Lupenone improves motor dysfunction in spinal cord injury mice through inhibiting the inflammasome activation and pyroptosis in microglia via the nuclear factor kappa B pathway
Fudong Li, Xiaofei Sun, Kaiqiang Sun, Fanqi Kong, Xin Jiang, Qingjie Kong
Abstract Spinal cord injury-induced motor dysfunction is associated with neuroinflammation.Studies have shown that the triterpenoid lupenone, a natural product found in various plants, has a remarkable anti-inflammatory effect in the context of chronic inflammation.However, the effects of lupenone on acute inflammation induced by spinal cord injury remain unknown.In this study, we established an impact-induced mouse model of spinal cord injury, and then treated the injured mice with lupenone (8 mg/kg, twice a day) by intraperitoneal injection.We also treated BV2 cells with lipopolysaccharide and adenosine 5′-triphosphate to simulate the inflammatory response after spinal cord injury.Our results showed that lupenone reduced IκBα activation and p65 nuclear translocation, inhibited NLRP3 inflammasome function by modulating nuclear factor kappa B, and enhanced the conversion of proinflammatory M1 microglial cells into anti-inflammatory M2 microglial cells.Furthermore, lupenone decreased NLRP3 inflammasome activation, NLRP3-induced microglial cell polarization,and microglia pyroptosis by inhibiting the nuclear factor kappa B pathway.These findings suggest that lupenone protects against spinal cord injury by inhibiting inflammasomes.
Key Words: inflammasome; inflammation; lupenone; microglia; polarization; pyroptosis; spinal cord injury
Introduction
Spinal cord injury (SCI) is a severe condition.The trauma causes inevitable primary mechanical injuries, as well as secondary injury involving inflammation that contributes to cell death (Kong et al., 2020).Therefore,the prognosis of patients with SCI is closely associated with the degree of inflammation that they experience.Under normal conditions, microglia are responsible for maintaining homeostasis of the brain and spinal cord (Perry and Teeling, 2013).When inflammation occurs, microglia are rapidly activated and release cytokines, thereby promoting the clearance of pathogens and cellular debris (Okorji et al., 2016; Brownjohn et al., 2018).However, SCI tends to be accompanied by overactivation of microglia, leading to excessive inflammation.Inhibiting the inflammatory cascade helps to alleviate cell damage and improve motor function (Kong et al., 2020).
Inflammasomes initiate an inflammatory response, as first described by Martinon in 2002 (Martinon et al., 2002; Mortezaee et al., 2018), and the resulting inflammation largely results in cellular damage (Liu et al., 2020;Lun et al., 2022; Zhao et al., 2022).The well-characterized NOD-like receptor protein-3 (NLRP3) inflammasome is involved in the microglia-mediated inflammatory response (Liu et al., 2020).The NLRP3 inflammasome consists of NLRP3, apoptosis-associated speck-like protein (ASC), and caspase-1(Swanson et al., 2019).Importantly, activated NLRP3 leads to pyroptosis, a form of programmed inflammatory cell death (Swanson et al., 2019).Cleaved caspase-1 helps remove the carboxyl terminus from gasdermin D (GSDMD),converting it into GSDMDNterm(N-GSDMD).N-GSDMD then inserts into the cell membrane, forming a pore complex with a diameter of 10–14 nm.Many proinflammatory factors, including interleukin (IL)-1β and IL-18, translocate into the extracellular space via this pore (Grebe et al., 2018).
Glial cells in the spinal cord play a key role in inflammation by activating cells and releasing inflammatory cytokines (Wang et al., 2019; Li et al., 2022).Microglia are closely associated with physiologic and pathologic events,including development, tissue repair, neural environment maintenance,injury, and repair (Orihuela et al., 2016).Microglial cells exhibit one of two phenotypes that regulate neuronal function: proinflammation (M1) and anti-inflammation (M2) (Orihuela et al., 2016).In the context of tissue damage,activated microglia adopt the proinflammatory M1 phenotype.The activated M1 microglia secrete inflammatory factors that disrupt neuronal cell homeostasis (Shapouri-Moghaddam et al., 2018).Conversely, M2 microglial cells secrete anti-inflammatory cytokines that promote differentiation of oligodendrocyte progenitors and protect against neuronal damage (Miron et al., 2013).Promoting microglial M2 polarization and inhibiting M1 polarization are therefore promising therapeutic strategies for SCI.
