membrane,0.5 mL of the filtrate was added to a liquid vial containing 0.5 mL of ethanol for a quantitative analysis of AO7.Concentrations of AO7 were determined by high-performance liquid chromatography(Waters 2695).The measurement of dissolved Fe and Mn ions was carried out on an inductively coupled plasma mass spectrometer(ICP-MS,Agilent 7500).
3.Results and discussion
3.1.Character of catalysts
During the synthesis process,four samples were prepared withMnO2weightloadingsof10%(sample 1),15%(sample2),20%(sample 3),and 25%(sample 4).Zhao et al.(2012)demonstrated that MnO2adhered on the surface of Fe3O4in thenanocomposites,formingacore-shellnanoplate.Thetypical SEM andTEM imagesofsample3areshowninFig.1.Fig.1(a)shows that Fe3O4-MnO2nanocomposites have a uniform plate morphology.Meanwhile,Fig.1(b)showsthatamorphousMnO2adhered to the surface of Fe3O4nanoplates,forming an obvious core-shell structure.
The BET surface area and hysteresis loop of the four samples were measured,and the obtained results are listed in Table 1.As can be seen from the results,the variation tendency of the BET surface area and saturation magnetization were similar to those of previous studies.The surface area increased with the MnO2weight loading,while the saturation magnetization of samples had a negative correlation with the MnO2weight loading.
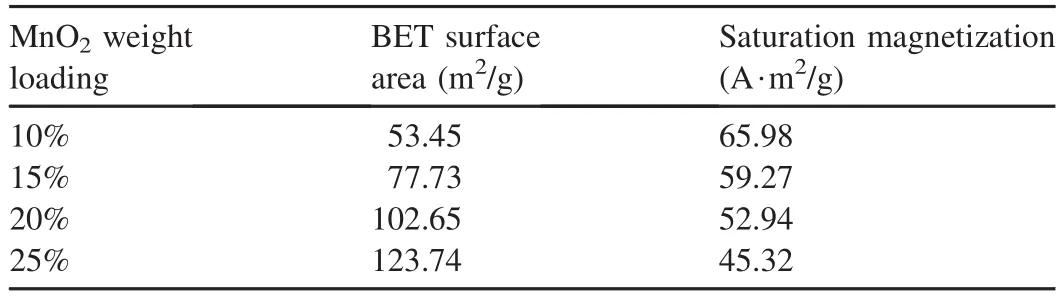
Table 1 Physiochemical properties of Fe3O4-MnO2magnetic nanocomposites.
3.2.Catalytic activity of Fe3O4-MnO2nanocomposites
The removal efficiency of AO7 in different reaction systems was investigated under the conditions of a pH value of 5.5,a temperature of 303 K,an initial AO7 concentration of 50 mg/L,a catalyst dosage of 0.6 g/L,and an H2O2dosage of 9 mL.The experimental results are shown in Fig.2,where C is the AO7 concentration,and C0is the initial AO7 concentration.Fig.2 shows that,when the Fe3O4-MnO2nanocomposites alone are added,the AO7 concentration in the solution does not change significantly over 120 min,which indicates that the Fe3O4-MnO2nanocomposites have little adsorption capacity for AO7.When the reaction system of Fe3O4/H2O2was used,the AO7 concentration decreased to 40.8 mg/L after 120 min,and the removal efficiency was 18.4%.In the reaction system of MnO2/H2O2,the removal efficiency of AO7 was much higher than that of the Fe3O4/H2O2system,and the removal efficiency was 79.2%over 120 min.When the Fenton catalyst was changed to Fe3O4-MnO2nanocomposites,the removal efficiency of AO7 was greatly improved.When the reaction time was 120 min,the concentration of AO7 was only 1.6 mg/L and the removal efficiency was 96.8%.Based on these results,the catalytic ability of Fe3O4-MnO2nanocomposites is higher than that of the two single components as catalysts,which indicates that there is a synergetic effect between Fe3O4and MnO2in the Fe3O4-MnO2nanocomposites,significantly enhancing the catalytic capacity of Fe3O4-MnO2nanocomposites.
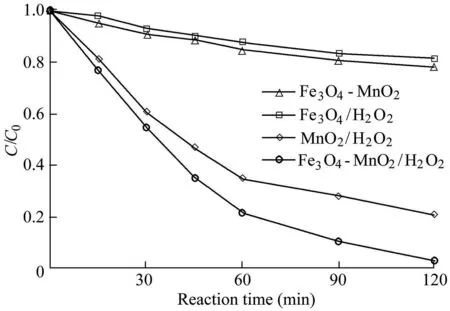
Fig.2.Degradation of AO7 in different systems.
