A New Species of Cyrtodactylus (Squamata:Gekkonidae) from the Karst Forests of Daweishan National Nature Reserve,Yunnan,China
Yinpeng ZHANG ,Xiaolong LIU ,Justin BERNSTEIN ,Jian WANG and Zhiyong YUAN1,*
1Key Laboratory of Freshwater Fish Reproduction and Development,Ministry of Education,Southwest University,Chongqing 400715,China
2Key Laboratory of Conserving Wildlife with Small Populations in Yunnan,Southwest Forestry University,Kunming 650224,Yunnan,China
3Michigan State University,East Lansing 48825,MI,USA
4Rutgers University-Newark,Boyden Hall,195 University Avenue,Newark 07102,NJ,USA
5College of Life Science and Technology,Honghe University,Mengzi 661199,Yunnan,China
Abstract Cyrtodactylus geckos are one of the most speciose and diverse groups of extant lizards known,distributed throughout the Asian and Pacific realms.Using molecular phylogenetic methods and supporting morphological data,we describe a new species of Cyrtodactylus in Daweishan National Nature Reserve,Yunnan Province,China.Cyrtodactylus hekouensis sp.nov. can be morphologically distinguished from its nearby congeners by the following characters:maximum SVL 92.3 mm and TL 98.5 mm;11-12 supralabials;11-12 infralabials;36-57 scale rows between the fifth supralabials;10-13 dorsal tubercles rows;3 postnasals on blunt and smooth front snout;precloacalfemoral pores in a continuous series of 33-39 (females with pitted scales) located under vent/cloaca and thighs in both sexes;precloacal groove absent;3/3 postcloacal tubercles;subdigital lamellae under the fourth finger 21 or 22,under the fourth toe 20-23;smooth midbody with smooth venter and tuberculate dorsal scale rows,tubercles from head to tail base;dorsal transverse patterns are generally large,bilaterally symmetrical.The results of the phylogenetic analysis recover specimens of this new species as sister to a clade containing C.wayakonei and C.martini.Uncorrected pairwise intraspecific distances were < 1%,and distances between our new species and other Cyrtodactylus species from nearby countries ranged from 14.2% to 26.8%.
Keywords China-Vietnam border,Yunnan Province,Cyrtodactylus hekouensis sp.nov.,Gekkonidae,molecular phylogeny,systematics,taxonomy
1.Introduction
As one of the most diverse genera in the Gekkonidae,Cyrtodactylus
contains almost 300 recognized species (Uetzet al
.,2020).New species are continuously being described each year through fieldwork in underexplored localities and the development of molecular techniques,especially DNA barcoding (Hebertet al
.,2003).A significant number ofCyrtodactylus
have been described from tropical karst forests in regions of Southeast Asia,including Myanmar,Malaysia,Laos,Vietnam,Thailand,Cambodia,Indonesia,Singapore,Timor Leste,Philippines,Brunei and China (e.g.,Pauwelset al
.,2016;Sitthivonget al
.,2019;Grismeret al
.,2020).Four species ofCyrtodactylus
are currently recognized in China (Wanget al
.,2020) includingC.cayuensis
(Li,2007;Agarwalet al
.,2018),C.tibetanus
(Boulenger,1905),C.zhaoermii
(Shi and Zhao,2010)andC.wayakonei
(Nguyenet al
.,2010).All of these species are in the Indo-Burma species group (Agarwalet al
.,2014),with the exception ofC.wayakonei
(Nguyenet al
.,2010).The Indo-Burma species group,including three of the four Chinese species,may represent a radiation of Himalayan species;the diversity of species from Yunnan,China in the Southeast Asian group (Brennanet al
.,2017;Nazarovet al
.,2018).Cyrtodactylus wayakonei
has only been recorded from Xishuangbanna (Yuan and Rao,2011),a biodiversity hotspot area in the Yunnan Province border area of China.Excluding this record,the knowledge ofCyrtodactylus
