Potential of polyphenols in curbing quorum sensing and biofilm formation in Gramnegative pathogens
Arnica F Lal, Shaminder Singh, Francisco C. Franco, Jr., Sonam Bhatia?
1Department of Pharmaceutical Science, SHALOM Institute of Health and Allied Sciences, Sam Higginbottom University of Agriculture, Technology and Sciences, Naini, Prayagraj, India
2Regional Centre for Biotechnology, NCR Biotech Science Cluster, 3rd Milestone, Faridabad-Gurugram Expressway, Faridabad - 121 001, Haryana,India
3Chemistry Department, De La Salle Univers, 2401 Taft Ave., Manila 0922, Philippines
ABSTRACT
KEYWORDS: Polyphenols; Quorum sensing; Biofilm;Recombinant polyphenolic compounds; Sensitizers; Antibiotic resistance
1. Introduction
The plant kingdom contains a heterogeneous group of substances called phenolic compounds produced during their metabolism.Polyphenols are claimed to be present in higher plants as metabolites which are highly specific and play a vital role in response to any external stimuli made by the pathogenic microorganism that can damage plants through their sensory properties like color,astringency, bitterness, and roughness. These phenolic metabolites possess activity against various human diseases; thus, they play a crucial role in promoting human health.
The polyphenolic scaffold comprised a benzene ring with at least one hydroxyl group attached to it and based on their chemical structure; polyphenols are i) phenolic acids, ii) flavonoids, and iii)non-flavonoids depending on the groups present on the chemical entity (Figure 1).
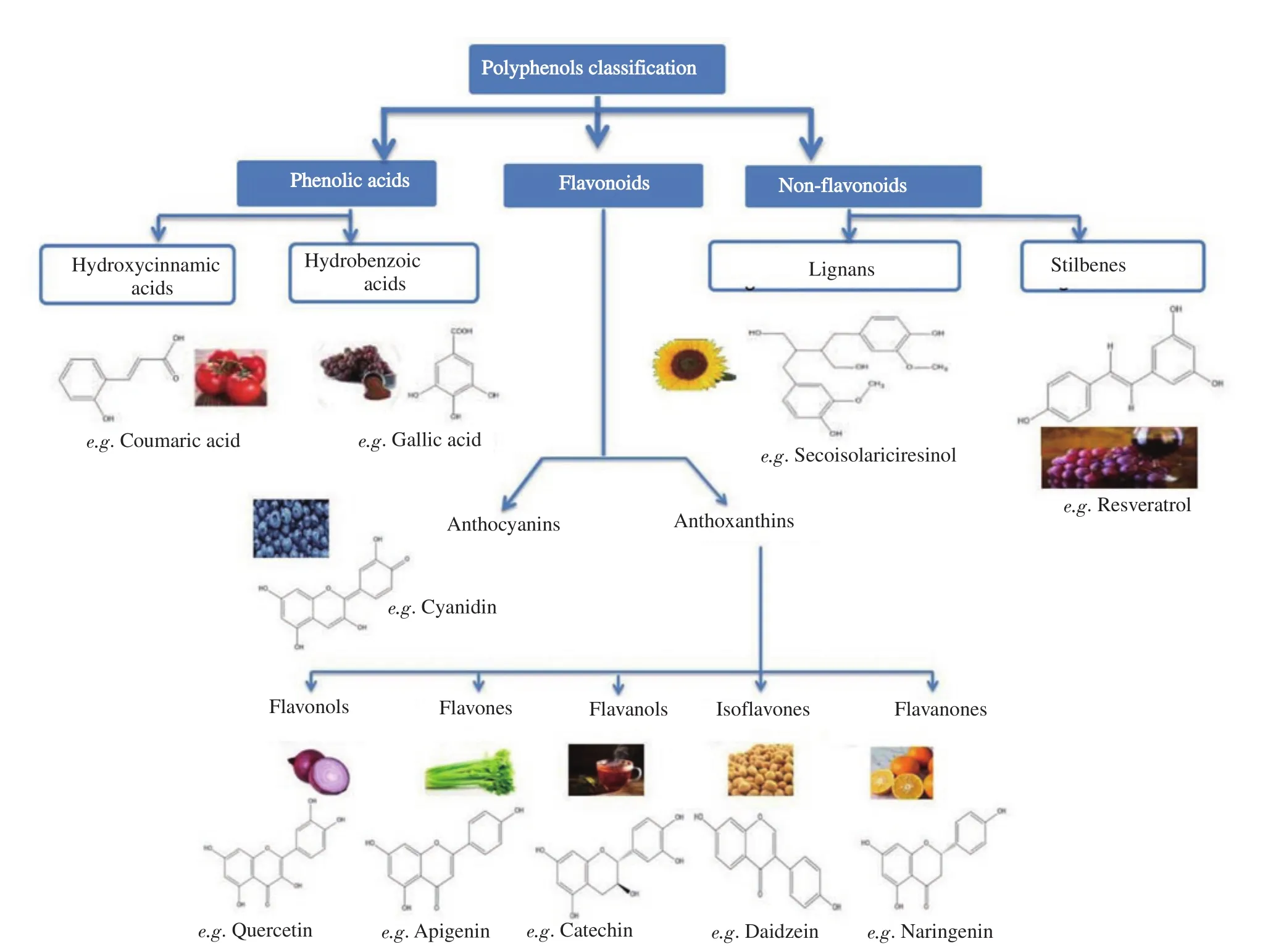
Figure 1. Polyphenol classification.
