Impact of increasing one-carbon metabolites on traumatic brain injury outcome using pre-clinical models
Sanika M.Joshi, Theresa Currier Thomas, Nafisa M.Jadavji,,4,5,*
Abstract Traumatic brain injury is a major cause of death and disability worldwide, affecting over 69 million individuals yearly.One-carbon metabolism has been shown to have beneficial effects after brain damage, such as ischemic stroke.However, whether increasing one-carbon metabolite vitamins impacts traumatic brain injury outcomes in patients requires more investigation.The aim of this review is to evaluate how one-carbon metabolites impact outcomes after the onset of traumatic brain injury.PubMed, Web of Science, and Google Scholar databases were searched for studies that examined the impact of B-vitamin supplementation on traumatic brain injury outcomes.The search terms included combinations of the following words: traumatic brain injury, dietary supplementation,one-carbon metabolism, and B-vitamins.The focus of each literature search was basic science data.The year of publication in the literature searches was not limited.Our analysis of the literature has shown that dietary supplementation of B-vitamins has significantly improved the functional and behavioral recovery of animals with traumatic brain injury compared to controls.However, this improvement is dosage-dependent and is contingent upon the onset of supplementation and whether there is a sustained or continuous delivery of vitamin supplementation post-traumatic brain injury.The details of supplementation post-traumatic brain injury need to be further investigated.Overall,we conclude that B-vitamin supplementation improves behavioral outcomes and reduces cognitive impairment post-traumatic brain injury in animal model systems.Further investigation in a clinical setting should be strongly considered in conjunction with current medical treatments for traumatic brain injury-affected individuals.
Key Words: folic acid; nicotinamide; one-carbon metabolism; riboflavin; traumatic brain injury;vitamin B12; vitamin B2; vitamin B3
Introduction
Traumatic brain injury (TBI) is a major cause of mortality and morbidity worldwide.In 2016, TBI was responsible for causing 8.1 million years of living with disability worldwide, and an annual global prevalence of 69 million.The global burden of TBI is found to be three times greater in low-to-middleincome countries compared with high-income countries.TBI is an important public health concern, especially among young people in both developed and developing countries, primarily due to increases in global motor-vehicle usage (Maas et al., 2008; GBD 2016 Traumatic Brain Injury and Spinal Cord Injury Collaborators, 2019).Road traffic injury, accounting for a majority of TBI, is listed by the World Health Organization as one of the top ten causes of disability-adjusted life years as of 2019.In fact, 56 percent of TBIs in lowerincome countries were estimated to be due to traffic accidents compared with 25 percent in high-income countries (MRC CRASH Trial Collaborators et al., 2008; Roozenbeek et al., 2013; GBD 2016 Traumatic Brain Injury and Spinal Cord Injury Collaborators, 2019).As the population ages, falls are also becoming another increasingly common cause of TBI among the elderly,who are more prone to hospitalization and fatality from TBI compared to younger individuals (Maas et al., 2008; MRC CRASH Trial Collaborators et al.,2008; Roozenbeek et al., 2013; GBD 2016 Traumatic Brain Injury and Spinal Cord Injury Collaborators, 2019).Survivors of TBI often live with significant permanent disabilities, and the substantial costs of clinical management for TBI patients have placed a considerable socioeconomic burden on both the healthcare system and society as a whole (Seifert, 2007).Thus, there is a pressing need to develop and implement comprehensive prevention strategies and treatments for TBI to mitigate its impact on individuals and society.
TBI is a complex, heterogeneous disease that can be classified in terms of its pathophysiology as primary injury or secondary injury (Ghajar, 2000; Maas et al., 2008; Ng and Lee, 2019).TBI’s primary injury occurs at the time of trauma and is a mechanical injury directly or indirectly to the brain through focal damage, diffuse, or a combination.However, the neurological damage that follows TBI is not solely due to the primary injury of TBI but rather a complex set of molecular, cellular, and physiological changes that occur afterward,known as secondary injury (Ghajar, 2000; Maas et al., 2008; Lucke-Wold et al.,2018; Ng and Lee, 2019).The cascades of secondary injury are characterized by mitochondrial dysfunction, neurotransmitter-mediated excitotoxicity,oxidative stress, secondary ischemia, vascular injury, and the apoptotic cell death of neurons and glia (Ng and Lee, 2019).In fact, in-hospital fatalities primarily occur due to the secondary damage that ensues following TBI because normally well-tolerated systemic events such as hypotension or hypoxia can aggravate neuronal injury in cells already vulnerable from the initial TBI (Chesnut et al., 1993; Jiang et al., 2002; Schreiber et al., 2002;McHugh et al., 2007).
Secondary damage to the brain is delayed, often taking months or even years to develop after the initial injury, which provides an ideal window-ofopportunity for treatments (Scrimgeour and Condlin, 2014; Lucke-Wold et al., 2018).Therefore, the current research on TBI has shifted its focus to understanding and treating the underlying pathophysiological mechanisms that occur during secondary injury (Scrimgeour and Condlin, 2014; Lucke-Wold et al., 2018).Over the past three decades, clinical trials have had limited success in the development of neuroprotective treatment for secondary injury, and as a result, considerable research has been primarily focused on identifying new therapeutic targets associated with these processes(Scrimgeour and Condlin, 2014; Lucke-Wold et al., 2018; Ng and Lee, 2019).