in Yunnan is lacking,despite the abundance of suitable limestone karst forests habitats along the country’s border.The China Vietnam border area is a well-known biodiversity hotspot,harboring a striking diversity of species and endemism (Myerset al
.,2000).However,a majority of this region is understudied,especially low latitudes,tropical evergreen forests,and broadleaved forests in southern Yunnan.Recently,several field expeditions were conducted by Chinese scholars,and several subsequent descriptions of new species or new country records were published,such asParamesotriton deloustali
(Zhanget al
.,2017),Tylototriton ziegleri
(Jianget al
.,2017),Amolops wenshanensis
(Yuanet al
.,2018) andNidirana chapaensis
(Yuanet al
.,2019).It is likely that candidate new species might be found around the China-Vietnam border area.For example,the Daweishan National Nature Reserve in Hekou,China,near the border of Vietnam,contains well-preserved tropical forests,where herpetological research has been limited.In the past few years,we collected several specimens ofCyrtodactylus
whose morphological characters were distinct from their congeners.We used molecular (COI) data and made a detailed morphological assessment to compare the Hekou specimens to describedCyrtodactylus
species,especially species from nearby countries.Our results demonstrate that this species has a distinct morphology and high interspecific distances from its congeners;we describe this new species ofCyrtodactylus
herein.Additionally,we use molecular data to taxonomically identifyCyrtodactylus
from Xishuangbanna,Yunnan.2.Materials and Methods
2.1.Sampling
Fieldwork was conducted with permits in a karst limestone forest area of Daweishan National Nature Reserve,Yunnan Province,China,by Yinpeng Zhang,Xiaolong Liu,Jian Wang and Zhiyong Yuan in from 2016 to 2018.Thirteen specimens (3 males,10 females) were collected.Specimens were fixed with 10% formalin solution and subsequently stored in 75% ethanol for voucher preservation.Prior to fixing,liver tissues were dissected out from the specimens and stored in 95%ethanol.All specimens were preserved in Southwest Forestry University,Kunming,Yunnan,China.2.2.Morphology
Measurements were taken by using a slide caliper from five well preserved samples (to the nearest 0.1 mm)due to 8 specimens were preserved in poor condition with some critical scalations missing,following the protocols of Nguyenet al
.,(2010).Eight specimens were not measured,as multiple scalation characters were missing due to poor preservation quality.Abbreviations are as follows:snout-vent length (SVL);tail length (TL);maximum head length (HL);maximum head width (HW);maximum head height (HH),from top of skull to underside of jaws;greatest diameter of orbits (OD);snout to eye distance (SE),from tip of snout to anterior corner of eye;eye to ear distance (EE),from anterior edge of ear opening to posterior corner of eye;axilla to groin distance (AG).The following scale characters were counted:supralabials(SL);infralabials (IL);nasal scales surrounding nare,from rostral to labial (excluding the rostral and labials),i.e.nasorostral,supranasal,postnasals (N);granular scales surrounding dorsal tubercles (GST);number of ventral scales within longitudinal rows at midbody (V);number of tubercles at dorsum of midbody (DTR);scales around midbody (MS);number of ocular scales around ocular area (OS);scale rows between fifth supralabials (SR5);numbers of continuous precloacal-femoral pores (females with pitted scales) (PP);postcloacal tubercles(PAT);subdigital lamellae under the fourth finger (LF4);subdigital lamellae under the fourth toe (LT4).Bilateral scale counts were given as left/right.Femoral and precloacal pores were counted with a digital microscope (Nikon SMZ800N).