3.3.Optimization of different MnO2weight loadings of Fe3O4-MnO2nanocomposites
For Fe3O4-MnO2core-shell nanocomposites,a higher Fe/Mn molar ratio means a lesser thickness of the shell MnO2.For a H2O2catalytic reaction at near-neutral pH conditions,the catalytic ability of MnO2is stronger than that of Fe3O4,so the nanocomposites with greater MnO2weight loading may have a greater catalytic ability.In the experiment,four kinds of Fe3O4-MnO2core-shell materials with various MnO2weight loadings were synthesized and compared in terms of their catalytic ability.In the experiment,the catalyst dosage was 600 mg/L,the H2O2dosage was 9 mL,the temperature was 303 K,the initial AO7 concentration was 50 mg/L,and the pH value was 5.5.
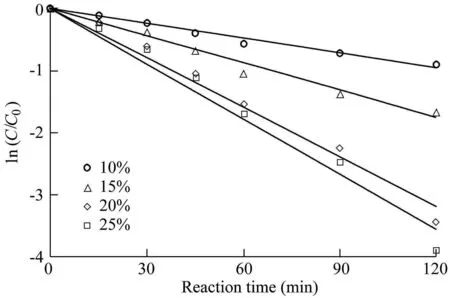
Fig.3.Influence of Fe3O4-MnO2core-shell nanocomposites with various MnO2weight loadings on degradation of AO7.
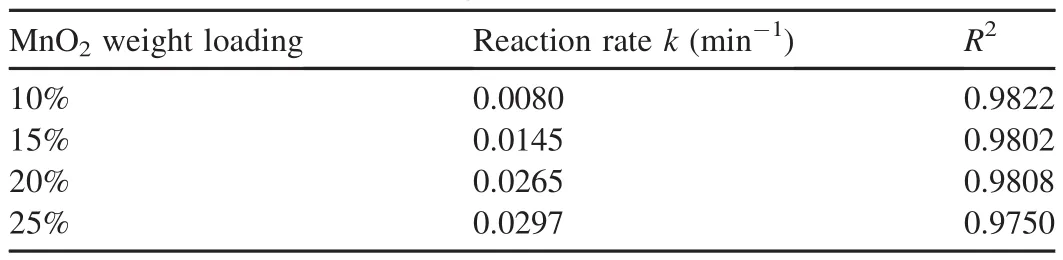
Table 2 Reaction rates calculated from Fig.3.
The experimental results are shown in Fig.3,and the reaction rates calculated from Fig.3 are listed in Table 2,where R2is the coefficient of determination.The results show that the removal efficiency of AO7 was lowest when the MnO2weight loading was 10%.Meanwhile,the removal efficiency of AO7 increased when the MnO2weight loading increased.When the MnO2weight loading increased from 10%to 20%,the reaction rate increased from 0.0080 min-1to 0.0265 min-1,and the removal efficiency of AO7 increased from 59.2%to 96.8%.However,when the MnO2weight loading increased from 20%to 25%,the reaction rate of AO7 increased gradually from 0.0265 min-1to 0.0297 min-1,and the removal efficiency increased from 96.8%to 98.0%.The results show that the catalytic ability of Fe3O4-MnO2core-shell nanocomposites is enhanced with the increase of MnO2weight loading.On the other hand,the increase of the MnO2weight loading from 20%to 25%leads to a slight growth in the reaction rate.
Our previous study showed that the complex Fe-Mn oxides between Fe3O4and MnO2in the Fe3O4-MnO2core-shell nanocomposites had greater catalytic ability than single components(Liu et al.,2015a).When the MnO2weight loading is low,the surface of Fe3O4is not completely covered by MnO2,and the amount of complex Fe-Mn oxides is less.With the increase of the MnO2weight loading,the interaction between Fe3O4and MnO2increases,so when the MnO2weight loading increases from 10%to 20%,the catalytic ability increases.At MnO2weight loading of 20%,the surface of Fe3O4may be completely covered by MnO2.When the content of MnO2further increases,the shell of MnO2thickens,which increases the number of active sites but hinders the contact between the Fe3O4core and the complex Fe-Mn oxides and H2O2,so the catalytic ability of nanocomposites with MnO2weight loading of 25%shows little growth compared with those with MnO2weight loading of 20%.Therefore,the optimal MnO2weight loading is 20%.In the experiments described below,nanocomposites with MnO2weight loading of 20%were used as catalysts.
3.4.Effects of parameters on catalytic ability of Fe3O4-MnO2/H2O2system
The effects of catalyst dosage,oxidant dosage,temperature,and initial pH on the removal efficiency of AO7 in the Fe3O4-MnO2/H2O2system were also investigated.