1.1. Polyphenols classification
1.1.1. Phenolic acids
Phenolic acids are the secondary metabolites that contain two groups: benzoic acids and cinnamic acids. The benzoic acids are the simplest phenolic acids with seven carbon atoms (C6-C1) and are most abundant. Cinnamic acids consist of nine carbon atoms (C6-C3). Their characteristic feature is the presence of a benzenic ring,a carboxylic group, and one or more hydroxyl or methoxyl groups.The human diet contains about one-third of the phenolic compounds as phenolic acids. Many researchers have found that phenolic acids and their esters have high antioxidant activity, especially hydroxybenzoic acid, hydroxycinnamic acid, caffeic acid, and chlorogenic acid[1]. Phenolic acids are abundantly present in cereals,legumes, oil seeds, fruits, vegetables, beverages, and herbs[2].
1.1.2. Flavonoids
Flavanoids consist of benzo-γ-pyrone structure and are hydroxylated phenolic substances that are synthesized by plants in response to microbial infection. Flavonoids have aglycones, glycosides, and methylated derivative portions[3]. Flavonoid phenols contain a backbone of 15 carbon atoms comprising two benzene rings joined by a heterocyclic ring. These compounds have the general structure C6-C3-C6 and are categorized into three groups according to the structure of the heterocycle: flavonols, dihydroflavonols, flavones;anthocyanidins; and flavanols. Catechins and condensed tannins are categorized under flavanols, which are again classified as procyanidins and delphinidins[4].
1.1.3. Non-flavonoid phenols
Non-flavonoid phenols comprise benzoic acids (C6-C1), cinnamic acids (C6-C3), lignans, stilbenes (C6-C2-C6) and coumarins.Hydroxybenzoic and hydroxycinnamic acids also come under the phenolic acid category[5]. Tannins, particularly hydrolyzable tannins,are made of gallic acid and ellagic acid esters of glucose or related sugars. Hydrolyzable tannins are of two types: gallotannins or ellagitannins. The term “hydrolyzable” means that the ester linkage is more susceptible to hydrolysis under normal conditions than the linkages of condensed tannins[6].
Our research group focuses on the potential of polyphenolic compounds that can curb quorum sensing (QS) in Chromobacterium violaceum (C. violaceum) and Pseudomonas aeruginosa (P.aeruginosa), which would be proved as quorum sensing inhibitors(QSI)[7-9].
1.2. Pharmacokinetic and pharmacodynamic of polyphenols
Polyphenols are generally present as ester, glycosides, or polymeric forms, which are not readily absorbed by the gastrointestinal tract[10]; thus, their hydrolysis is the first step before their absorption.These enzymatic reactions of metabolism include oxidation,hydrolysis, and reduction reactions (phase Ⅰ); however, in phase Ⅱmethylation, glucuronidation, or sulfation are major reactions. In the small intestine, phenolics exists as a conjugated molecule. There are many transporters (SGLT1, MRP, P-gp, BRCP, SLC, LPH, etc.)[11](Figure 2) present in the small intestine to facilitate the absorption of polyphenols. The structural feature of a polyphenol determines its intestinal absorption. Supposedly, the high molecular weight causes low recovery in urine, and glycosylation mediates the absorption through the gut barrier; glucosides are much readily absorbed[12]than rhamnosides[13,14]. Polyphenols are readily metabolized either in tissues, after absorption through the gut barrier, and the nonabsorbed fraction or fraction re-excreted in the bile by the colonic microflora. All polyphenols are conjugated to form O-glucuronides,sulfate esters, and O-methyl ether[15]. CYP450 metabolism includes glucuronides and sulfates-associated reactions, out of which CYP enzyme isoforms 1A2, 3A4, 3A5 2B6, 2C8, 2C19, 2C9, 2D6 and 2E1 are involved. The active reaction of CYP450 conjugation enzymes and efflux transporter proteins contributes to the bioavailability[16].
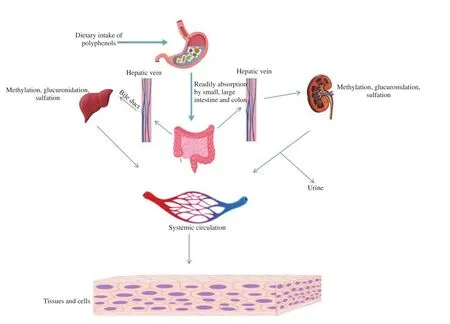
Figure 2. Depiction of absorption, distribution, metabolism, and excretion of polyphenolic compounds.
2. QS in Gram-negative pathogens and its mechanisms
QS, defined as cell-to-cell communication, is used by bacteria to detect changes in their environment and accordingly to implement specific strategies that allow adaptation to environmental stress.Through this process, bacterium generates regulatory mechanisms that help to make collective decisions about the expression of a specific set of genes. QS mechanisms amplify bacterial virulence by stimulating the expression of disease-causing attributes, such as motility, biofilm formation, and secretion of virulence agents of pathogens[17].
Autoinducers, which are low-molecular-weight signaling compounds, are produced by bacteria and are released into the environment. N-acyl-homoserine lactones (AHLs) are the most widely studied signaling compounds of lactone derivatives of fatty acids used by Gram-negative bacteria[18].
The QS system mainly consists of autoinducers, signal synthase,signal receptor, signal response regulator, and regulated genes(which form the so-called QS regulon)[19].
Bacteria use QS systems that are of three main types:
a) the LuxR/I-type systems, primarily used by Gram-negative bacteria;
b) the peptide signaling systems used primarily by Gram-positive bacteria;
c) the LuxS/AI-2 signaling used for interspecies communication.