2.3.Molecular analyses
Four specimens of the gecko species with unique morphology were used for molecular analyses,and one sample ofCyrtodactylus
collected from Xishuangbanna was also used to identify its taxonomic affilitation (Table 1).We sequenced a~650 bp fragment of mitochondrial cytochrome c oxidase subunit 1 (COI) to assess if theCyrtodactylus
specimens from Daweishan National Nature Reserve are distinct from other known species in the genus.This approach has been successfully used before in delimitingCyrtodactylus
geckos(Brennanet al
.,2017).The primers used were Chmf4:5-TYT CWA CWA AYC AYA AAG AYA TCG G-3 and Chmr4:5-ACY TCR GGR TGR CCR AAR AAT CA-3 (Cheet al
.2012).Amplification of 25 μL polymerase chain reactions(PCR) were executed on an Eppendorf Master cycler gradient thermocycler.Amplification of genomic DNA began with an initial denaturation for 2 min at 95 °C followed by 95 °C for 35 s,annealing at 50 °C for 35 s,and extension at 72 °C for 150 s with 4 s added to the extension per cycle for 32 cycles for COI.Amplified products were sequenced using an ABI 3730 automated sequencer.Considering our samples were collected near Vietnam and Laos,40 COI sequences ofCyrtodactylus
species (and one sequence ofCyrtopodion
for our outgroup) from previous studies(Nguyenet al
.,2013,2014,2015,2017;Ziegleret al
.,2013;Schendieret al
.,2014;Leet al
.,2016;Luuet al
.,2016a,2016b;Brennanet al
.,2017;Connetteet al
.,2017;Nazarovet al
.,2018;Pauwels,2018)from Southeast Asian specimens were downloaded from Genbank (National Center for Biotechnology Information) to explore the relationships with other known species (Table 1).All 46 COI sequences were initially aligned by using Geneious Basic(Kearseet al
.,2012),and then optimized by MEGA X (Kumaret al
.,2018).Mean uncorrected genetic distances (p
distances)between testedCyrtodactylus
species were calculated by MEGA X (Kumaret al
.,2018).Phylogenetic trees were inferred by Maximum Likelihood (ML) and Bayesian inference (BI).JModelTest 2 (Guindon and Gascuel,2003;Darribaet al
.,2012)was used to build the model of sequence evolution that best fit each partition.The model TrN+I+G was selected as the best fit model to the data.Metropolis coupled Markov Chain Monte Carlo (MCMCMC) analyses were run with 1 cold chain and 3 heated chains for 3 000 000 generations and sampled every 1 000 generations.Four independent MCMCMC runs were performed as result and 1 500 trees were discarded as burnin.The potential scale reduction factor (PSRF > 1) and the average standard deviation of split frequencies (ASDSF < 0.01) statistics generated by MrBayes were used to evaluate topological and branch-length convergence,respectively.Confidence of tree topology was assessed by posterior probability (PPr)(Huelsenbeck and Ronquist,2001).Maximum Likelihood analyses were conducted through RAxML NG v0.9.0.(Kozlovet al
.,2019) under a GTR+GAMMA model,the recommended model from RAxML authors.Branch supports (bootstrapsupports [BS]) were determined based on 1000 bootstrap replicates.We consider relationships with PPr ≥ 0.95 and BS ≥70 to be strongly supported.
Table 1 Specimens used in the study,with respective voucher numbers,localities,and GenBank accession numbers for COI.“/” mean the locality is unknown.
3.Results
Genetic Differentiation
Five new generated COI sequences ofCyrtodactylus
including four specimens from Daweishan National Nature Reserve and one unknownCyrtodactylus
collected from Xishuangbanna,Yunnan,China were obtained (GenBank ID:MW067125,MW067126,MW067127,MW067128,MW067129).The final alignments of the examined mtDNA COI gene fragment consisted of~650 sites without any gaps.The ML and BI analyses recovered similar topologies at strongly supported species relationship;conflicting topologies were found between relationships that received low support (BS < 70%,PPr < 0.95).Some clades in both trees were recovered with low support by both analyses,which is most likely due to COI having fewer informative sites than other mitochondrial genes,such as the commonly used NADH dehydrogenase 2 (ND2;Brennenet al
.,2017).More relationships in the BI analysis were recovered with strong support than in the ML analysis,and both analyses strongly recover the same sister relationships between species or pairs of species (Figure 1).Our sample collected from Xishuangbanna was sister toC.martini
from Vietnam,with an uncorrected pairwise distance of 2.8%.The new samples from Hekou form a strongly supported clade with an uncertain phylogenetic placement with regards to theC.wayakonei
andC.pulchellus
groups (BI analysis).In the ML analysis,the Hekou specimens are recovered as reciprocally monophyletic to the clade containingC.wayakonei
+C.
sp.Xishuangbanna+C.martini
with poor support.Uncorrected pairwise distance between the new samples from Hekou and otherCyrtodactylus
species ranged from 14.2% (withC.martini
) to 26.8% (withC.badenensis
) (Table S1).However,though we find support of the new species as a distinct clade,the relationships of these species in the analyses are poorly supported and require more data.Cyrtodactylus hekouensis
sp.nov.