The triterpenoid lupenone (Lup) is a natural product found in various plant families (Na et al., 2009; Lee et al., 2020).Lup has many complicated activities, such as anti-inflammatory, anti-diabetic, and anti-tumor functions(Xu et al., 2014, 2018, 2020).Its anti-inflammatory activity has been reported in the context of edema and chronic inflammation (Xu et al., 2020).Lup can also alleviate SH-SY5Y cell death (Lee et al., 2020).We therefore hypothesized that Lup could inhibit inflammation after SCI.The aim of this study was to investigate the role of Lup in treating SCI and to explore the potential mechanisms underlying its regulation of neuroinflammation.
Methods
Animals
The experiments were approved by the Shanghai Changzheng Hospital Ethics Committee (approval No.2020SL062) in November 2020.All experiments were designed and reported according to the Animal Research: Reporting ofIn VivoExperiments (ARRIVE) guidelines (Percie du Sert et al., 2020).To eliminate estrogen interference, adult male C57/BL6 mice (6–8 weeks, 20–25 g) obtained from the Zhejiang Academy of Medicinal Sciences (Hangzhou,China; license No.SCXK (Zhe) 2019-0002) were used for the experiments.The animals were randomly assigned to the sham, sham + Lup, SCI, and SCI+ Lup groups (n= 20/group).A schematic diagram is presented in Figure 1A.Anesthesia was induced with 4% inhaled isoflurane (Yaji Biological Technique,Shanghai, China) and maintained with 2% isoflurane.Then, a laminectomy at T10 was performed to expose the spinal cord.The weight drop technique using a rod (5 g, 68099II, Reaworld, Shenzhen, Guangdong, China) was used to induce spinal cord trauma from a height of 6.5 cm.Mice in the sham group and sham + Lup group were subjected to laminectomy without SCI.
Drug treatment
The three-dimensional chemical structure of Lup is shown in Figure 1B.Lup(Selleck, Houston, TX, USA, E002001) was dissolved in dimethyl sulfoxide and diluted with normal saline.Animals in the sham + Lup and SCI + Lup groups received intraperitoneal injections of Lup (8 mg/kg, twice daily), as previously described (Xu et al., 2022).The other groups received the same volume (100μL) of a mixture of normal saline and dimethyl sulfoxide.
Cell treatment and cell viability measurements
BV2 cells (Rio de Janeiro Cell Bank, Rio de Janeiro, Brazil, Cat# 0356, RRID:CVCL_0182) were cultured in 96-well plates at a density of 0.5 × 105per well.Lipopolysaccharide (LPS) and adenosine 5′-triphosphate (ATP) were used to induce neuroinflammation in cells, which is regarded as a classic model of inflammation (Fan et al., 2020; Liao et al., 2020; Tastan et al., 2021).The cells were incubated with Lup (0, 1, 5, 10, 20, 100, or 200 μM) for 2 hours, then LPS (1 μg/mL; Cat# GC205009, Servicebio, Shanghai, China) and ATP (5 mM;Cat# D7378, Beyotime, Shanghai, China) (Nam et al., 2018; Lee et al., 2020;Tastan et al., 2021).Next, the cell culture medium containing Lup, LPS, and ATP was replaced with fresh medium, after which various concentrations of Lup (0, 1, 5, 10, 20, 100, or 200 μM) were added to the plates for 24 hours.In addition, to assess the role of the NLRP3 inflammasome in BV2 cells, CY-09 (10 μM; Cat# HY-103666, MedChemExpress, Shanghai, China) was used to specifically inhibit the NLRP3 inflammasome.Cell viability was assessed using a Cell Counting Kit-8 (CCK-8; Dojindo, Kumamoto Prefecture, Japan) according to the manufacturer’s instructions.The optical density value at 450 nm was determined using a microplate reader (Molecular Devices, Sunnyvale, CA,USA).