Besides the above signaling systems, the AI-3/epinephrine/norepinephrine is also considered an interkingdom signaling system[20]. The degradation/inhibition of QS signal molecules (AHL)is called QS inhibition or quorum quenching. The inhibition of QS can be accomplished by the following: (a) enzymatic destruction of QS signal molecules, (b) the development of QS antibodies to signal molecules, (c) agents that block QS known QSI.
Strategies of quorum quenching involve (Ⅰ) inhibition of AHL signal generation, (Ⅱ) inhibition of AHL signal dissemination, (Ⅲ)and inhibition of AHL signal reception[21].
2.1. Mechanism of QS in Gram-negative bacteria
For orderly communication, bacteria must detect, interpret, and integrate extracellular chemical information (autoinducers) and convert it to bring about changes in their gene expression[22]. In Gram-negative bacteria, biosynthesis of AHL lactones (autoinducers in Gram-negative bacteria) is much significant to bring about the functioning of QS. AHLs are small molecules with a neutral charge that are synthesized by the AHL synthase and can diffuse rapidly across the membrane to bind with receptors that are DNA-binding transcription factors known as “R-proteins”. This phenomenon accomplishes when the autoinducer level reaches a threshold level to bring a chemical change within the cell-this binding triggers several transcriptional genes that are essential for bacteria behavior and coordination[23]. Thus, three components, i.e., a signal generator LuxI (synthase), the cognate receptor LuxR (“R proteins”), and acyl-L-homoserine lactone, are involved in QS circuits of Gramnegative bacteria. We discuss the two network architectures using Pseudomonas spp. and Chromobacterium spp. as examples.
2.1.1. QS circuits in P. aeruginosa
Their primary QS signal molecule is N-(3-oxododecanoyl)-L-homoserine lactone (3-oxo-C12-HSL) synthesized by LasI synthase and targeted upon intracellular LasR-type receptor producing complex (3-oxo-C12-HSL)-LasR (Figure 3). When population[24]density and QS advances, the LasI becomes RhlI and makes another autoinducer-N-butyryl-L-homoserine lactone (C4-HSL), that binds to protein RhlR receptor. LasR and RhlR receptors homodimerize that cause upregulation or downregulation of transcription factors for phenotype genes, which constitute 10% of the genome. There is also a third type QS system in P. aeruginosa named-PqsR, whose autoinducer is 2-heptyl-3-hydroxy-4(1H)-quinolone transformed from 2-heptyl-4(1H)-quinolone by protein PqsH; HHQ biosynthesized by protein PqsA-D and PqsE[25]. This QS system is known as Pseudomonas quinolone signal (PQS) based arrangement for a cell to cell signaling[26]. P. aeruginosa is a complex organism with multi QS models and signals in which the (3-oxo-C12-HSL)-LasR complex causes expression of LasI apart from RhlR (regulates both AHL QS circuits positively), while the RhlR- C4-HSL complex controls its autoinduction with no effect on the LasR system. Additionally, LasR and RhlR have both positive and negative regulation effects, respectively,in the expression of genes involved in PqsR. While PQS regulates its production, it increases the RhlR expression but has no direct regulatory activity on the LasR system[27].
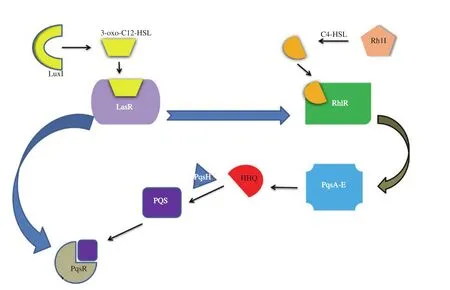
Figure 3. Quorum sensing (QS) circuit of Pseudomonas aeruginosa consists of three QS system namely, (1) LasR system with 3-oxo-C12-HSL (N-3-oxododecanoyl-L-homoserine lactone; autoinducer) and LuxI substrate, (2) RhlR system with C4-HSL (N-butyryl-L-homoserine lactone; autoinducer) and Rh1I substrate, (3) PqsR system with PQS [2- heptyl-3-hydroxy-4(1H)-quinolone; autoinducer] and PqsA-E substrate. All three systems are co-related to each other for QS regulation.
A recent study on QS in P. aeruginosa lists thirteen transcription factors, of which ten are repressed and three are activated Rhl-directed or Las-directed functions[28]. Surprisingly, RhlR acts as a critical QS component in P. aeruginosa that controls virulence gene expression[29].RhlI is activated by either LasR or PqsR, having a pre-requisite that the RhlR is bound to C4-HSL, so at least one of the autoinducers is sufficient for pathogenicity. There is a massive difference in the strains of P. aeruginosa; in the wild-type strain, the autoinducer requirement gets satisfied by the Las system. However, strains from patients infected with cystic fibrosis are reported frequently to have mutated lasR[30].The phosphate starvation protein PhoB plays an alternative for LasR through the activation of Integrated Quorum Sensing system (IQS)production. PhoB functions under phosphate limiting conditions, which is a common stressor that bacteria experiences during infection having ambABCDE as a signal synthase molecule[31]. To stop this chronic infection, a study by Lee et al. reported that IQS is an integrated circuit that connects the central las system and phosphate-stress response mechanism that attenuates pqs and rhl regulatory systems thereby targeting IQS and decreasing the virulence traits to a more significant extent[32].