In the experiment regarding catalyst dosage,the H2O2dosage was 9 mL,the initial pH value was 5.5,the temperature was 303 K,the initial AO7 concentration was 50 mg/L,and the dosages of the catalysts were 0.2,0.4,0.6,and 0.8 g/L,respectively.The experimental results are shown in Fig.4,and the reaction rates calculated from Fig.4 are listed in Table 3.
As can be seen from the results provided above,the reaction rate of AO7 was 0.014 min-1when the catalyst dosage was 0.2 g/L.When the dosage reached 0.6 g/L,the reaction rate increased to 0.0265 min-1.However,when the dosage increased to 0.8 g/L,the reaction rate decreased to 0.0215 min-1.The main reason is that when the catalyst dosage is less than 0.6 g/L,the increase of the catalyst dosage cannot increase the number of active sites in the system,resulting in increases in the decomposition of H2O2and the generation of hydroxyl radicals,which are favorable for the degradation of AO7.When the catalyst dosage is further increased,the collision probability of the nanoparticles in the solution is greatly increased,which causes the nanoparticles to gradually agglomerate and obscures some active sites(Zhang et al.,2013).On the other hand,the increase of the solid particles also affects the mass transfer rates of H2O2and AO7 in the solution(Xu and Wang,2011),thus leading to a decrease in the catalytic ability.
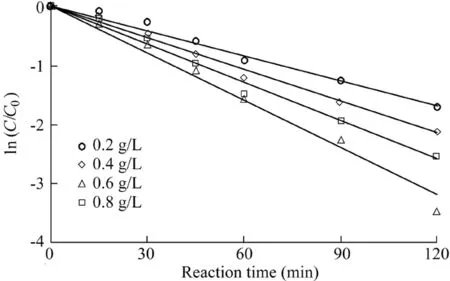
Fig.4.Influence of catalyst dosage on degradation of AO7.
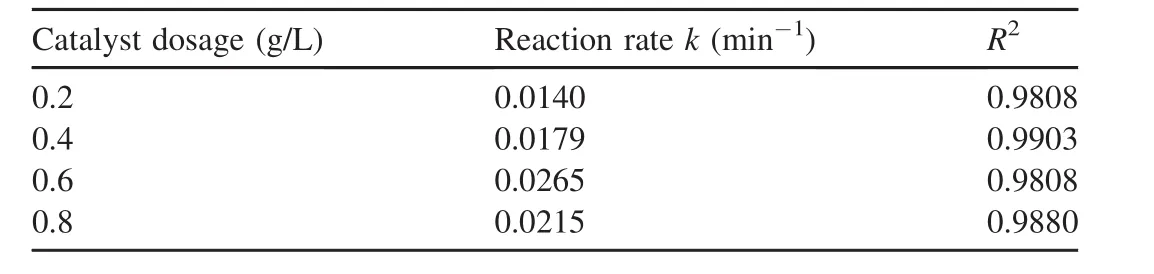
Table 3 Reaction rates calculated from Fig.4.
For the Fenton system,the dosage of H2O2should be moderate,because the deficiency of the oxidant dosage may impede the complete degradation of the pollutants.In addition,as the dosage is too large,the generated hydroxyl radicals will continue to react with H2O2,leading to a decrease in the pollutant removal efficiency(Luo et al.,2010).In the experiment,the effects of the H2O2dosage on the removal effi-ciency of AO7 were investigated.The reaction conditions were as follows:the catalyst dosage was 600 mg/L,the temperature was 303 K,the initial AO7 concentration was 50 mg/L,and the pH value was 5.5.The experimental results are shown in Fig.5.When the H2O2dosage was between 3 and 9 mL,the increase of H2O2dosage increased the removal efficiency of AO7.When the dosage of H2O2increased from 9 to 15 mL,the removal efficiency of AO7 showed a slight increase,indicating that some H2O2may become a radical quencher,leading to a weak rise in the AO7 removal efficiency.
The pH of the solution has a strong influence on the Fenton reaction system(Pignatello et al.,2006).The degradation of AO7 with the initial pH values of 3.5,5.5,and 7.5 was investigated.Other reaction conditions were as follows:the catalyst dosage was 600 mg/L,the H2O2dosage was 9 mL,the temperature was 303 K,and the initial AO7 concentration was 50 mg/L.The removal efficiency of AO7 was highest at the initial pH value of 3.5,and reached 90.4%at 15 min(Fig.6).When the initial pH values of the solution were 5.5 and 7.5,the removal efficiencies of AO7 over 120 min were 96.8%and 77.2%,respectively.It can be seen that a lower pH leads to greater degradation of AO7.However,AO7 can be degraded efficiently over a wide range of pH,showing that the reaction system is suitable for application in practical engineering.