A compelling area of interest in the treatment of TBI has been the utilization of diet, specifically one-carbon (1C) metabolite vitamin supplementation post-injury.1C metabolism is a complex network of biochemical reactions that are involved in the transfer and utilization of 1C units responsible for the production of functional compounds like amino acids, purines, pyrimidines,and methyl groups, which are essential for a variety of cellular processes(Figure 1; Kennedy, 2016; Lyon et al., 2020).Vitamins B6, B9 (folic acid/folate),B12, and other B vitamins play critical roles in this metabolic process (Kennedy,2016; Lyon et al., 2020).B vitamins are vital for the maintenance of human health and deficiencies, or excess of B vitamins can lead to neurological defects, anemia, aberrant immune responses, and cancer (Kennedy, 2016;Vonder Haar et al., 2016; Lyon et al., 2020).
One-carbon metabolism plays a critical role in brain function, including neurotransmitter synthesis, and DNA and RNA methylation (Mattson and Shea, 2003; Kennedy, 2016; Lyon et al., 2020).The metabolism of folate and other one-carbon nutrients is involved in the regulation of homocysteine,an amino acid associated with cognitive decline, dementia, and depression(Mattson and Shea, 2003; Kennedy, 2016; Lyon et al., 2020).Alterations in 1C metabolism can lead to a buildup of homocysteine, which has been shown to cause synaptic dysfunction and neuronal death (Mattson and Shea, 2003)and has also been reported to worsen TBI outcomes (Lauretta et al., 2022).Homocysteine promotes DNA damage, activation of apoptotic signaling cascades, enhanced Ca2+influx, and endoplasmic reticulum stress, thereby contributing to the development and progression of various psychiatric and neurodegenerative conditions (Mattson and Shea, 2003).Additionally, high levels of homocysteine can lead to an imbalance in the gut microbiome and trigger a range of systemic disorders, further exacerbating TBI pathology(George et al., 2021).Disturbances in 1C metabolism could also impair neural plasticity and exacerbate the damage caused by the injury, potentially affecting the brain’s ability to adapt and recover (Mattson and Shea, 2003;Lyon et al., 2020).Thus, maintaining optimal levels of 1C nutrients is essential for neuronal homeostasis, proper cognitive functioning, and the preservation of a healthy gut microbiome.
In essence, understanding the contributions of various B vitamins in 1C is imperative in assessing the impact of 1C metabolite supplementation following TBI.This review explores the therapeutic effects of increasing levels of B2, B3, B6, B9, and B12 vitamins on the outcomes after TBI.Our review will focus on a comprehensive analysis of pre-clinical studies.Specifically, we discuss the results of pre-clinical nutraceutical interventions, highlighting the need for further investigation in this area.
Search Strategy
PubMed, Web of Science, and Google Scholar databases were searched for studies that examined the impact of B-vitamins on TBI outcomes.The search terms included combinations of the following words: traumatic brain injury,dietary supplementation, one-carbon metabolism, and B-vitamins.The year of publication was not limited.Since there are few studies in this area, all studies that assessed the impact of any B-vitamins on TBI were included in this review.Table 1 includes a summary of all articles included in this review and their main findings.
Riboflavin
Riboflavin, vitamin B2, is a powerful antioxidant present in milk, dairy products, and dark green leafy vegetables (Powers, 2003; Kennedy, 2016).The two flavoprotein coenzymes, flavin adenine dinucleotide and flavin mononucleotide, are the most biologically active forms of B2, serving as electron carriers in a variety of redox reactions (Powers, 2003).Additionally,flavin adenine dinucleotide and flavin mononucleotide are also involved in synthesizing and recycling vitamins and heme proteins, aiding in the metabolism of essential fatty acids and iron absorption, and regulating thyroid hormones (Kennedy, 2016).As a part of 1C metabolism, B2 acts as a coenzyme with methylenetetrahydrofolate reductase (MTHFR) in the folate cycle, which catalyzes the conversion of 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate.B2 also rate-limits the recycling of methionine synthase in the methionine cycle by providing a methyl group for the remethylation of homocysteine to form methionine (García-Minguillán et al.,2014).When there is a deficiency of riboflavin, the activity of MTHFR and methionine synthase can be impaired, leading to a variety of negative health outcomes, including DNA damage, impaired red blood cell formation, birth defects, and increased levels of homocysteine (Mattson and Shea, 2003;Kennedy, 2016; Vonder Haar et al., 2016; Lyon et al., 2020).The dysregulation of B2 may have broader consequences for brain function, as it plays critical roles in the production of neurotransmitters and the maintenance of neuronal health.