2.1.2. QS circuit in C. violaceum
It is an aquatic bacterium that can infect humans and cause abscesses, septicaemias in the lungs, and the liver[33]. The C.violaceum QS system consists of CviI/CviR. The CviI/CviR circuit controls virulence, with the AHL bound ligand, and induce CviR conformation that prevents DNA binding[34]. QS in C. violaceum regulates violacein pigment production, antibiotic, hydrogen cyanide, some enzymes, and biofilm formation[35]. C. violaceum has many genes involved in violacein production, such as vioD,vioC, vioA, and vioB genes, which are arranged in an operon system mediated by AHL[36]. The much-studied trait is the production of purple pigment violacein[37]. Products of vioABCD operon generate violacein from tryptophan[36]. CviR controls the vioA promoter in C.violaceum (regulated directly), and other promoters[34]. C. violaceum phenotypes that depend on AHL include biofilm formation and chitinase production[38].
In C. violaceum ATCC 31532 strain, the cognate signal is C6-HSL, synthesized by CviI. AHSLs with acyl chain lengths from C4 to C8 can activate vioA transcription when bound to CviR.In contrast, AHSLs having C10 to C14 acyl chains are weak or completely inactive agonists. On the other hand, C10-HSL is an active CviR antagonist[34,39]. Overall, the C. violaceum QS system regulates (i) multiple genes that cause chitinases production[38],(ii) vioA promoter that produces violacein (a purple pigment that is water-insoluble)[40], and (iii) genes that cause cyanide production and degradation[41]. Apart from these regulatory genes mentioned above, several other genes regulated directly by CviR receptor in C. violaceum ATCC12472 strain namely CV_0577 (putative transcriptional regulator that encodes transcriptional regulator for the upstream process), CV_0578 (guanine deaminase encoder), CV_4240(extracellular chitinase encoder) and CV1432 (a type Ⅵ secretion system gene having a putative role)[35].
3. Importance of QS inhibition
QS facilitates bacterial communication and coordination, with various characteristic features such as motility, toxin production,biofilm formation, etc. of bacteria established to be under the control of QS. In the singular state, bacteria do not have any adverse effect on the host immune system; neither are resistant to the action of antibiotics. While they attain a complex consortium such as biofilm,it becomes challenging to degrade them; hence large doses of antibiotics are needed to combat the infections. Cholera bacteria use a QS mechanism to disperse the bacteria present in biofilm for the spread of infections[42]. Researches have shown that biofilm infections from methicillin-resistant Staphylococcus aureus (MRSA)have become prone to the antibiotic. Vancomycin shows resistance against biofilm infections[43].
Hence, there is a pressing priority to find an antibiotic alternative,of natural origin. In the past decades, natural products are observed as the burgeoning field in QSI and adjuvants to antibiotics with 80% of their contribution to drug discovery and possessing high approval rates from the past two decades[44]. QSIs work in different ways explained above in section 2 to stop the infections and have several advantages over conventional antibiotics such as limited interference in the bacterial mechanism[45]. QS is the integral mechanism for bacterial pathogenicity, and LuxI/LuxR homologs are present in more than 100 Gram-negative bacterial species and over 200 different Gram-negative bacteria with AHLs as signal transduction[46].
Combination/adjuvant therapies are applied to provide better results against the resistant forms by using amphotericin B with the QSI against clinical isolates of Candida albicans for biofilm infections[47,48]. Another approach of adjuvant therapies reported with tobramycin and QSI like garlic extract against infections caused by resistant strains of P. aeruginosa[49]. Preceding the post-antibiotic era, the scientific community has to look for alternatives that would inhibit the “bacterial-communication” and reduce virulence production among infectious agents.
4. Polyphenols as QSIs isolated from natural and recombinant sources
The autoinducers threshold level facilitates communication between the bacteria due to which several phenotypic characteristics express and lead to infections through the bacterial population. QSI controlling these infections are also known as QQ. These QSIs are obtained either from natural or recombinant sources. For instance,quercetin, a naturally occurring polyphenol, can inhibit biofilm formation (95%) in P. aeruginosa bacterial strains and inhibit twitching motility. Quercetin was the only compound that showed an anti-biofilm effect at the concentration of 0.5 minimum inhibitory concentration (MIC) compared with other polyphenols [(+)-catechin,caffeic acid, and morin][50]. Another natural compound, diterpene phytol, is also proved effective in inhibiting the biofilm formation,twitching, and flagella motility in P. aeruginosa at a concentration of 0.5 MIC. It was observed that the treated bacteria did not produce the twitching zone, and the resulting colony formed was almost round and smooth with regular edges[51]. Table 1 below describes some of the polyphenols from natural sources that act as QSI.
Metabolic engineering also produces polyphenols that act as QSIs under the influence of different microbes shown in Table 2.Recombinant technology introduces the specific genetic makeup in an organism which offers improved phenotype, such as improving biosynthesis capabilities of secondary metabolites[60].
5. Biofilm generation and polyphenols as biofilm inhibitors
A biofilm consists of bacterial cell clusters with a network of internal channels or hollows in the extracellular polysaccharide and glycoprotein matrix, allowing nutrients and oxygen from the bulk liquid to the cells. Thus biofilm is defined as a microbially derived immobile community characterized by cells that bind to the surface and embed into a matrix of extracellular polymeric substance (EPS)and thereby exhibit an altered phenotype relating to the growth rate and gene transcription. Biofilms are more resistant to antimicrobials than bacteria, as they have a barrier that prevents contact with antimicrobial agents[68,69].
The formation and development of biofilm are affected by many factors, including the specific microorganism, material surface properties, and environmental parameters, such as temperature, pH,and nutrient levels[69].
Biofilm formation consists of: (a) transport of cells from the surface and adsorption of bacteria at the surface, (b) EPS and cell to cell signaling molecules production and irreversible adsorption of cells,(c) biofilm maturation, (d) detachment of some biofilm cells, and (e)and biofilm recolonization (Figure 4)[70].
