“Starry liver” - Von Meyenburg complex clinical case presentation and differential diagnosis discussion: A case report
Kateryna Priadko, Marco Niosi, Luigi Maria Vitale, Chiara De Sio, Marco Romano, Ilario De Sio
Kateryna Priadko, Marco Niosi, Luigi Maria Vitale, Marco Romano, llario De Sio, Department of Precision Medicine and Hepato-Gastroenterology Unit, University Hospital and Università degli Studi della Campania Luigi Vanvitelli, Naples 80138, Italy
Chiara De Sio, Internal Medicine Unit, Camilliani Hospital, Casoria 80026, Italy
Abstract BACKGROUND Von Meyenburg complex (VMC) (i.e., biliary hamartoma) is a rare congenital disorder characterized by multiple dilated cystic bile ducts, without clear trends in sex or age predominance. Due to the low number of published cases and the lack of recognized guidelines, the management of such patients remains a clinical challenge.CASE SUMMARY We present a case of symptomatic VMC that was diagnosed after imaging and histopathological examinations. Considering the patient’s condition, a conservative treatment strategy was chosen. Instrumental, laboratory, and clinical follow-up demonstrated the stable condition of the patient receiving conservative treatment.CONCLUSION VMC is a potentially non-life threatening condition, but its recognition is crucial for the management of patients.
Key Words: Biliary hamartoma; Von Meyenburg complex; Liver polycystic disease; Ultrasonography imaging; Magnetic resonance imaging; Case report
lNTRODUCTlON
Biliary hamartoma or Von Meyenburg complex (VMC) is a congenital disorder that is characterized by multiple dilated cystic bile ducts. The discovery of such lesions in most cases is incidental, with an estimated incidence in the general population of 6%[1]. The formation of hamartomas has a genetic background and consists of remodeling of primitive ductal plate configurations[1]. The distinctive feature of these cystic hepatic lesions is that they do not communicate with biliary tracts, and are usually small (up to 1.5 cm), dimensionally similar with each other, and countless, producing a “starry sky” configuration[1]. Although in most cases hamartomas do not cause symptoms, VMC can have diverse clinical presentations, such as abdominal pain, fever, jaundice[2], and in rare cases, severe portal hypertension[3]. The condition can be diagnosed by ultrasonography (US), computed tomography (CT) and magnetic resonance imaging (MRI), contrast-enhanced magnetic resonance cholangiopancreatography (MRCP) in particular, where it appears as multiple irregularly-shaped lesions of about 10 mm diameter[2]. Multiple liver cystic lesions may cause diagnostic uncertainties, such as when mimicking liver metastasis on imaging[4]. In such cases, liver biopsy with subsequent histopathological evaluation is recommended[1]. Notably, blood analysis has not proven to be very useful due to the variability of findings and low specificity.
Complications of the condition include possible calcifications[5], portal hypertension[3], recurrent cholangitis with infectious complications[2] and malignization[1]. Currently no specific treatment is available, except treatment for symptomatic patients developing the abovementioned complications. Surveillance by US, MRI and/or MRCP is necessary, but clinical recommendations lack data on the frequency of check-ups[1].
Considering the variability of symptoms, imaging results, and possible inconclusiveness of histological evaluation, the knowledge of differential diagnostic features of the condition is essential to establish the correct diagnosis.
Here, we present a clinical case report, which is discussed from the point of differential diagnosis in order to combine the latest knowledge of clinicians, radiologists, and pathologists on biliary hamartoma.
CASE PRESENTATlON
Chief complaints
A 57-year-old Caucasian woman complained of pain in the right hypochondriac region.
History of present illness
Fifteen days after a trip to Egypt, the patient was referred to a local hospital where her temperature was recorded at 38.5 °C. Laboratory examination showed increased cholestasis indices (alkaline phosphatase and gamma-glutamyl transferase > 5-6 times higher than the normal range) and acute inflammation of gall bladder as discovered during US. The patient was transferred to our unit for a second opinion.
History of past illness
The patient’s medical history was unremarkable.
Personal and family history
The patient’s family history was unremarkable.
Physical examination
Physical examination did not reveal any abnormality apart from painful sensations during palpation of the right hypochondriac area.
Laboratory examinations
Increased cholestasis indices were confirmed, and erythrocyte sedimentation rate and C-reactive protein were notably increased. Antinuclear, antimitochondrial, anti-smooth muscle actin, perinuclear antineutrophil cytoplasmic, and anti-Saccharomyces cerevisiaeantibodies, and viral hepatitis markers were negative. Alpha fetoprotein, carcinoembryonic antigen, and carbohydrate antigen 19-9 were within normal limits.