Hekou Bent-Toed Gecko (Figures 2-5)
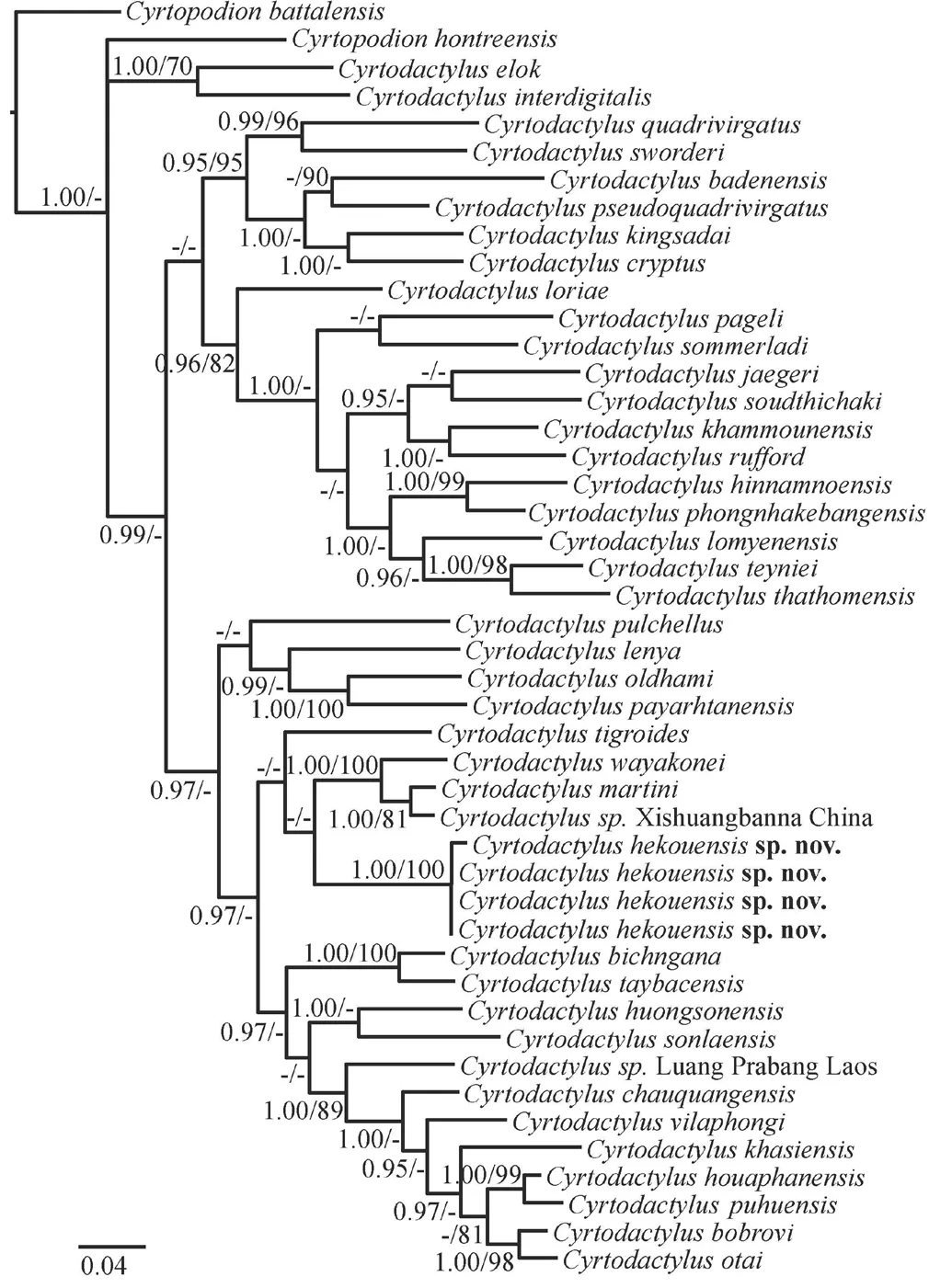
Figure 1 Bayesian phylogenetic tree of Cyrtodactylus inferred from mitochondrial COI.Numbers above and below branches indicate bootstrap support values (BS ≥ 70%) and Bayesian posterior probabilities (PPr≥ 95%),respectively;low support values are denoted by “-”.