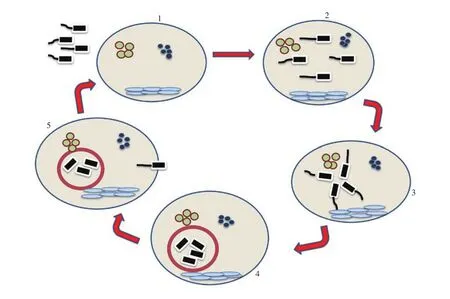
Figure 4. Schematic representation of biofilm-formation steps. Biofilm formation involves five steps: (1) initial attachment, (2) irreversible attachment, (3)microcolony development by proliferation, (4) biofilm formation, and (5) dispersal.
Biofilm formation also impacts the performance of antibiotics,which usually leads to antimicrobial resistance. These include series of activities: direct molecule inactivation, altered target sensitivity,low drug concentration at target, and efflux pumps; all of these contribute to antimicrobial resistance. The factors that contribute to biofilm resistance are as follows: (a) impaired diffusion, (b) lysis and, neutralization of enzymes, (c) slow growth, (d) presence of non-dividing cells and, (e) alteration in membrane function[71-73].Research studies conclude that polyphenols can act as a substitute for antibiotics and successfully involved in the inhibition of biofilm,their mechanism of action involves: inhibition of formation of the matrix, cell adhesion, and attachment, inferring extracellular matrix generation, and decreasing virulence gene production,thus contributing as a biofilm inhibitor[74,75]. Several plant-basednatural products combat infections. For example, cranberry works by glucan-binding proteins against cariogenic and periodontalpathogenic bacteria, destroying the extracellular matrix, lowering carbohydrate production, hydrophobicity, proteolytic activities,and retarding co-aggregation involved in biofilm formation[76].Cinnamaldehyde affects the DNA-binding ability of the LuxR transcriptional protein of Escherichia coli and Vibrio spp. that alters biofilm structure, swimming motility, stress response and virulence factors[77,78]. Phloretin compound shows anti-biofilm and antifimbria production activity by interfering with the toxin genes[hlyE and stx(2)], autoinducer-2 importer genes (lsrACDBF), curli genes (csgA and csgB)[79], and prophage genes in Escherichia coli O157:H7 bacterial strains, and also by efflux protein genes against Staphylococcus aureus (S. aureus) RN4220 and SA1199B bacterial strains[80]. Quercetin has many benefits like inhibiting biofilm formation, blocking of SrtA gene[81], and shows an effect on sialic acid production against Streptococcus pneumoniae. Further, this compound also impairs LasI[82-86], LasR, RhlI and RhlR production in P. aeruginosa. Quercetin also has a role in creating an imbalance in pH of biofilm of Streptococcus mutans and blocking glycolytic[87],protein translation-elongation, and membrane folding in Enterococcus faecalis[88]. Table 3 lists some of the polyphenolic constituents that are capable of inhibiting biofilms in Gram-negative pathogens.
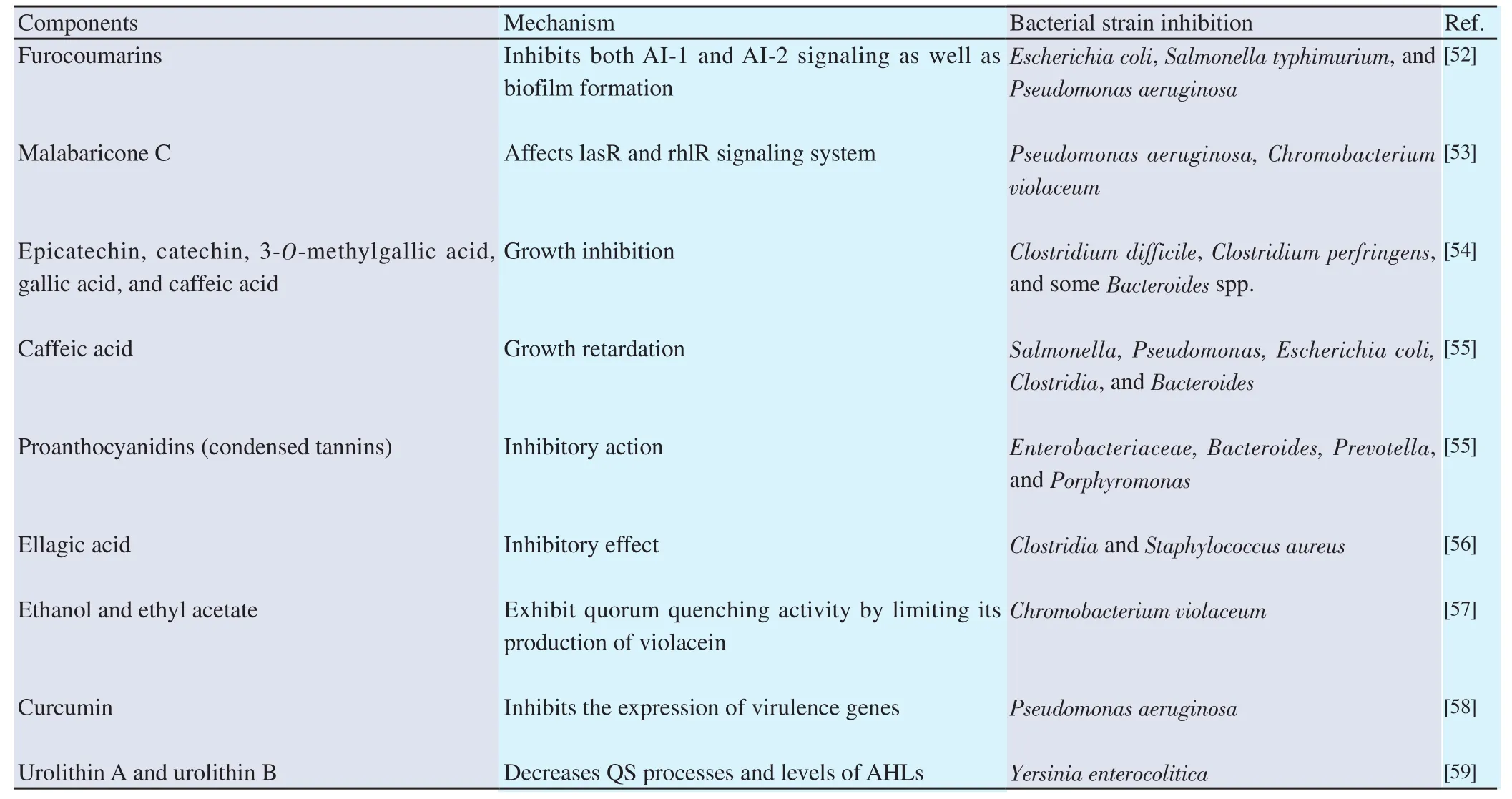
Table 1. Polyphenol based QSI from the natural sources.