The mechanism of action of B2 that holds clinical promise in protecting tissues from injury is its ability to reduce oxidative damage (Hultquist et al., 1993; Barbre and Hoane, 2006; Vonder Haar et al., 2016).Pre-clinical studies assessing the impact of B2 on TBI outcome have found that 7.5 mg/kg dosage administered after bilateral cortical contusion was optimal for significantly reducing cognitive and behavioral impairments post-injury(Hoane et al., 2005).In this study, rats were assigned to either B2 or saline treatment conditions that received contusion injuries or sham procedures;drug treatment was administered 15 minutes and 24 hours following injury(Hoane et al., 2005).The rats were examined on several tests to measure sensorimotor performance (bilateral tactile removal test) and cognitive ability(acquisition of reference and working memory) in the Morris water maze.The administration of B2 following injury significantly reduced behavioral impairments observed on the bilateral tactile removal test and improved the acquisition of both reference and working memory tests compared to saline-treated rats (Hoane et al., 2005).The study’s findings indicate that B2 administration significantly improved behavioral outcomes and reduced lesion volume, edema formation, and the expression of glial fibrillary acidic protein (GFAP) following traumatic brain injury (Hoane et al., 2005).However, the study notes that more research is needed to determine the efficacy of riboflavin in clinical settings.Additional studies investigating the impact of combination treatment of 7.5 mg/kg B2 with 1 mmol/kg of magnesium following brain injury significantly reduced initial impairment and facilitated recovery on the vibrissae forelimb placing test; however, lowdose combination treatments of magnesium and B2 or individual treatments were equally effective in improving recovery on the tactile adhesive removal test (Barbre and Hoane, 2006).These studies have yielded promising results,suggesting that B2 may have therapeutic potential for TBI treatment.However,further research is necessary to determine the optimal dosage, timing, and efficacy of B2 administration for TBI treatment, as well as the impact of administering B2 at alternative time points post-injury and in different brain injury mechanisms and the long-term benefit of early supplementation postinjury (Hoane et al., 2005; Barbre and Hoane, 2006; Vonder Haar et al., 2016).
Nicotinamide
In recent years, there has been a growing interest in exploring the potential benefits of nicotinamide (NAM), the amide form of vitamin B3, as a neuroprotective agent after TBI (Hoane et al., 2006b, c; Vonder Haar et al., 2016).Nicotinamide is naturally obtained from dietary sources, such as meat, poultry, nuts and seeds, or supplements.It serves as a precursor for two vital enzymes, namely, β-nicotinamide adenine dinucleotide (NAD+) and nicotinamide adenine dinucleotide phosphate (NADP+) (Maiese, 2021).These enzymes play crucial roles in several metabolic pathways related to energy metabolism, oxidative stress, apoptosis, and autophagy.NAM serves as an essential cofactor in the folate/tetrahydrobiopterin cycles, including the enzymes dihydrofolate reductase and methylenetetrahydrofolate reductase,as well as the methionine cycle’s S-adenosylhomocysteine hydrolase in 1C metabolism (Kennedy, 2016; Lyon et al., 2020).Therefore, it is imperative to consider the impact of NAM’s role in 1C metabolism to understand how it can effectively serve as a nutritive intervention following TBI.
Animal studies have investigated NAM’s potential for improving functional recovery and reducing tissue damage after TBI.One study found that NAM administration prevented the development of behavioral and working memory deficits in rats at 15 minutes and 24 hours post-injury (Hoane et al., 2003, 2006c), while another showed that it reduced blood-brain barrier damage and apoptosis expression in the tissue surrounding the lesion cavity,leading to a reduction in neuronal cell loss and lesion cavity expansion (Hoane et al., 2006a).A separate study found that NAM mononucleotides (NMN), a direct precursor for NAD+, when administered post-injury in rats demonstrated neuroprotective effects by reducing neuronal death, preserving hippocampal structure, alleviating brain edema, and improving neurological function (Zhu et al., 2023).NMN treatment mitigated the activation of tumor necrosis factor-α and interleukin (IL)-1β in TBI rats, and decreased the levels of GFAP and Iba-1, indicating reduced astrocytosis and microgliosis, and decreased neuronal apoptosis, suggesting that the improvement in neurological function may be attributed to the alleviation of neuroinflammation by NMN (Zhu et al.,2023).