Imaging examinations
Abdominal US scanning showed a coarse echostructure with irregularity of the liver surface, similar to that of a cirrhotic liver. Considering the marked inhomogeneity of the liver structure revealed by US scan, further contrast-enhanced US (CEUS) was performed (Figure 1), which showed some small areas of contrast washout during the portal phase. Due to the high suspicion of cirrhosis associated with neoplastic areas, an US-guided liver biopsy was performed (Figure 2). Histological examination hypothesized the diagnosis of liver hamartomas (small clusters of dilated biliary ducts surrounded by fibrous stroma with epithelial lining of biliary ducts formed by a single layer of cuboidal or flattened biliary epithelium) (Figure 3). Consequent MR cholangiography sequences enhanced with gadoliniumbased contrast showed multiple hypointense nodular lesions on T1-weighted images, hyperintense lesions on T2-weighted images, and no enhancement in arterial phase after Gadolinium infusion. The lesions did not communicate with the biliary tract and a typical “starry sky” image was recorded (Figure 4). Based on the above-mentioned findings, the diagnosis of biliary hamartoma, first described by Von Meyenburg in 1918, was established.
FlNAL DlAGNOSlS
VMC (i.e.,biliary hamartoma).
TREATMENT
The proposed treatment approach was conservative and included biliary salts (ursodeoxycholic acid 15 mg/kg of body weight), daily, with approved prolongation at each subsequent follow-up visit.
OUTCOME AND FOLLOW-UP
Further clinical, laboratory, and instrumental check-ups performed every 6 mo to date, showed a clinically stable condition with no progression noted on US imaging, as well as normal serum liver tests.
DlSCUSSlON
Biliary hamartoma or VMC belongs to a heterogeneous group of congenital diseases defined as “fibrocystic liver diseases.” Such diseases are caused by anomalous development of ductal plate during embryogenesis. In addition to VMC, other fibrocystic diseases include congenital liver fibrosis, Caroli’s disease (CD), polycystic liver disease (PCLD), and choledochal cysts[6].
From the embryogenetic point of view, in VMC as well as PCLD, malformation of the ductal plate is involved in little intrahepatic biliary tracts causing loss of continuity with the remaining biliary tree[7]. The consequence of such malformation is the absence of communication between typical cysts of the PCLD or VMC and biliary tree, which is different from some other “fibropolycystic liver diseases,” such as CD. In CD, communication between cystic formations and the biliary tree is preserved since malformation of the ductal plate takes place at another time during embryogenetic development, hence involving other biliary ducts than those in PCLD or VMC.
The diagnosis of PCLD can be made by the identification of more than 20 Liver cysts on imaging modalities such as US, CT, and/or MRI, which do not communicate with the biliary tree. In cases where doubts exist regarding whether there is communication between cystic formation and the biliary tree, and consequently, the differential diagnosis among PCLD, VMC and CD is not possible, liver-specific contrast-enhanced MR cholangiography (functional MRI) will be of use. On such images, in the liver phase, biliary tracts are opacified with the contrast, and consequently, in the absence of communication between biliary tracts and cysts, the latter will not be contrasted, unlike biliary tracts. While in the case of communication between cystic lesions and the intrahepatic biliary tree, the cystic formation is opacified, allowing the diagnosis of CD to be established[5]. In our presented case, MR cholangiography sequences allowed us to exclude CD, as the communication between cystic formation and biliary ducts was not preserved. Contrast-enhanced CT or MRI allowed us to study the vessels as well, allowing identification of the last distinctive feature of VMC and PCLD from CD (i.e.,“central dot sign” - tiny dots with strong contrast enhancement of the portal vein in the venous phase within the dilated hepatic bile duct).
In biliary hamartoma as well as PCLD, cystic hepatic lesions that characterize the disease do not communicate with biliary tracts; however in VMC, such findings are usually smaller (up to 1.5 cm), countless, and dimensionally similar with each other compared with typical cystic formations in PCLD[4]. Therefore, in MR cholangiography, in addition to the lack of communication between hamartomas and biliary tracts, the typical formation of VMC is defined as a “starry sky,” as can be seen in Figure 4.