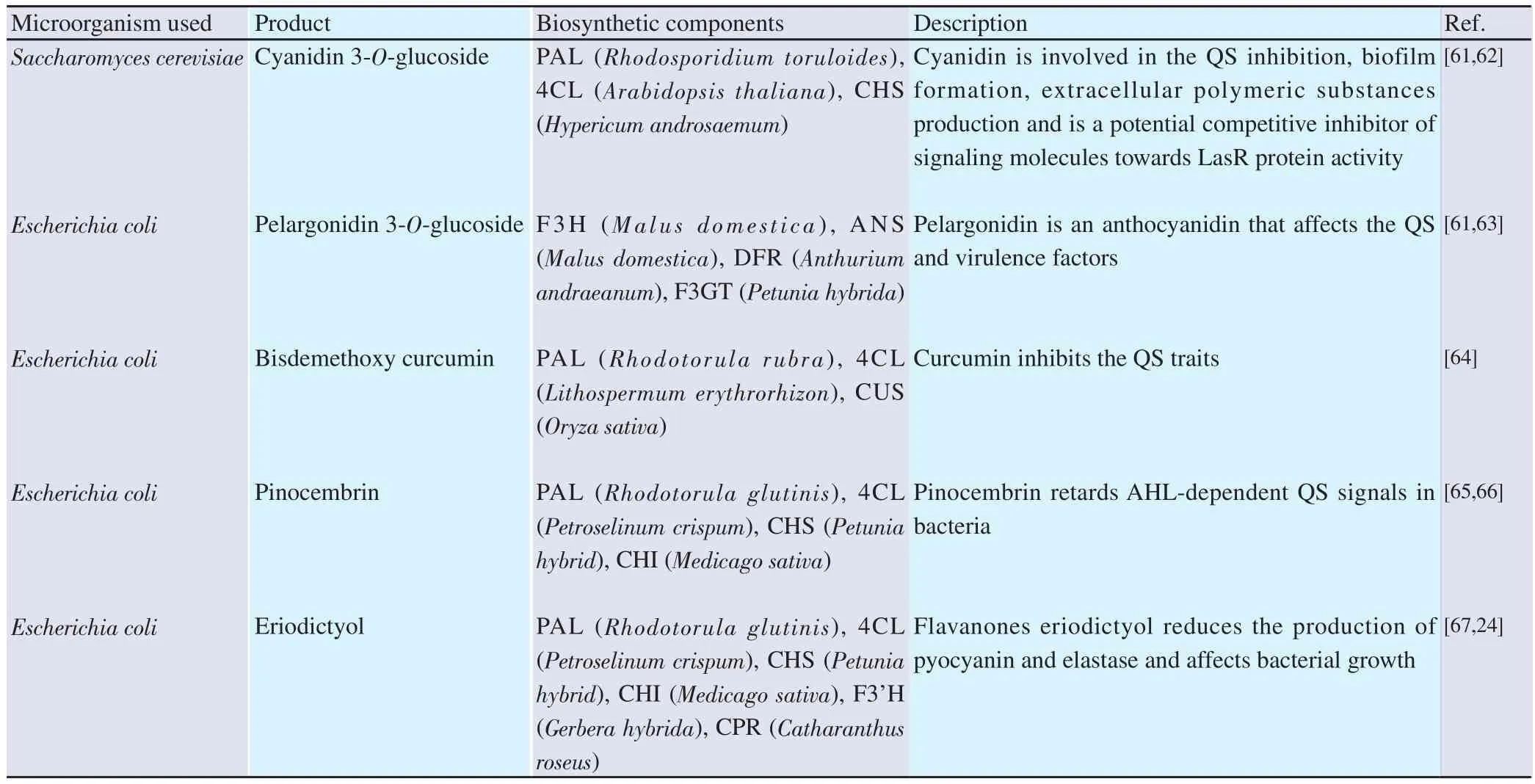
Table 2. QSIs from recombinant products.