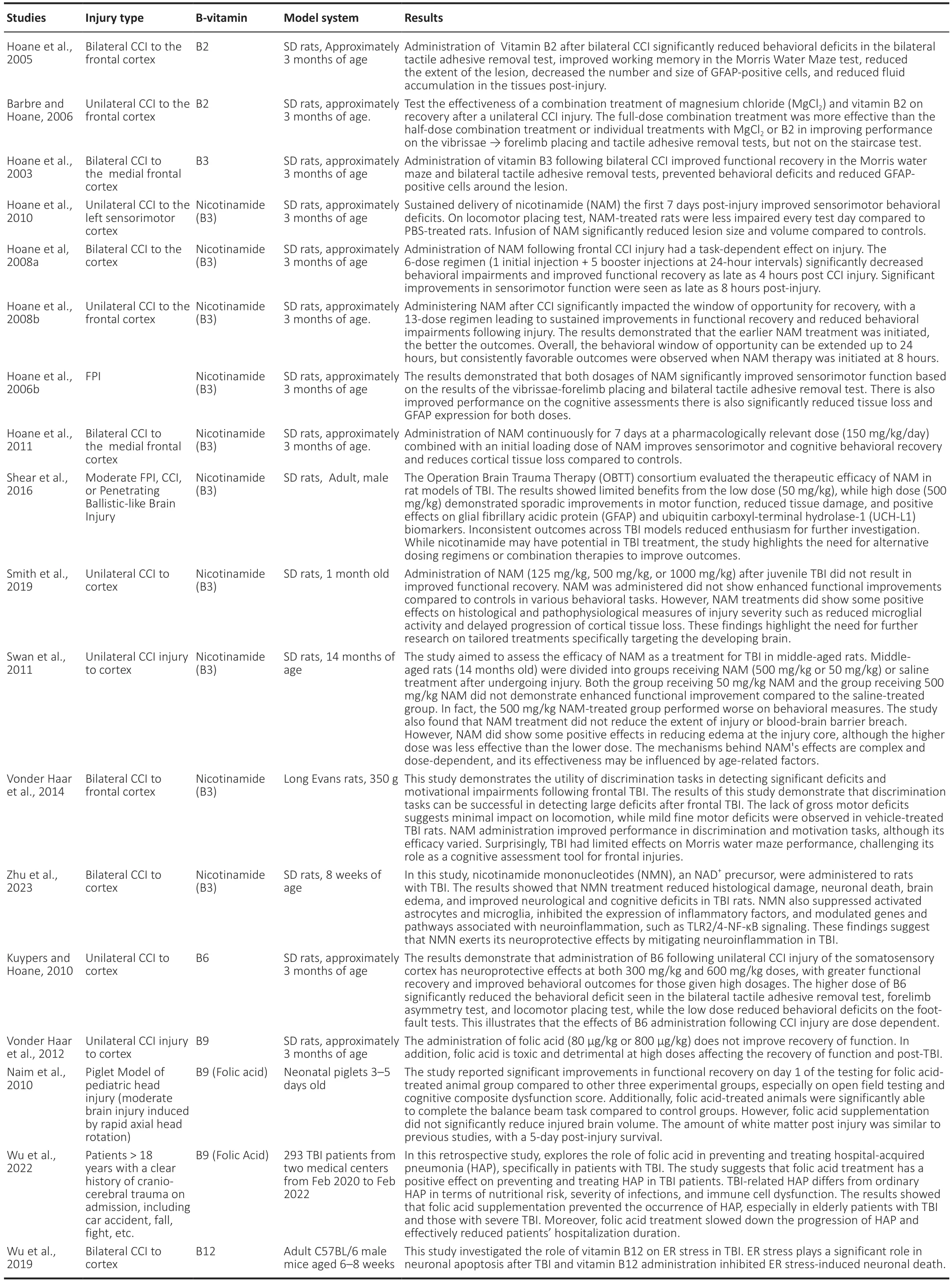
Table 1 |Summary of pre-clinical studies reviewed to assess the impact of B-vitamins on traumatic brain injury outcome
NAM’s pharmacokinetics have also been examined, with some studies finding that higher dosages (500 mg/kg) are more effective in improving cognitive tasks, such as the Morris water maze.In comparison, lower dosages (50 mg/kg)are still effective in improving functional recovery on behavioral tests, such as the bilateral tactile removal test and vibrissae-forelimb placing (Hoane et al.,2006c).Although some studies have demonstrated the lack of therapeutic effect and even negative outcomes associated with a low dose of NAM (50 mg/kg),higher doses (500 mg/kg) of nicotinamide exhibited some modest yet notable benefits including improvements in motor function, reduced tissue damage, and positive effects on specific biomarkers (Shear et al., 2016).Additionally, NAM has been found to modulate reactive astrocytosis, which is believed to play a crucial role in the pathogenesis of TBI.NAM administration has been shown to reduce the number of reactive astrocytes and GFAP expression, leading to improved tissue integrity and neuronal survival (Holland et al., 2008).
Furthermore, NAM appears to have multiple mechanisms of action that contribute to its neuroprotective effects in TBI.It may prevent ATP depletion and inhibit the destructive enzyme poly [ADP-ribose] polymerase, which can help to restore energy levels and suppress the detrimental effects of poly [ADP-ribose] polymerase activation after injury (Maiese and Chong,2003; Vonder Haar et al., 2011).NAM may also reduce oxidative stress and activate anti-apoptotic pathways, contributing to neuronal preservation(Maiese and Chong, 2003; Maiese, 2021).It affects gene expression in several functional categories and cell signaling pathways involving neurotransmitters,neuropeptides, growth factors, and ion channels, modulating acute pathophysiological processes and aiding in recovery of function (Anderson et al., 2013).Ribonucleic acid (RNA) sequencing showed that NMN treatment even exhibited the capacity to reverse the expression of 792 genes out of 1589 genes changed in TBI (Zhu et al., 2023).Notably, NMN effectively reduced the levels of CCL2, proinflammatory cytokines including IL-6, IL-11, and IL1rn, as well as Toll-like receptors TLR2 and TLR4 (Zhu et al., 2023).These findings strongly suggest that the inhibition of TLR2/4-nuclear factorκB signaling serves as the underlying molecular mechanism through which NAM exerts its beneficial effects, leading to a reduction in brain damage and protection of nerves in TBI (Zhu et al., 2023).