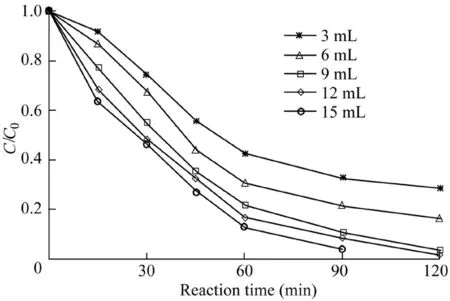
Fig.5.Influence of H2O2dosage on degradation of AO7.
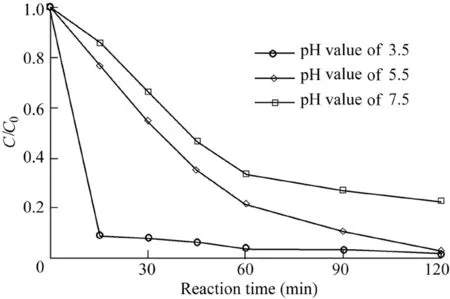
Fig.6.Influence of pH on degradation of AO7.
The reaction temperature is a critical factor in the Fenton process,because a higher temperature can enhance the reaction rate(Saputra et al.,2013a).Therefore,the effect of temperature on AO7 degradation was investigated and the results are shown in Fig.7 and Fig.8.The reaction conditions were as follows:the catalyst dosage was 600 mg/L,the H2O2dosage was 9 mL,the initial AO7 concentration was 50 mg/L,and the pH value was 5.5.It can be seen that the effect of temperature on the reaction rate and AO7 removal efficiency was positive.The removal efficiency was 97.2%over 90 min and the reaction rate was 0.0381 min-1when the reaction temperature was 313 K,much higher than the result when it was 293 K.The activation energy of the reaction system calculated from the data in Fig.8 was 31.47 kJ/mol,revealing that the chemical reaction rate was the limiting factor in the Fe3O4-MnO2/H2O2system rather than mass transfer(Xu and Wang,2012).
3.5.Stability test
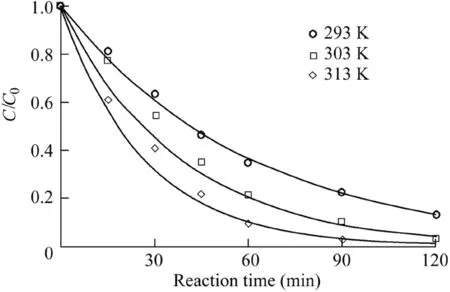
Fig.7.Effects of temperature on degradation of AO7.
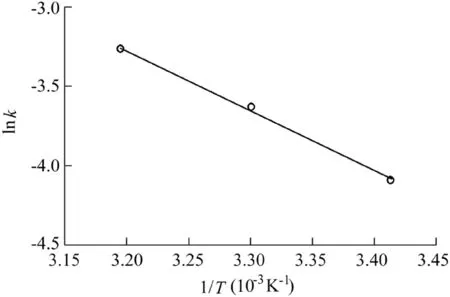
Fig.8.Relationship between lnk and 1/T.
The stability and reusability of the Fe3O4-MnO2nanocomposites were evaluated through cycle catalytic reactions.The reaction conditions were as follows:the catalyst dosage was 600 mg/L,the H2O2dosage was 9 mL,the temperature was 303 K,the initial AO7 concentration was 50 mg/L,and the pH value was 5.5.After each reaction,the used catalysts were washed with pure water and added into the system.The results are shown in Fig.9.As can be seen,with the increase of the number of cycle runs,the removal efficiency of AO7 decreased slightly.After seven runs,the removal efficiency of AO7 decreased from 96.8%to 83.1%,while the removal efficiency of total organic carbon(TOC)decreased from 46.5%to 31.6%.Both of these changes demonstrated the stability of Fe3O4-MnO2nanocomposites in the heterogeneous Fenton process.In addition,the decrease of the removal efficiency of AO7 may have been due to the adsorption of intermediates on the surface of catalysts(Saputra et al.,2013b).On the other hand,no detectable Fe and Mn ions appeared in any of the seven runs.These results demonstrate the stability of the Fe3O4-MnO2nanocomposites in the heterogeneous Fenton process.
3.6.Mechanism of catalysis
In order to explore the species of generated radicals during the catalytic reactions,radical quenching experiments were carried out.TBA was used as a scavenger of hydroxyl radicals(with a reaction rate of 5.2 × 108L/(mol·s))(Huang et al.,2015),while chloroform was used as an O2·-scavenger(with a reaction rate of 3 × 1010L/(mol·s))(Wang et al.,2011).The reaction conditions were as follows:the catalyst dosage was 600 mg/L,the H2O2dosage was 9 mL,the temperature was 303 K,the initial AO7 concentration was 50 mg/L,and the pH value was 5.5.As shown in Fig.10(a),compared with the removal efficiency of 96.8%in the absence of a scavenger,the removal efficiency of AO7 decreased to 67.6%and 36.3%with the addition of TBA into the system from 2 mmo/L to 4 mmol/L.The results show that HO·was generated in the catalytic reaction and was the dominant reactive oxygen species for the AO7 degradation.Meanwhile,the results shown in Fig.10(b)demonstrate that the addition of chloroform into the system had little influence on the AO7 removal.O2·-was produced in the reaction but its role in the AO7 degradation was limited.