6. Polyphenol as sensitizing agents for old antibiotics
Since the discovery of penicillin made by Fleming in 1928,antibiotics have acquired a major role in combating infections for both outpatient and hospitalized patients. The abuse and misuse of antibiotics from all over the globe have led to a serious problem of drug resistance, especially ineffectiveness against multi-drug resistant bacteria. This led to an increase in morbidity and mortality rates both among developed and developing countries. The most serious problem is that there is no guaranteed therapy for multidrug resistant infections[105]. Over the last 20 years, polyphenolshave attracted researcher’s attention to develop therapeutic agents that can act as adjuvants to the existing antibiotics and help in decreasing the antibiotic dose, increasing drug efficacy, and simultaneously decrease adverse effects. Kaempferol and quercetin,which are flavonoids of phenolic groups, were found to be excellent β-lactamase inhibitors when used with rifampicin against clinical rifampicin-resistant and methicillin-resistant S. aureus isolates. In addition to β-lactamase inhibitory activity, kaempferol and quercetin have inhibitory catalytic activity against topoisomerase of bacteria and hence used in combination with ciprofloxacin causing cessation and cell death of S. aureus[106]. The mechanism of this synergism can take place by (i) active site modification inside bacterial cell,(ii) enzyme inhibition or modification of antibiotics, (iii) rise in membrane permeability (iv) efflux pumps inhibition, etc.[106,107].There are several synergistic combinations reported of antibiotics and polyphenols, where polyphenols act as a “sensitizing” agent to increase the action on resistant bacterial strains, listed in Table 4 below.
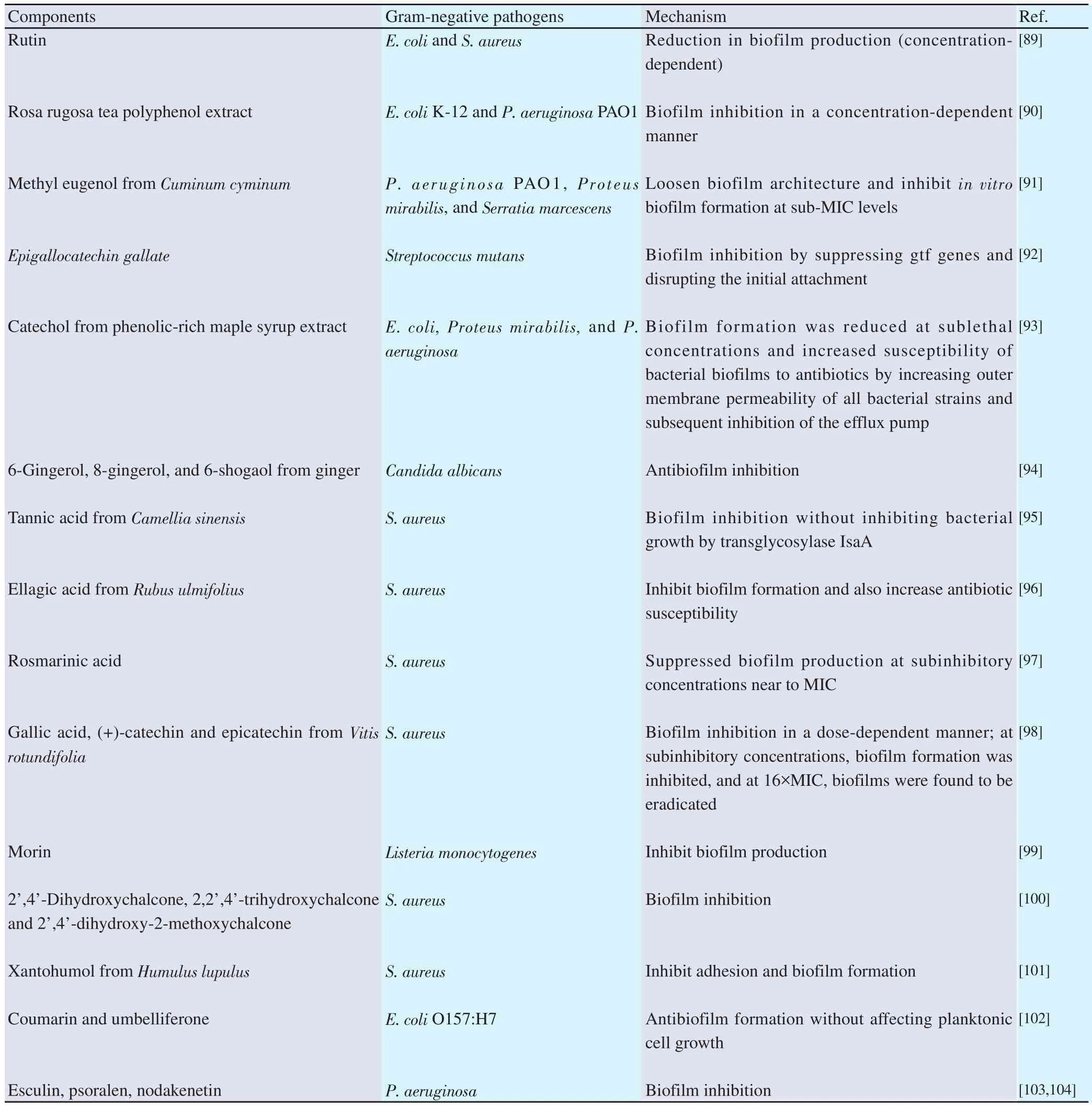
Table 3. Polyphenols combating biofilm formation along with their mechanism.