Recent studies have explored various aspects of NAM treatment, such as the appropriate time window, benefits of continuous infusion, optimal dose, and duration of treatment.The available evidence suggests that NAM administered early after TBI results in improved recovery of sensorimotor function and has a window of opportunity of up to 4 hours for positive cognitive outcomes (Hoane et al., 2008a); however, NAM therapy administered up to 8 hours post-TBI also showed a preservation of corticaltissue loss compared to the control group (Hoane et al., 2008b).Continuous treatment for seven days at a dose of 150 mg/kg/day can improve multiple aspects of behavioral recovery and reduce tissue loss (Vonder Haar et al.,2011).A long-term treatment regimen of 50 mg/kg of NAM starting at clinically relevant time points may prove efficacious in human TBI, with sustained, steady-state levels of treatment being maximally effective (Goffus et al., 2010).Administering NAM at 4-hour and 8-hour time points postinjury, with a dosage of 50 mg/kg for up to 12 days, resulted in significant improvements in functional recovery and preserved cortical tissue based on histological analysis (Hoane et al., 2008b).Notably, even when administered as late as 8 hours after TBI, prolonged NAM administration significantly enhanced sensorimotor function across various behavioral measures and reduced tissue loss in the injured cortex (Hoane et al., 2008b).While the optimal time for NAM treatment following TBI may vary depending on the task, it can be extended up to 24 hours post-injury (Hoane et al., 2008b).Overall, extending the duration of NAM therapy not only enhances tissue sparing and behavioral and functional recovery, but also extends the window of opportunity for effective treatment, leading to improved outcomes in TBI.
Age is another critical factor to consider in predicting outcomes in administering NAM treatment post-injury.While previous studies have highlighted the clinical relevance and potential benefits of NAM treatment,they have largely overlooked the specific needs of children and adolescents who face higher rates of hospitalization and long-term disabilities.In one study, juvenile rats were administered varying doses of NAM (125 mg/kg,500 mg/kg, or 1000 mg/kg) at specific time intervals following injury (Smith et al., 2019).Surprisingly, the NAM-treated animals did not exhibit improved functional outcomes compared to the control group across a range of behavioral tasks (Smith et al., 2019).Although behavioral improvements were not observed, the NAM-treated animals did show some positive effects.They experienced a slower rate of cortical loss and a reduction in microglial activity compared to the control group (Smith et al., 2019).However, these effects did not translate into a significant reduction in overall cortical volume loss or improved behavioral outcomes (Smith et al., 2019).It is clear that the preclinical efficacy of NAM treatment for cortical contusion injuries in juvenile animals differs from what has been observed in adult rat models.When examining the effect of NAM therapy on TBI outcomes in middle-aged rats,the findings are even more discouraging.NAM therapy shows limited efficacy and, in fact, may exacerbate the recovery process in middle-aged animals,particularly at higher dosages (Swan et al., 2011).To gain a comprehensive understanding of how age impacts the efficacy of TBI treatments, future preclinical evaluations should include a larger sample of juvenile and middleaged animals.This approach is crucial in developing tailored therapies that effectively address the unique characteristics and needs of the developing brain, which undergoes constant changes.By recognizing age-related differences and refining treatment strategies, we can better optimize TBI interventions and improve outcomes for patients of all ages.
NAM treatment has the potential to be an effective therapeutic strategy for improvements in cognitive and behavioral outcomes post-TBI.However,the optimal timing, dose, and duration of treatment may depend on various factors, including the type and severity of injury, the specific behavioral deficits being targeted, and the age and health status of the patient.Moreover, expanding the range of behavioral assessments in TBI research to include fear learning and social interaction can provide valuable insights into potential treatment approaches (Vonder Haar et al., 2014).Notably, a study revealed that discrimination tasks successfully identify deficits in frontal TBI,primarily associated with motivation, while gross motor functions remain relatively unaffected (Vonder Haar et al., 2014).Although NAM administration exhibits varying efficacy in enhancing discrimination and motivation, further investigation is warranted (Vonder Haar et al., 2014).Future studies are needed to refine the treatment protocol and determine the safety and efficacy of NAM treatment in human TBI.
Folates
Folates (vitamin B9) play a critical role in the central nervous system development and are essential for functioning in all stages of life, from development to adulthood (Mattson and Shea, 2003).Folates are obtained mostly through our diet from food like leafy greens, and liver, and can be taken in supplement form, which is referred to as folic acid.The importance of folic acid was first discovered in pregnant women deficient in vitamin B9 who were found to have a higher risk of neural-tube defects.During childhood, inborn errors of folate transport and metabolism were shown to have developmental delay, motor and gait abnormalities, cognitive and behavioral deterioration, seizures, and signs of myelination or failure of remyelination (Reynolds, 2002).In adulthood, folic acid deficiency contributes to neuropsychiatric disorders, such as dementia and depression, increasing the risk of Alzheimer’s disease and vascular dementia, especially in geriatric populations (Reynolds, 2002).