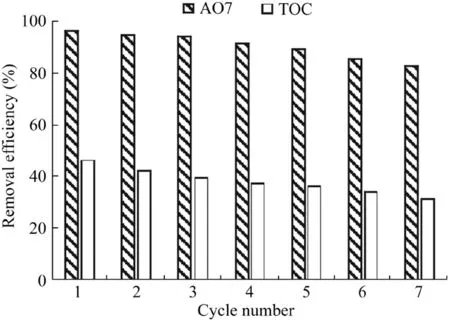
Fig.9.Stability of Fe3O4-MnO2core-shell nanocomposites in repeated batch AO7 and TOC degradation experiments.
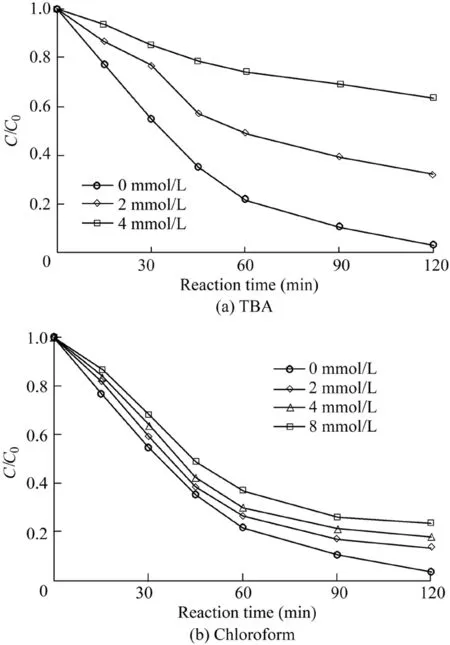
Fig.10.Influence of two radical scavengers on degradation of AO7.
Based on the results and studies reported by Jaafarzadeh et al.(2015),a catalytic mechanism is proposed(Fig.11).
First,H2O2is adsorbed onto the surface of catalysts(Eq.(1)),and then it generates HO2·,while≡Mn4+is reduced to ≡Mn2+(Eq.(2)).Meanwhile,≡Fe3+in Fe3O4can be reduced to≡Fe2+by H2O2and generate HO2·(Eq.(3)).The generated≡Fe2+can obtain electrons from≡Mn4+for transfer to≡Fe3+(Eq.(4)),while≡Mn4+is transferred to≡Mn2+.Furthermore,≡Mn2+species may adhere to H2O2and be oxidized to≡Mn4+(Eq.(5)),while HO·is released into the solution.The HO2· in the solution may decompose to H+and O2·-(Eq.(6)).Meanwhile,HO·can oxidize AO7 to generate intermediate(Eq.(7)).
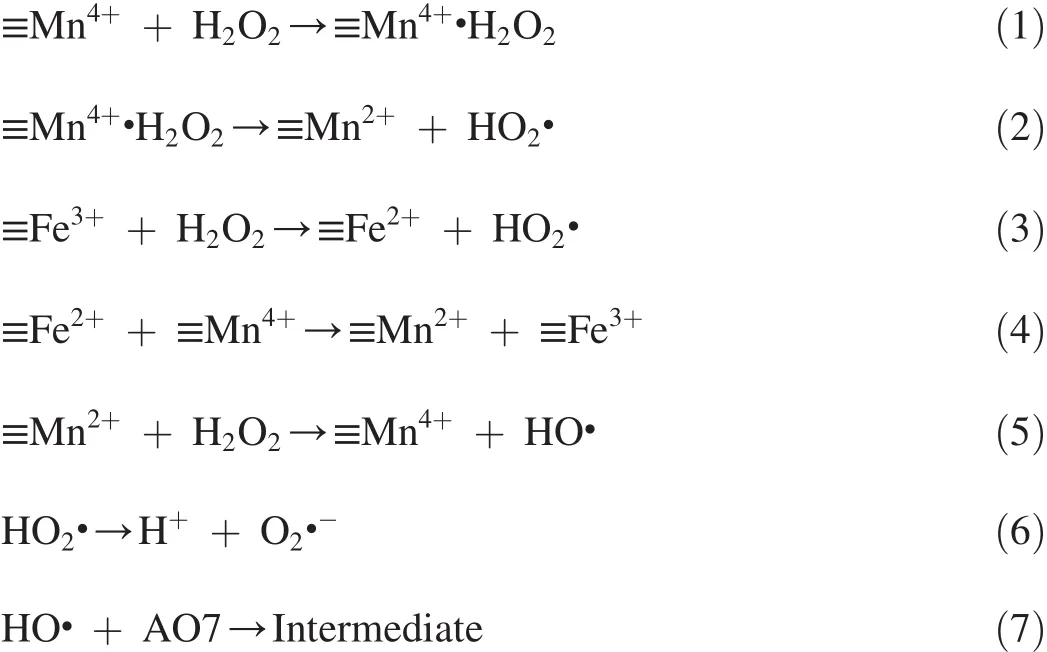
4.Conclusions
Fe3O4-MnO2core-shell nanocomposites were prepared and used for thefirst time as a heterogeneous Fenton catalyst for catalytic oxidation of AO7 in an aqueous solution.The experimental results showed that Fe3O4-MnO2nanocomposites had a greater catalytic ability than Fe3O4or MnO2used alone.The removal efficiency of AO7 was 96.8%over 120 min.The Fe3O4-MnO2nanocomposites had the greatest catalytic ability when used with a MnO2weight loading of 20%.Catalystdosage,H2O2dosage,initialpH,and temperature of the reaction had strong effects on the AO7 degradation.The chemical reaction rate was the limiting factor in the Fe3O4-MnO2/H2O2system rather than mass transfer according to thermodynamic calculation.The Fe3O4-MnO2nanocomposites showed a high degree of stability and reusability.The hydroxyl radicals were the main radicals in the catalytic system.Based on the experimental results and studies,a mechanism for the reaction process in the Fe3O4-MnO2/H2O2system has been proposed.