Figure 2 Holotype of Cyrtodactylus hekouensis sp.nov. (SFU002507):Dorsum (A);precloacal region (B);subdigital lamellae under left hindfeet toes (C);pair of chin-shields under dentary (D).Photo credit:Yinpeng ZHANG.

Figure 3 Dorsal color variations of Cyrtodactylus hekouensis sp.nov.Photo credit:Xiaolong LIU.
Holotype:SWFU002507,adult male,collected by Yinpeng ZHANG,07/24/2018,from Daweishan National Nature Reserve,Hekou County,Honghe Autonomous Prefecture,Yunnan Province,China.(N 22.673459°,E 103.943534°,~160 m).Paratypes:SWFU001578,adult male collected by Zhiyong YUAN;SWFU002879,SWFU002880,SWFU002881,all gravid female adults collected by Zhiyong YUAN and Yinpeng ZHANG,07.24.2018.Collected from Daweishan National Nature Reserve,Honghe Autonomous Prefecture,Yunnan Province,China (N 22.673459°,E 103.943534°,~160 m).
Diagnosis
A moderate sizedCyrtodactylus
,with a slender trunk and bent toes,C.hekouensis
is distinguished from other describedCyrtodactylus
species by the combined characteristics:maximum SVL 92.3 mm and TL 98.5 mm;11-12 supralabials;11-12 infralabials;36-57 scale rows between the fifth supralabials;10-13 dorsal tubercles rows;3 postnasals on blunt and smooth front snout;precloacal-femoral pores in a continuous series of 33-39 (females with pitted scales) located under vent/cloaca and each side of thigh in both sexes;precloacal groove absent;3/3 postcloacal tubercles;subdigital lamellae under the fourth finger 21 or 22,under the fourth toe 20-23;smooth midbody with smooth venter and tuberculate dorsal scale rows,tubercles from head to tail base;dorsal transverse patterns are generally large,bilaterally symmetrical.Holotype Description
Male collected from Daweishan National Nature Reserve,Honghe Autonomous Prefecture,Yunnan Province,China,with total length 149.8 mm (SVL 77.0 mm,TL 72.8 mm with tail tip regenerated),slender,elongate trunk.
Head with short and smooth snout,some tuberculate scales start to regularly distribute into scales rows behind end of ocular scale to the tail base;nasal surrounded by 3 postnasals and connected with one large rectangular front lip scale;11 supralabials;12 infralabials;43 scale rows behind fifth supralabials;1infralabials connect with a large pair of chin shields;elongated supralabials reach to the end of ocular,5and 6supralabials enlarged in middle;a narrow row of scales separating the supralabials from the infraoculars;always one wide dark brown band with yellow granular scales starts from nasal and crosses eye;external ears located below the dark brown band behind eyes.
Dorsal scales from head to tail base,flat and smooth,with irregularly located tuberculate scales around trunk of body and above venter mostly around black dorsum patterns;the greatest diameter of the orbits is 12.2 mm;129 scales around the midbody;wrinkles slightly developed until the gular scale;lateral skin fold with no tubercles;10 rows of dorsum tubercles;9 granular scales surrounding dorsal tubercles;71 ventral scale rows,smooth,subimbricated round,larger than dorsal scales;three rows of enlarged scales presented in front of region of precloacal pores;37 precloacal femoral pores series located in enlarged precloacal scales (9 precloacal pores plus in 14+14 femoral pores on each side under thighs),continuous;precloacal groove absent.
Fore and hind limbs stout and slender;small scales covered dorsum;small scales covered dorsum fore and hind limbs,with some tubercles;scales on ventral fore and hind limbs rectangular;fingers and toes with no webbing;subdigital lamellae:finger I with 15,finger II with 18,finger III with 19,finger IV with 21,and finger V with 21 subdigital scales (left measured);toe I with 16,toe II with 18,toe III with 18,toe IV with 22,and toe V with 22 subdigital scales covered (left measured);relative length of fingers (right and left) is IV > III >V > II > I;relative length of toes (right and left) is IV > V > III >II > I.
Tail regenerated at tip;3 postcloacal tubercles on each side of the tail base;tubercles concentrated in dorsum tail base in 6-8 scale rows;5 dark dorsum tail bands.