Folic acid is best known for its role as a key regulator in 1C metabolism where it acts as a substrate for nucleotide synthesis and as a methyl group donor,promoting the regeneration of methionine from homocysteine (Mattson and Shea, 2003).Its neuroprotective effects of folic acid are thought to be because of its ability to maintain low levels of homocysteine, a cytotoxic amino acid that induces DNA damage, apoptosis, and oxidative stress in the cell.Folate and homocysteine together play a significant role in affecting fundamental processes involving neuroplasticity, stem cell proliferation, synaptic plasticity,cell survival, and even differentiation into neural and glial cells (Mattson and Shea, 2003).
Epidemiological and pre-clinical studies have connected folate deficiency and increased homocysteine levels to neurodegenerative disorders such as Alzheimer’s disease, Parkinson’s disease, stroke, and even neuropsychiatric disorders such as depression and dementia (Ebara, 2017).The role of folic acid supplementation in improving neurodevelopment and enhancing neuroprotection has even been evident spinal cord injury and stroke (Naim et al., 2010).
Pre-clinical studies investigating the effects of folic acid supplementation on improving neurological outcomes after TBI have not been widely studied.One experimental study investigated whether folic acid would reduce brain injury and improve neurological outcomes in a swine model of pediatric TBI(Naim et al., 2010).There were four experimental groups of 3-to-5 day old female piglets; two groups were injured and two groups were uninjured(control).One of the injured groups was injected with 80 μg/kg of folic acid 15 minutes after the procedure and then 6 days daily following the injury(Naim et al., 2010).The second injured group received saline at the sametime points (Naim et al., 2010).The two control groups received either folic acid (80 g/kg) or saline at the same time points, following a sham procedure.Behavioral testing occurred on days 1 and 4 following injury; the piglets were euthanized on day 6 following injury and their brains were perfusion fixed for histological analysis (Naim et al., 2010).Significant findings include improved functional outcome on day 1 of testing in the experimental group given folic acid in open-field exploration and the cognitive composite dysfunction score,a measure of the overall neurobehavioral performance of the piglet (Naim et al., 2010).This suggested early improvement in motor function following folic acid supplementation following TBI.Additionally, groups that received B9 had shorter latencies in completing the beam task on day 1 and made fewer errors when completing the glass barrier testing on day 4, which suggested improved motor function and visual problem-solving.However, histological analysis revealed that folic acid supplementation failed to reduce brain volume, despite 6 days of treatment with folic acid (Naim et al., 2010).
Another study evaluated the effects of a higher dose (800 μg/kg) of folic acid supplementation on neuronal recovery of function following a unilateral controlled cortical impact injury in 3.5-month-old male rats (Vonder Haar et al., 2012).The rats were divided into 4 groups; three groups were injured and were injected with either 80 μg/kg of folic acid, 800 μg/kg of folic acid,or saline thirty minutes following injury.The fourth group was injected with saline at the same time point following a sham procedure.All groups received booster injections every 24 hours for 14 days consecutively.Behavioral testing was followed by histological analysis of brain tissue was performed to determine lesion size and neuronal loss.Results showed that folic acid does not improve recovery of function at either dose, and there was significant neuronal loss in the group given high dosages (Vonder Haar et al., 2012).
Overall, experimental studies investigating the effects of folic acid supplementation on TBI outcomes have shown promising results, with early functional recovery observed in one of the studies (Naim et al., 2010).However, these benefits may be limited to an immediate, initial period, and the possibility of longer treatment durations not being beneficial in improving TBI outcomes has been revealed by both studies (Naim et al., 2010; Vonder Haar et al., 2012).Moreover, caution must be exercised when considering high doses of folic acid as they have been shown to have particularly detrimental effects and may lead to poor TBI outcomes.As such, additional experimental research studies in this area will focus on potential mechanisms of the beneficial effect of folic acid, long-term neurological outcomes, shorter treatment durations, and optimal dosages.
Vitamin B6
Vitamin B6 is a water-soluble vitamin, naturally present in many foods such as fruits, grains, and vegetables, and is critical for performing a wide variety of enzymatic reactions (Vonder Haar et al., 2016).Pyridoxal-5-phosphate and pyridoxamine-5-phosphate are the most active coenzyme forms of B6 among the six vitamer forms (Vonder Haar et al., 2016).Pyridoxine serves as a coenzyme in over 150 enzymatic reactions, most of which are involved in amino acid synthesis, transamination, and degradation.Its role as an essential rate-limiting cofactor in the synthesis of neurotransmitters such as dopamine, serotonin, gamma-butyric acid, noradrenaline, and melatonin is critical.Therefore, even mild deficiencies in pyridoxine can lead to changes in sleep, behavior, and cardiovascular function due to altered neurotransmitter synthesis (Vonder Haar et al., 2016; Lyon et al., 2020; Office of Dietary Supplements, 2023).