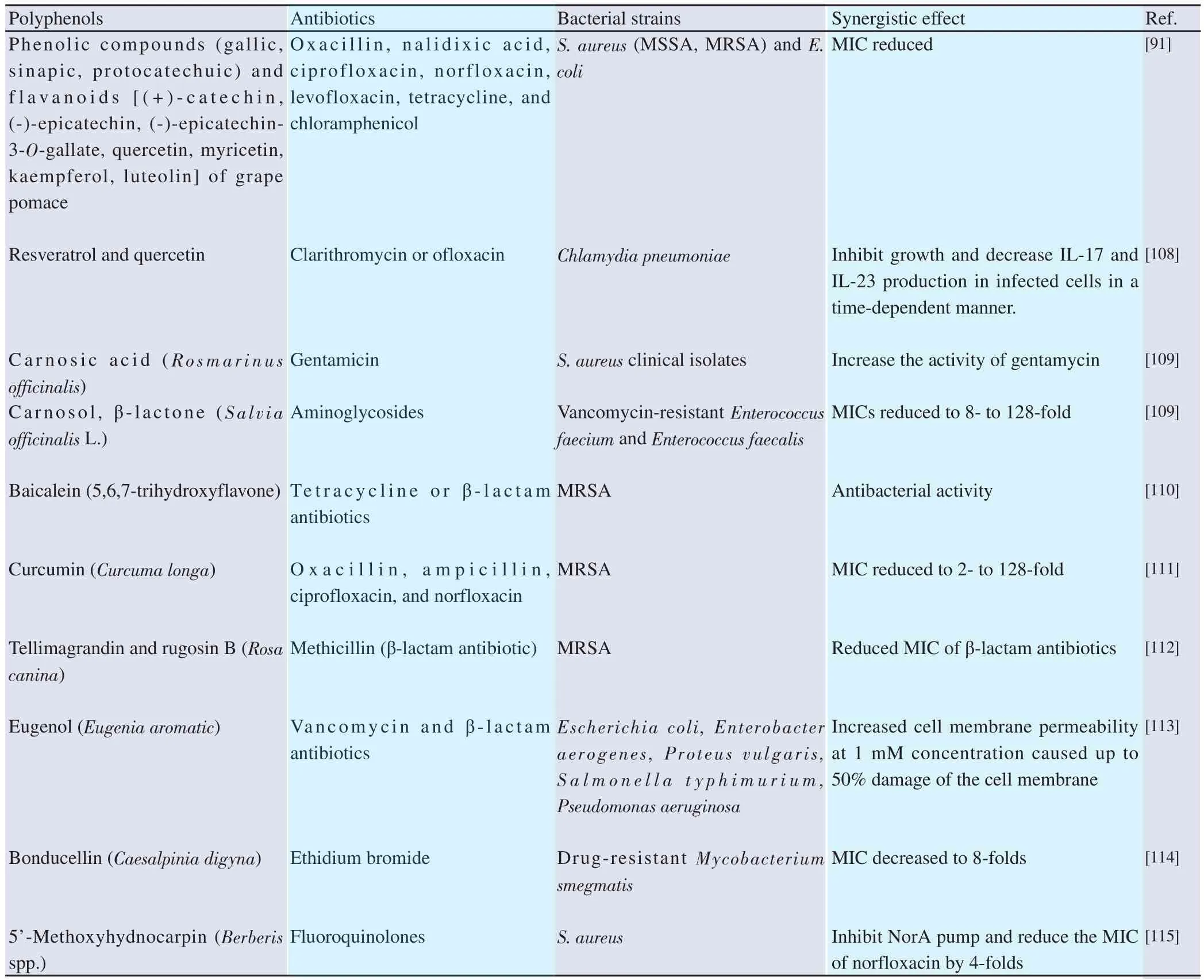
Table 4. Polyphenol compounds reported to act in synergism with old antibiotics.
Besides the naturally occurring polyphenols, marine sources have also contributed towards the anti-QS activity. The methanolic and acetone extract of freshwater sponge Ochridaspongia rotunda were found to be effective against the P. aeruginosa PA01 bacterial strain.Satisfying results were observed, including reduced pyocyanin production, anti-biofilm activity, and reduction in twitching and flagella motility were reported[116]. Another Mediterranean sponge named Dysidea avara, its secondary metabolite component,sesquiterpene hydroquinone avarol showed moderate anti-QS effects with 75% reduction in P. aeruginosa PAO1 biofilm formation,39% reduction in pyocyanin production and reduction in twitching and protrusions motilities which proves to be effective as QSI[117].Hence, polyphenolic compounds extracted from natural sources are equally effective as antibiotics in decreasing the pathogenic load by combating the QS signals and thereby decreasing the virulence traits.
7. Conclusion
This review is an overview of the scientific literature on QS and antibiofilm activity of selected plant-based and recombinant polyphenolic compounds. Several polyphenols have been reported as antimicrobial and antibiofilm agents, but their journey towards clinical application may still be long enough due to their pharmacokinetics incompatibilities, which can be overcome by different nanotechnological application and intervention.Polyphenols have proven to be effective in controlling virulence and should be put forward in clinical testing to evolve a new area beyond antibiotics in controlling bacterial infections. These polyphenolic scaffolds can play a crucial role as sensitizing agents/adjuvant therapeutics which can enhance the anti-bacterial action of antibiotics and prove effective in overcoming the problem of drug resistance.
Conflict of interest statement
We declare that there is no conflict of interest.
Authors’ contributions
LAF contributed to the design and writing of the manuscript and it was formatted and proofread by BS, SS, and FFC Jr.
 Asian Pacific Journal of Tropical Biomedicine2021年6期
Asian Pacific Journal of Tropical Biomedicine2021年6期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Total flavonoids from Saussurea involucrata attenuate inflammation in lipopolysaccharide-stimulated RAW264.7 macrophages via modulating p65, c-Jun,and IRF3 signaling pathways
- Immunostimulatory effect of ethanol extract of Chondracanthus tenellus in RAW 264.7 macrophages in vitro
- Ginseng ameliorates pulmonary toxicity induced by silicon dioxide nanoparticles in rats
- Caffeic acid and protocatechuic acid modulate Nrf2 and inhibit Ehrlich ascites carcinomas in mice