Holotype,fixed with 10% formalin solution and transferred to 75% ethanol,light brown dorsally and dark gray ventrally.Coloration in live animals can be described as follows:light,yellow brown coloration with large blotches on dorsal surface of head,neck,dorsal,extremities and tail,head color slightly lighter;wide dark brown band starts from nasal and crosses eye;five irregular bands on dorsal,patterns on dorsal are generally large,and bilaterally symmetrical;some small irregular dorsal patterns present in between those bands,closed to belly;light whitish pink belly;five dark brown irregular transversal bands on tail completely encircled,with regenerated tail tip;light whitish gray tail venter.
Variation
Paratypes are similar to the holotype in morphological characteristics.For measurements and scalations variations see Table 2.Individuals show variation in color and blotch pattern at dorsal tail bases,dorsal limb surfaces,and midbody dorsal surfaces.The dark dorsal tail bands vary in numbers but are lost in the regenerated tails of the type specimens.Dark blotches on midbody dorsal surfaces less or more symmetrical and meet near spine (Figure 3).Dark blotches on dorsal head surfaces variable in position and size,with blotch size variable between and within specimens.Background coloration of specimens ranges from light tan to a moderate to dark brown,with darker blotches.The number of precloacal femoral pores show variation between different specimens,ranging from 33 to 39.For measurements and scalations,see Table 2.

Table 2 Morphological measurements and scalation from holotype and paratypes of Cyrtodactylus hekouensis sp.nov. specimens.Only the maximum value was given for continuous data (i.e.,distances).M:males;F:females;*:regenerated or broken tails;**:snout broken,no scales of sufficient quality to measure or count;m:mean;min:minimum;max:maximum;s:standard deviation.
Distribution
C.hekouensis
only known fromthe type locality,Daweishan National Nature Reserve,Honghe Autonomous Prefecture,Yunnan Province,China.
Etymology
The species is named after the county of the type locality(“Hekou”=河口in Chinese).
Natural history
All specimens were found and collected in a karst (i.e.,limestone) river valley with low density of vegetation near a stream at night from 20:00 to 24:00.Specifically,allC.hekouensis
in this study were found on karst edges near a road side of Daweishan National Nature Reserve,about 1.0-1.5 m height from the ground (Figure 5).Geckos were observed on the cliff sides or limestone burrow edges within high moisture.All collected females were gravid with two large,developed eggs,both~10 mm in length and 7-8 mm in diameter.
Figure 4 Dorsal view of live C.hekouensis sp.nov. from Yunnan,China.Photo credit:Zhiyong YUAN.

Figure 5 Type locality of Cyrtodactylus hekouensis sp.nov.,Daweishan National Nature Reserve,Hekou County,Honghe Autonomous Prefecture,Yunnan Province,China.Photo credit:Yinpeng ZHANG.
The specimens ofC.hekouensis
were collected from a tropical broadleaf evergreen forest.The locality is located next to the Nanxi River (part of the Red River system),close to the China-Vietnam border.Day time temperatures generally rises in the range of 34-41 degrees centigrade in one day and the limestone surfaces are typically dry during the daytime from June to August;night temperatures drops to around 28-30 degrees centigrade,on average.Limestone surfaces show moisture by condensation of water vapor from groundwater,the Nanxi River,plant transpiration and other sources.Comparisons
Species ofCyrtodactylus
endemic to tropical karst forest are often limited to small areas.Thus,rather than compareC.hekouensis
with all species ofCyrtodactylus
,we mainly focused on species from north and central regions of Laos,Vietnam,and Myanmar,which distribute geographically near China,as this is whereC.hekouensis
been found.C.hekouensis
sp.nov.
can be differentiated fromC.huongsonensis
(Luu,Nguyen,Do and Ziegler,2011) by having more ventral scale rows (68-72 vs.41-48),and having more continuous precloacal-femoral pores (continuous 33-39 vs.6-8 precloacal plus in 15-17 femoral pores in separate).C.hekouensis
sp.nov.
can be distinguished fromC.sonlaensis
(Nguyen,Pham,Ziegler,Ngo and Le,2017) by havingpresenting precloacal-femoral pores in females (absent inC.sonlaensis
).C.hekouensis
sp.nov.