Moreover, in the case of a patient who has liver lesions suspected of VMC or PCLD, it is necessary to obtain a thorough family history, as in approximately 90% of cases, PCLD is associated with autosomal dominant polycystic kidney disease (ADPKD) or autosomal dominant polycystic liver disease (ADPLD), which should be excluded to confirm isolated PCLD due to an inheritance pattern of ADPKD/ADPLD being autosomal-dominant[8,9]. The diagnosis of ADPLD in the setting of liver cysts is based on a family history of polycystic liver with a requisite number of liver cysts for a given age. Notably, liver cysts in ADPLD are often greater in quantity and size than those in ADPKD; hence, studies emphasize the necessity of the differential diagnosis between ADPKD and ADPLD as well[9]. An Italian study group reported a clinical case of a 54-year-old kidney transplant recipient with ADPKD in whom VMC was not previously recognized but visualized on routine ultrasound scan and confirmed with MRI 4 years after renal transplantation[10]. The authors emphasized that the similar pathological pathways of the two conditions as well as immunosuppressive therapy in the patient could lead to increased risk of malignization of the lesions; thus, thorough surveillance of such patients, preferentially by CEUS or MRI over routine US, is recommended[10]. It is worth mentioning that the use of contraceptive steroids or female hormone replacement therapy by postmenopausal women is another independent risk factor for developing PCLD, which should be considered during medical history collection while no similar considerations have been published for VMC[8].
In Table 1, we present the main differential diagnostic criteria of PCLD, CD, and VMC using a PubMed search with terms such as ‘biliary hamartoma,’ ‘Von Meyenburg complex,’ ‘Caroli’s disease,’ and ‘polycystic liver disease.’
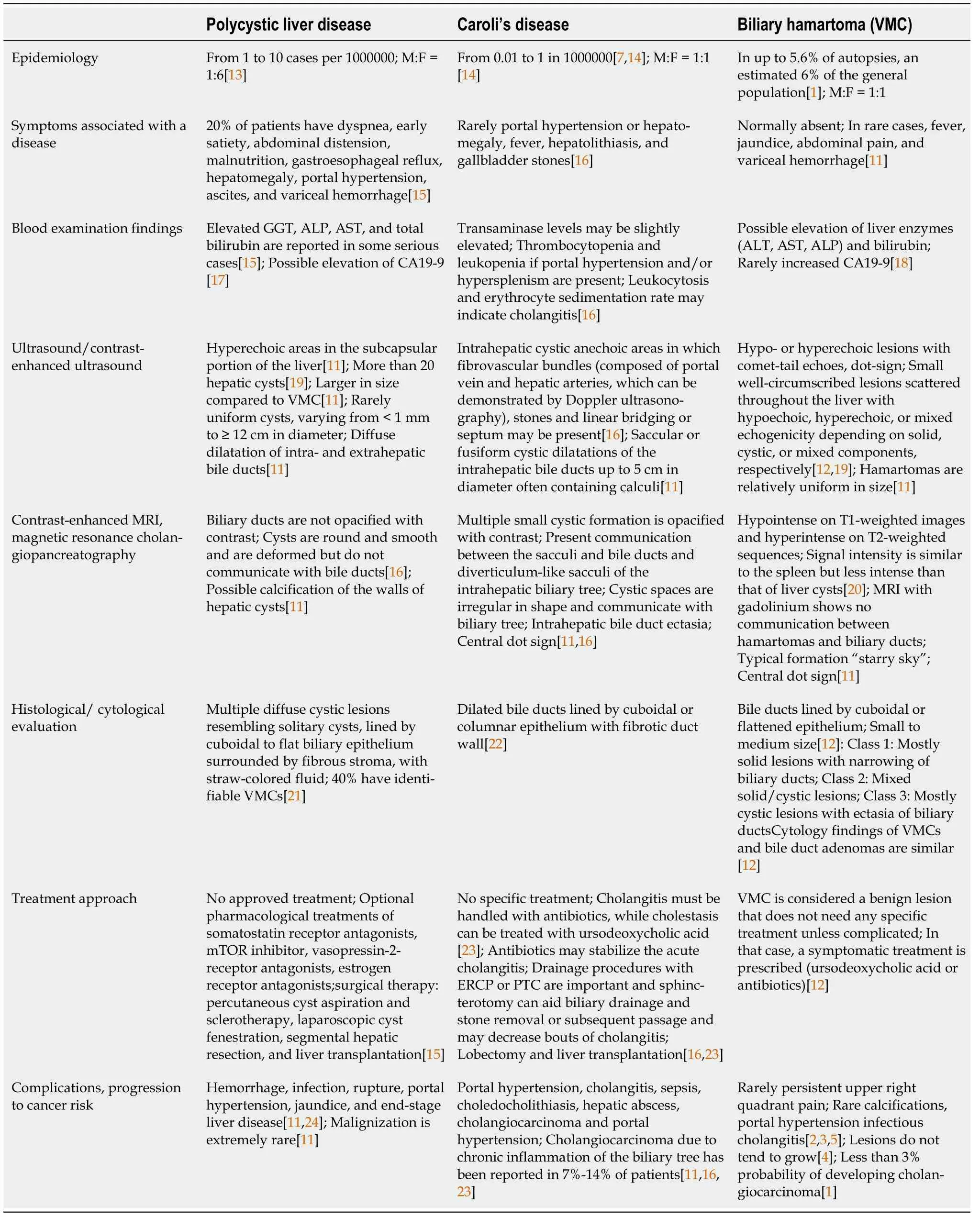
Table 1 Differential diagnosis criteria of polycystic liver disease, Caroli’s disease, and biliary hamartoma
By contrast, peribiliary cysts are cystic formations that are small in size (up to 20 mm) and localized along intrahepatic biliary ducts of the large caliber, in peribiliary spaces, with possible involvement of extrahepatic biliary tracts. Peribiliary cysts in the majority of cases are associated with liver cirrhosis, portal hypertension, portal thrombosis, and polycystic disease predominantly of the kidneys[3]. These little cysts do not show communication with corresponding biliary ducts; therefore, functional MR cholangiography is an accurate method to exclude biliary-cyst communication[3,11].
Liver-specific contrast-enhanced functional MR cholangiography is the most sensitive method for the diagnosis of intra- and extrahepatic biliary pathways and liver cystic lesions, allowing evaluation of their connection with the biliary tree[1,3,11].