In addition to its role in neurotransmitter synthesis, B6 is involved in glucose regulation, hemoglobin production, and immune functioning,with decreased levels of pyridoxal-5-phosphate serving as a biomarker for severe inflammation.Most importantly, pyridoxine is involved in several phases of one-carbon metabolism.During the folate cycle,tetrahydrofolate (THF) is converted to 5,10-methyleneTHF, which requires pyridoxal-5-phosphate as a cofactor.The production of 5,10-methyleneTHF is crucial for not only regenerating dihydrofolate, but also producing deoxythymidine monophosphate as a byproduct, which is necessary for nucleotide synthesis.B6 is also necessary for converting homocysteine to cysteine via the transsulfuration pathway, a crucial process for methionine recycling.Considering the role of homocysteine in neurodegenerative and cardiovascular diseases, B6’s involvement in regulating its production may be critical in affecting cognitive and behavioral outcomes following TBI.
One study evaluated the neuroprotective effects of pyridoxine following unilateral controlled cortical impact injury in an animal model (Kuypers and Hoane, 2010).Animals were administered with either 300 mg/kg or 600 mg/kg doses, 30 minutes post-injury and then given a booster dose after 24 hours (Kuypers and Hoane, 2010).The group given the higher dosage had significantly reduced behavioral deficit and performed better on the bilateral tactile adhesive removal test, forelimb asymmetry test, and locomotor placing test (Kuypers and Hoane, 2010).The group given the lower dosage, only showed improvement initially in the forelimb asymmetry test and foot-fault tests (Kuypers and Hoane, 2010).Overall, the study demonstrated that the effects of pyridoxine administration following unilateral controlled cortical impact injury are dose-dependent, with the higher dose showing significantly improved behavioral and anatomical outcomes (Kuypers and Hoane, 2010).
Although the number of studies related to vitamin B6 supplementation and traumatic brain injury is highly limited, there is an increased need to determine the ideal dosing regimen as high levels of pyridoxine are shown to be neurotoxic, while low levels as demonstrated by the study show very minimal neuroprotection post-TBI.The animals were also administered pyridoxine 30 minutes and 24 hours post-injury, which is very difficult to replicate in a clinical setting where initial administration of pyridoxine may be hours after injury.Therefore, there is a need to establish a window of opportunity for pyridoxine administration to secure its full benefits.Further studies are needed to determine the optimal dosage and administration of pyridoxine in TBI cases.
Vitamin B12
Vitamin B12 is produced by bacteria and archaea via aerobic and anaerobic pathways (Lyon et al., 2020).These bacteria are often ingested through diet, with meat, eggs, and dairy products serving as primary sources of B12(Kennedy, 2016).Vitamin B12 is paramount for the normal functioning and metabolism for many organ systems.The most well-established functional roles of B12 are red blood cell synthesis and prevention of megaloblastic anemia, development and myelination of the central nervous system,cognitive functioning and prevention of dementia, and the regulation of homocysteine levels (Lyon et al., 2020; Office of Dietary Supplements,2022).Thus, the disruption of B12 regulation in the body can have long-term consequences across multiple organ systems.
Of the metabolically active forms of vitamin B12, 5-methylcobalamin, and 5-deoxyadenosylcobalamin function as cofactors for cystolic methionine synthase and mitochondrial methylmalonyl CoA mutase, respectively (Lyon et al., 2020).The methionine cycle requires 5-methylcobalamin as a cofactor to donate a methyl group to regenerate methionine from homocysteine, which is necessary for protein incorporation and the synthesis of the universal methyl donor S-adenosylmethionine (Lyon et al., 2020).In addition, 5-mTHF is converted back to tetrahydrofolate as a result of this reaction which further continues the production of de novo nucleotide synthesis (Lyon et al., 2020).
Methylmalonyl CoA mutase, on the other hand, catalyzes the conversion of methylmalonyl CoA to succinyl CoA, which then enters the citric acid cycle playing a key role in the oxidation of fatty acids and catabolism of ketogenic amino acids (Lyon et al., 2020).Thus, deficiencies in B12 can have significant biological consequences only for one-carbon metabolism, but also for energy and redox metabolism.
Although the role of vitamin B12 on neurological function has been established, there are a minimal number of studies conducted till date that have recognized vitamin B12 as a form of treatment for TBI-affected patients.It has been reported that systemic administration of vitamin B12 has been promoted in the recovery process from peripheral nerve damage in rats (Baltrusch, 2021).Furthermore, vitamin B12 was found to serve as a superoxide scavenger contributing to neuronal cell growth (van de Lagemaat et al., 2019).One randomized controlled trial showed that vitamin B12 treatment in Chilean elderly subjects with vitamin B12 deficiency at screening improved conductivity in myelinated peripheral nerves (Brito et al., 2016).Endoplasmic reticulum stress plays a significant role in neuronal apoptosis post-TBI, but vitamin B12 downregulates endoplasmic reticulum stress-related apoptosis by rescuing microtubule stabilization, remyelination, and myelin reparation (Wu et al., 2019).This study demonstrated through pre-clinical research that vitamin B12 enhances the survival of nerve cells post-TBI.This pre-clinical study warrants the need for additional studies to establish preclinical efficacy and the impact of vitamin B12 treatment on behavioral and cognitive functioning post-TBI.