Costa,R.C.C.,Moura,F.C.C.,Ardisson,J.D.,Fabris,J.D.,Lago,R.M.,2008.Highly active heterogeneous Fenton-like systems based on Fe0/Fe3O4composites prepared by controlled reduction of iron oxides.Appl.Catal.B Environ.83,131-139.https://doi.org/10.1016/j.apcatb.2008.01.039.
Cui,H.,Huang,H.,Fu,M.,Yuan,B.,Pearl,W.,2011.Facile synthesis and catalytic properties of single crystalline β-MnO2nanorods.Catal.Commun.12(14),1339-1343.https://doi.org/10.1016/j.catcom.2011.05.013.
Gao,L.,Zhuang,J.,Nie,L.,Zhang,J.,Zhang,Y.,Gu,N.,Wang,T.,Feng,J.,Yang,D.,Perrett,S.,Yan,X.,2007.Intrinsic peroxidase-like activity of ferromagnetic nanoparticles.Nat.Nanotechnol.2(9),577-583.https://doi.org/10.1038/nnano.2007.260.
Gogoi,A.,Navgire,M.,Sarma,K.C.,Gogoi,P.,2017.Fe3O4-CeO2metal oxide nanocomposite as a Fenton-like heterogeneous catalyst for degradation of catechol.Chem.Eng.J.311,153-162.https://doi.org/10.1016/j.cej.2016.11.086.
He,J.,Yang,X.,Men,B.,Wang,D.,2016.Interfacial mechanisms of heterogeneous Fenton reactions catalyzed by iron-based materials:A review.J.Environ.Sci.39,97-109.https://doi.org/10.1016/j.jes.2015.12.003.
Hou,L.,Zhang,Q.,J′er^ome,F.,Duprez,D.,Zhang,H.,Royer,S.,2014.Shapecontrolled nanostructured magnetite-type materials as highly efficient Fenton catalysts.Appl.Catal.B Environ.144,739-749.https://doi.org/10.1016/j.apcatb.2013.07.072.
Huang,R.,Liu,Y.,Chen,Z.,Pan,D.,Li,Z.,Wu,M.,Shek,C.,Wu,C.M.L.,Lai,J.K.L.,2015.Fe-species-loaded mesoporous MnO2superstructural requirements for enhanced catalysis.ACS Appl.Mater.Interfaces 7(7),3949-3959.https://doi.org/10.1021/am505989j.
Jaafarzadeh,N.,Kakavandi,B.,Takdastan,A.,Kalantary,R.R.,Azizi,M.,Jorfi,S.,2015.Powder activated carbon/Fe3O4hybrid composite as a highly efficient heterogeneous catalyst for Fenton oxidation of tetracycline:Degradation mechanism and kinetic.RSC Adv.5(103),84718-84728.https://doi.org/10.1039/C5RA17953J.