While most patients with VMC remain asymptomatic, the elevation of inflammatory factors and liver function parameters (i.e.,gamma-glutamyl transferase, alkaline phosphatase, aspartate aminotransferase, alanine aminotransferase) in serum could represent the only available biomarkers suggestive of the pathology. The approach to management of patients with VMC varies from regular follow-up, as in cases with asymptomatic course, to active treatment, as in cases of symptomatic or complicated disease course and which might include administration of ursodeoxycholic acid and/or antibiotics[12], as in our presented case.
CONCLUSlON
Biliary hamartoma is a predominantly asymptomatic liver formation that is often diagnosed incidentally. Some studies have proven that with time, the function of the affected liver can be altered, although the formation bears a low risk of malignization. Thus, the knowledge of diagnostic features and differential diagnostic criteria are crucial for choosing the correct surveillance method, as currently no available international guidelines exist for standardizing the clinical diagnoses or guiding clinicians in the treatment approach and follow-up for VMC.
FOOTNOTES
Author contributions:De Sio I, Vitale LM, Niosi M, and De Sio C performed and interpreted the imaging findings and managed the patient; Priadko K reviewed the literature and drafted the manuscript; De Sio I contributed to manuscript drafting; Romano M was responsible for the revision and final approval of the manuscript; all authors issued final approval for the version to be submitted.
lnformed consent statement:Informed written consent was obtained from the patient for publication of this report and any accompanying images.
Conflict-of-interest statement:All authors have no conflicts of interest to declare.
CARE Checklist (2016) statement:The authors have read the CARE Checklist (2016), and the manuscript was prepared and revised according to the CARE Checklist (2016).
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:Italy
ORClD number:Kateryna Priadko 0000-0003-3551-1006; Marco Niosi 0000-0002-7820-3137; Luigi Maria Vitale 0000-0003-2881-907X; Marco Romano 0000-0002-3271-349X; Ilario De Sio 0000-0001-5016-6955.
S-Editor:Wang LL
L-Editor:A
P-Editor:Wang LL
 World Journal of Hepatology2022年7期
World Journal of Hepatology2022年7期
- World Journal of Hepatology的其它文章
- Retraction Note: Screening and identification of bioactive compounds from citrus against non-structural protein 3 protease of hepatitis C virus genotype 3a by fluorescence resonance energy transfer assay and mass spectrometry
- Challenge of managing hepatitis B virus and hepatitis C virus infections in resource-limited settings
- Gut microbiota contribution to hepatocellular carcinoma manifestation in non-alcoholic steatohepatitis
- Hepatitis B virus markers in hepatitis B surface antigen negative patients with pancreatic cancer: Two case reports
- Volumetric assessment of hepatic grafts using a light detection and ranging system for 3D scanning: Preliminary data
- Hepatitis C virus burden: Treating and educating people without prejudice