Clinical Data
We were interested in determining whether the preclinical results translated to the clinical setting.In our search of the literature we were able to find a few studies investigating the impact of 1C metabolites on TBI outcomes.One study did show that increased levels of homocysteine can worsen outcomes after TBI (Lauretta et al., 2022).Furthermore, folic acid deficiency has been associated with a poor prognosis for patients with pneumonia (Wu et al., 2022).With hospital-acquired pneumonia being the most common complication of TBI, folic acid supplementation has been found to shorten hospitalization stay, reduce the need for tracheostomy, and effectively treat and prevent the occurrence of hospital-acquired pneumonia (Wu et al.,2022).Another study in military TBI found that S-adenosylmethionine can improve post-concussive sequelae in affected patients (Schieffler and Matta,2022).Clinical studies conducted in Russia have shown the positive effects of Cytoflavin, which is a combination of inosine, nicotinamide, riboflavin, and succinic acid on outcomes of TBI including vertigo and dizziness (Lavrick et al.,2020), intensive care (Lebedeva et al., 2014), as well as in patients that are of advanced age (Lebedeva et al., 2014).Lastly, we searched ClinicalTrials.gov and found three clinical trials evaluating the role of 1C supplementation on TBI outcomes, one is completed and two are still in progress.
Conclusion and Future Directions
There is not a comprehensive understanding of the role of TBI secondary injury cascades.We want to suggest that when investigating and implementing therapies for TBI, it is imperative to consider long-term outcome measures.TBI has enduring effects on individuals, encompassing cognitive impairments, behavioral changes, and functional limitations.While short-term measures may provide initial insights into immediate improvements or changes following TBI, they may not capture the complete trajectory of recovery or uncover potential delayed consequences.By evaluating long-term outcomes, researchers can gain deeper insights into the sustained impact of TBI and assess the effectiveness of interventions or treatments over an extended duration.Furthermore, long-term outcome measures enable the assessment of critical factors such as cognitive function,quality of life, resumption of work or daily activities, and overall well-being among TBI patients.Moreover, studying long-term outcomes facilitates the identification of potential complications or secondary conditions that may manifest months or even years after the initial injury.This knowledge is invaluable in designing comprehensive treatment plans, developing targeted interventions, and enhancing long-term care strategies for individuals affected by TBI.
Maintaining adequate levels of B-vitamins, including B2, B3, B6, B9, and B12,is vital for promoting brain function and safeguarding against neurological damage.The preclinical studies reviewed have demonstrated that increasing these B-vitamin levels can lead to improved behavioral outcomes including reduced cognitive impairment following TBI.However, given the complexity of TBI as a serious condition, a comprehensive treatment approach is necessary to promote recovery after injury.The evidence presented in this review demonstrates that B-vitamin supplementation shows promise as a complementary therapy for TBI-affected patients.The data from preclinical studies that B-vitamin supplementation could be integrated as part of a broader treatment strategy rather than a standalone solution.Biomarker development for TBI and B-vitamin needs could also be developed to determine which vitamin a patient needs and whether the treatment is effective, homocysteine could potentially be this marker along with another 1C metabolite.
Future research should focus on determining the optimal dosage, timing,and mode of administration (sustained vs.continuous) of B-vitamins, as well as exploring their potential synergistic effects with other therapeutic interventions.Additionally, understanding the “window of opportunity” for supplementation and considering individual variations based on factors such as sex, race, and age are important areas of investigation.Differences in sex are especially important due to the variations in the absorption of certain B vitamins between males and females, potentially influenced by hormonal and physiological factors.Sex hormones, such as estrogen, can impact B vitamin metabolism, including folate and vitamin B12.Genetic factors, like MTHFR gene polymorphisms, contribute to variations in folate metabolism (Bennettet al., 2021; Ali et al., 2022).Hormonal contraceptives can also alter B-vitamin metabolism.These differences have clinical implications, such as the risk of neural tube defects during pregnancy.Further research is needed to fully understand sex-specific B-vitamin metabolism and guide supplementation recommendation to enhance recovery for individuals with TBI.
Overall, the encouraging findings from pre-clinical studies and the potential benefits of B-vitamin supplementation highlight the promising nature of this research area in improving outcomes for individuals affected by TBI within a clinical setting.
Author contributions:Writing – original draft,investigation,writing –review and editing:SMJ;writing – review and editing:TCT;conceptualization,investigation,writing – original draft,writing – review and editing,visualization,supervision,project,and administration:NMJ.All authors approved the final version of the manuscript.
Conflicts of interest:The authors declare no conflicts of interest.
Data availability statement:Not applicable.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
 中國(guó)神經(jīng)再生研究(英文版)2024年8期
中國(guó)神經(jīng)再生研究(英文版)2024年8期
- 中國(guó)神經(jīng)再生研究(英文版)的其它文章
- The big data challenge – and how polypharmacology supports the translation from pre-clinical research into clinical use against neurodegenerative diseases and beyond
- P-aminobenzoic acid promotes retinal regeneration through activation of Ascl1a in zebrafish
- Lupenone improves motor dysfunction in spinal cord injury mice through inhibiting the inflammasome activation and pyroptosis in microglia via the nuclear factor kappa B pathway
- Two-photon live imaging of direct glia-to-neuron conversion in the mouse cortex
- Ferroptosis mechanism and Alzheimer’s disease
- Neutrophil extracellular traps mediate neuroimmunothrombosis