Kim,E.,Oh,D.,Lee,C.,Gong,J.,Kim,J.,Chang,Y.,2017.Manganese oxide nanorods as a robust Fenton-like catalyst at neutral pH:Crystal phasedependent behavior.Catal.Today 282,71-76.https://doi.org/10.1016/j.cattod.2016.03.034.
Liu,J.,Zhao,Z.,Shao,P.,Cui,F.,2015a.Activation of peroxymonosulfate with magnetic Fe3O4-MnO2core-shell nanocomposites for 4-chlorophenol degradation.Chem.Eng.J.262,854-861.
Liu,J.,Zhao,Z.,Ding,Z.,Fang,Z.,Cui,F.,2016.Degradation of 4-chlorophenol in a Fenton-like system using Au-Fe3O4magnetic nanocomposites as the heterogeneous catalyst at near neutral conditions.RSC Adv.6(58),53080-53088.https://doi.org/10.1039/C6RA10929B.
Liu,J.,Zhou,J.,Ding,Z.,Zhao,Z.,Xu,X.,Fang,Z.,2017.Ultrasound irritation enhanced heterogeneous activation of peroxymonosulfate with Fe3O4for degradation of azo dye.Ultrason.Sonochemistry 34,953-959.https://doi.org/10.1016/j.ultsonch.2016.08.005.
Liu,W.,Wang,Y.,Ai,Z.,Zhang,L.,2015b.Hydrothermal synthesis of FeS2as a high-efficiency Fenton reagent to degrade alachlor via superoxidemediated Fe(II)/Fe(III)cycle.ACS Appl.Mater.Interfaces 7(51),28534-28544.https://doi.org/10.1021/acsami.5b09919.
Luo,W.,Zhu,L.,Wang,N.,Tang,H.,Cao,M.,She,Y.,2010.Efficient removal of organic pollutants with magnetic nanoscaled BiFeO3as a reusable heterogeneous Fenton-like catalyst.Environ.Sci.Technol.44(5),1786-1791.https://doi.org/10.1021/es903390g.
Munoz,M.,de Pedro,Z.M.,Casas,J.A.,Rodriguez,J.J.,2015.Preparation of magnetite-based catalysts and their application in heterogeneous Fenton oxidation:A review.App.Catal.B:Environ.176-177,249-265.https://doi.org/10.1016/j.apcatb.2015.04.003.
Nidheesh,P.V.,2015.Heterogeneous Fenton catalysts for the abatement of organic pollutants from aqueous solution:A review.RSC Adv.5(51),40552-40577.https://doi.org/10.1039/C5RA02023A.
Pan,W.,Zhang,G.,Zheng,T.,Wang,P.,2015.Degradation of p-nitrophenol using CuO/Al2O3as a Fenton-like catalyst under microwave irradiation.RSC Adv.5(34),27043-27051.https://doi.org/10.1039/C4RA14516J.
Pignatello,J.J.,Oliveros,E.,MacKay,A.,2006.Advanced oxidation processes for organic contaminant destruction based on the Fenton reaction and related chemistry.Crit.Rev.Environ.Sci.Technol.36(1),1-84.https://doi.org/10.1080/10643380500326564.
Ramirez,J.H.,Costa,C.A.,Madeira,L.M.,Mata,G.,Vicente,M.A.,Rojas-Cervantes,M.L.,Martín-Aranda,R.M.,2007.Fenton-like oxidation of Orange II solutions using heterogeneous catalysts based on saponite clay.Appl.Catal.B Environ.71(1),44-56.https://doi.org/10.1016/j.apcatb.2006.08.012.
Saputra,E.,Muhammad,S.,Sun,H.,Ang,H.M.,Tad′e,M.O.,Wang,S.,2013a.Different crystallographic one-dimensional MnO2nanomaterials and their superior performance in catalytic phenol degradation.Environ.Sci.Technol.47(11),5882-5887.https://doi.org/10.1021/es400878c.
Saputra,E.,Muhammad,S.,Sun,H.,Ang,H.,Tad′e,M.O.,Wang,S.,2013b.A comparative study of spinel structured Mn3O4,Co3O4and Fe3O4nanoparticles in catalytic oxidation of phenolic contaminants in aqueous solutions.J.Colloid Interface Sci.407,467-473.https://doi.org/10.1016/j.jcis.2013.06.061.
Segura,Y.,Martínez,F.,Melero,J.A.,Molina,R.,Chand,R.,Bremner,D.H.,2012.Enhancement of the advanced Fenton process(Fe0/H2O2)by ultrasound for the mineralization of phenol.Appl.Catal.B Environ.113-114,100-106.https://doi.org/10.1016/j.apcatb.2011.11.024.
Soon,A.N.,Hameed,B.H.,2011.Heterogeneous catalytic treatment of synthetic dyes in aqueous media using Fenton and photo-assisted Fenton process. Desalination 269(1-3), 1-16. https://doi.org/10.1016/j.desal.2010.11.002.