can be distinguished fromC.otai
(Nguyen,Le,Pham,Ngo,Hoang,Pham and Ziegler,2015) by having presenting femoral pores (absent inC.otai
).C.hekouensis
sp.nov.
can be distinguished fromC.bobrovi
(Nguyen,Le,Pham,Ngo,Hoang,Pham and Ziegler,2015) by having more precloacal-femoral pores (33-39 vs.5 in males and absent in females),and numbers of postcloacal tubercles (3/3 vs.1/1+2/3).C.hekouensis
sp.nov.
can be differentiated fromC.jaegeri
(Luu,Calame,Bonkowski,Nguen and Ziegler,2014) by having fewer postcloacal tubercles (3/3 vs.male 5/6),and dorsal patterns (bilaterally symmetrical patterns vs.wide bands).C.hekouensis
sp.nov.
can be differentiated fromC.puhuensis
(Nguyen,Yang,Le,Nguyen,Orlov,Hoang,Nguyen,Jin,Rao,Hoang,Che,Murphy and Zhang,2014) by having more ventral scale rows (68-72 vs.36),and having femoral pores (absent inC.puhuensis
).C.hekouensis
sp.nov.
can be differentiated fromC.soudthichaki
(Luu,Calame,Nguyen,Bonkowski and Ziegler,2015) by having fewer postcloacal tubercles (3/3 vs.4/4-5/5);more precloacal-femoral pores (33-39 vs.29);and dorsal body patterns (bilaterally symmetrical patterns vs.5 transverse bands).C.hekouensis
sp.nov.
can be distinguished fromC.soni
(Le,Nguyen,Le and Ziegler,2016) by different formations of precloacal-femoral pores (continuous 33-39 vs.males 6/7 precloacal+6-8 femoral,separate).C.hekouensis
sp.nov.
can be distinguished fromC.taybacensis
(Pham,Le,Ngo,Ziegler and Nguyen,2019) by having more ventral scale rows (68-72 vs.30-38),and more precloacalfemoral pores (33-39 vs.males 11-13+females 5/15 pitted+femoral absent).C.hekouensis
sp.nov.
can be differentiated fromC.houaphanensis
(Schneider,Luu,Sitthivong,Teynié,Le,Nguyen and Ziegler,2020) by having more ventral scale rows (68-72 vs.35);more precloacal-femoral pores (33-39 vs.6+femoral absent);and more postcloacal tubercles (3/3 vs.2/2).C.hekouensis
sp.nov.
can be differentiated fromC.ngoiensis
(Schneider,Luu,Sitthivong,Teynié,Le,Nguyen and Ziegler,2020) by having more precloacal-femoral pores (33-39 vs.males 7 precloacal+14 femoral+female absent).C.hekouensis
sp.nov.
can be distinguished fromC.vilaphongi
(Schneider,Nguyen,Le,Nophaseud,Bonkowski and Ziegler,2014) by having more ventral scale rows (68-72 vs.34-36);more postcloacal tubercles (3/3 vs.2/2);existence of precloacal-femoral pores (continuous pores 33-39 vs.precloacal pore unknown in male and absent in female).C.hekouensis
sp.nov.
can be distinguished fromC.martini
(Tri,2011) by having more postcloacal tubercles (3/3 vs.2/2);more ventral scale rows (68-72 vs.39-43);and more precloacalfemoral pores (continuous pores 33-39 vs.4 precloacal pores and femoral absent).C.hekouensis
sp.nov.
can be distinguished fromC.wayakonei
(Nguyen,Kingsada,R?sler,Auer and Ziegler,2010) by having more precloacal-femoral pores (continuous pores 33-39 vs.6-8);more postcloacal tubercles (3/3 vs.2/2);and by having different dorsal banded patterns (bilaterally symmetrical vs.reticulated).4.Discussion
Cyrtodactylus wayakonei
might be misidentified in China based on our phylogeny result of aCrytodactylus
specimen from Xishuangbanna.However,whileC.wayakonei
in China is only known from Yunnan (Yuan and Rao,2011),previous records of Xishuangbanna'sC.wayakonei
were established by morphological comparison only.According to our phylogenetic results,the sample from Xishuangbanna county (C.