Wang,H.,Zhao,Y.,Su,Y.,Li,T.,Yao,M.,Qin,C.,2017.Fenton-like degradation of 2,4-dichlorophenol using calcium peroxide particles:Performance and mechanisms.RSC Adv.7(8),4563-4571.https://doi.org/10.1039/C6RA26754H.
Wang,N.,Zhu,L.,Lei,M.,She,Y.,Cao,M.,Tang,H.,2011.Ligand-induced drastic enhancement of catalytic activity of nano-BiFeO3for oxidative degradation of bisphenol A.ACS Catal.1(10),1193-1202.https://doi.org/10.1021/cs2002862.
Xing,S.,Zhou,Z.,Ma,Z.,Wu,Y.,2011.Characterization and reactivity of Fe3O4/FeMnOx core/shell nanoparticles for methylene blue discoloration with H2O2.Appl.Catal.B Environ.107(3-4),386-392.https://doi.org/10.1016/j.apcatb.2011.08.002.
Xu,L.,Wang,J.,2011.A heterogeneous Fenton-like system with nanoparticulate zero-valent iron for removal of 4-chloro-3-methyl phenol.J.Hazard.Mater.186(1),256-264.https://doi.org/10.1016/j.jhazmat.2010.10.116.
Xu,L.,Wang,J.,2012.Magnetic nanoscaled Fe3O4/CeO2composite as an efficient Fenton-like heterogeneous catalyst for degradation of 4-chlorophenol.Environ.Sci.Technol.46(18),10145-10153.https://doi.org/10.1021/es300303f.
Yan,J.,Lei,M.,Zhu,L.,Anjum,M.N.,Zou,J.,Tang,H.,2011.Degradation of sulfamonomethoxine with Fe3O4magnetic nanoparticles as heterogeneous activator of persulfate.J.Hazard.Mater.186(2-3),1398-1404.https://doi.org/10.1016/j.jhazmat.2010.12.017.
Yin,R.,Guo,W.,Zhou,X.,Zheng,H.,Du,J.,Wu,Q.,Chang,J.,Ren,N.,2016.Enhanced sulfamethoxazole ozonation by noble metal-free catalysis based on magnetic Fe3O4nanoparticles:Catalytic performance and degradation mechanism.RSC Adv.6(23),19265-19270.https://doi.org/10.1039/C5RA25994K.
Zhang,G.,Gao,Y.,Zhang,Y.,Guo,Y.,2010.Fe2O3-pillared rectorite as an efficientand stable Fenton-like heterogeneouscatalystforphotodegradation of organic contaminants.Environ.Sci.Technol.44(16),6384-6389.https://doi.org/10.1021/es1011093.
Zhang,S.,Zhao,X.,Niu,H.,Shi,Y.,Cai,Y.,Jiang,G.,2009.Superparamagnetic Fe3O4nanoparticles as catalysts for the catalytic oxidation of phenolic and aniline compounds.J.Hazard.Mater.167(1-3),560-566.https://doi.org/10.1016/j.jhazmat.2009.01.024.
Zhang,T.,Zhu,H.,Crou′e,J.,2013.Production of sulfate radical from peroxymonosulfate induced by a magnetically separable CuFe2O4spinel in water:Efficiency,stability,and mechanism.Environ.Sci.Technol.47(6),2784-2791.https://doi.org/10.1021/es304721g.
Zhang,W.,Yang,Z.,Wang,X.,Zhang,Y.,Wen,X.,Yang,S.,2006.Largescale synthesis of β-MnO2nanorods and their rapid and efficient catalytic oxidation of methylene blue dye.Catal.Commun.7(6),408-412.https://doi.org/10.1016/j.catcom.2005.12.008.
Zhao,Z.,Liu,J.,Cui,F.,Feng,H.,Zhang,L.,2012.One pot synthesis of tunable Fe3O4-MnO2core-shellnanoplatesandtheirapplicationsforwaterpurification.J.Mater.Chem.22(18),9052-9057.https://doi.org/10.1039/C2JM00153E.
 Water Science and Engineering2017年4期
Water Science and Engineering2017年4期
- Water Science and Engineering的其它文章
- Preface for special section on flood modeling and resilience
- Preparation of 2D square-like Bi2S3-BiOCl heterostructures withenhanced visible light-driven photocatalytic performance for dye pollutant degradation
- Effects of urban grass coverage on rainfall-induced runoff in Xi'an loess region in China
- Effect of water-sediment regulation and its impact on coastline and suspended sediment concentration in Yellow River Estuary
- Flood management of Dongting Lake after operation of Three Gorges Dam
- A distributed eco-hydrological model and its application