sp.Xishuangbanna in our gene tree) is sister toC.martini
,and the two former specimens together are the sister group toC.wayakonei
by ML analysis (BS > 70%),which is consistent with the study of Brennanet al
.(2017) who also identified aCyrtodactylus
specimen from Xishuangbanna asC.
aff.martini
.Additionally,the uncorrectedp
distance results (Table S1)suggest that the samples from Xishuangbanna have a smaller genetic divergence fromC.martini
than toC.wayakonei
(C.
sp.Xishuangbanna andC.martini
:2.8%;C.
sp.Xishuangbanna andC.wayakonei
:6.8%).This result also corresponds with a study from Nazarovet al
.(2018),which acknowledged aCyrtodactylus
specimen from Xishuangbanna asC.
cf.martini.
Moreover,the morphological characters identified by Yuan and Rao (2011) from four specimens (male 2011R0010;females KIZ201101,KIZ201102,KIZ201103) may be inaccurate or represent variation.For example,precloacal-femoral pores data as a sexual character of lizards (Mayerlet al
.,2015) from both genders are not provided;supralabials and infralabials differed from the holotype description (males/females supralabials and infralabials 9-10 in Yuan and Rao’s [2011] studyvs.
supralabials 7-8 and infralabials 9-10 in type specimens description).The evaluation ofC.wayakonei
and distribution ofC.martini
in China should be discussed and supplemented with further morphological data by more specimens from these localities in future studies.The karstic habitats of southwestern China represent the world’s largest karst habitat system,and harbor an extraordinary diverse endemic flora and vertebrate fauna,which are in danger to anthropogenic threats (National Bureau Statistics of China,2011;Luoet al
.,2016).Fieldwork in these areas since 2015 has discovered eight amphibians and reptiles as new species or new locality records (B?hme,2003;Nicodemo and Bain,2007;Wanget al
.,2015;Chenet al
.,2018;Renet al
.,2018;Wanget al
.,2018;Zhanget al
.,2018;Yuanet al
.,2019).Although these recent discoveries provide a greater understanding of the reptilian biodiversity in this region,it also indicates that there is likely much biodiversity that has yet to be discovered.Most of these works were conducted in Yunnan Province of China near the international boundary with Vietnam,and there is a lack of survey and collecting work in other areas of this hotspot,such as the areas between northern Hanoi,Vietnam and Guangxi Province,China.Extensive collaboration between China and Vietnam to conduct comprehensive fieldworks on amphibians and reptiles will be crucial to discovering cryptic species and understanding the full diversity of this region,which is critical in the current time during which global extinction rates have increased (Pimmet al
.,2014).Acknowledgement
We thank Guiliang ZHANG and Guisheng ZHANG from the Hekou forestry department and Daweishan National Nature Reserve for their support in the processing of our sampling collection and their longtermed collaboration,respectively.We also thank Yanhong HE and Yixin XING for editing photographs.This work was supported by grants from the Second Tibetan Plateau Scientific Expedition and Research Program (2019QZKK0501),National Natural Science Foundation of China (31702008),Young talent project of China Association for Science and Technology(2019-2021QNRC001) and Yunnan Fundamental Research Project (202001AW070016,202005AC160046),Biodiversity Investigation,Observation and Assessment Program (2019-2023) of Ministry of Ecology and Environment of China to Z.Y.Y.and J.W.Appendix

 Asian Herpetological Research2021年3期
Asian Herpetological Research2021年3期
- Asian Herpetological Research的其它文章
- Diet of Juvenile Crocodylus porosus at Kuching Wetlands National Park,Sarawak,East Malaysia
- Transcriptome Sequencing Provides Evidence of Genetic Assimilation in a Toad-Headed Lizard at High Altitude
- Complete Mitochondrial Genome of the Steppe Ribbon Racer (Psammophis lineolatus):The First Representative from the Snake Family Psammophiidae and its Phylogenetic Implications
- Age Structure of Two Species of Odorous Frogs (Odorrana margaretae andOdorrana grahami)
- Effects of Life Histories on Genome Size Variation in Squamata
- Molecular Evolution of Visual Opsin Genes during the Behavioral Shifts between Different Photic Environments in Geckos