Plasmid transfection
The pcdna3.1 plasmid carrying full-length p65 was purchased from GenePharma (Shanghai, China).The viral packaging and titer tests were performed as previously described (Kong et al., 2020).Cells were plated in 24-well plates (3 × 105/well).After 24 hours, 100 ng of the recombinant plasmid was transfected into BV2 cells using Lipofectamine 2000 (GenePharma).After 4 hours, transfection ended.
Basso Mouse Scale
Hindlimb motor function was measured using the Basso Mouse Scale (BMS)before surgery and at 1, 3, 7, 14, 21, and 28 days post-operation (Basso et al.,2006).According to the BMS scoring system, a minimum score of 0 indicates no ankle movement, and a maximum score of 9 indicates complete functional recovery.
Louisville Swim Scale
Before SCI and at 7, 14, 21, and 28 days post-SCI, hindlimb function, trunk instability, and body angle were evaluated using the Louisville Swim Scale(LSS).Each mouse was assigned a score of 0 to 15: 0 indicates no hindlimb movement, and 15 indicates normal hindlimb movement (Smith et al., 2006).Each mouse was pre-trained to allow them to acclimatize to swimming in water.The average score for each mouse was used.
Footprint analysis
Footprint analysis was performed at 7, 14, 21, and 28 days after SCI as described earlier (de Medinaceli et al., 1982).The plantar surfaces of the limbs were brushed with nontoxic paint (forelimbs, red; hindlimbs, green).The mice were then tested in a confined walkway covered with paper.The test was repeated if the mouse turned around before reaching the end.For quantitative analysis, each mouse’s stride length (the distance from the start to the end of the back paw), angle of rotation (the angle between the third toe and the stride line), and interlimb coordination (the distance between the ipsilateral forelimb and hindlimb) were assessed.Each mouse was assessed three times per experiment, and the average result was used.
Immunofluorescence staining
Mice were subjected to inhalation anesthesia with 4% isoflurane (Cat# R510-22-10, Reaworld), then sacrificed and perfused via the left ventricle of the heart with phosphate-buffered saline and paraformaldehyde.Spinal cordtissue around the injury epicenter was obtained and fixed.After treatment in sucrose solution, the spinal cord tissues were submerged in optimum cutting temperature embedding agent (G6059, Servicebio), and 20-μm coronal frozen sections were prepared using a microtome (Leica CM1860, Heidelberger,Nussloch, Germany; Kong et al., 2021).The tissues were incubated with primary antibodies at 4°C overnight.The primary antibodies used in this study include anti-ionized calcium-binding adapter molecule 1 (IBA1; rabbit, 1:800,Cell Signaling Technology, Boston, MA, USA, Cat# 17198, RRID: AB_2820254),anti-inductible nitric oxide synthase (iNOS; rabbit, 1:200, Cell Signaling Technology, Cat# 13120, RRID: AB_2687529), and anti-arginase 1 (Arg1;rabbit, 1:200, Cell Signaling Technology, Cat# 93668, RRID: AB_2800207).Next, the sections were incubated with secondary antibodies for 50 minutes at 25°C (room temperature), including Cy3-labeled goat anti-rabbit IgG (1:200,Servicebio, Cat# GB21303, RRID: AB_2861435) and FITC-labeled donkey anti-rabbit IgG (1:200, Servicebio, Cat# GB22403, RRID: AB_2868508).The number of positive immunostained cells near the injury site was quantified.
For cell immunofluorescence staining, the cells were fixed and treated with Triton X-100 (Cat# GC204003, Servicebio), then blocked with bovine serum albumin (GC305006, Servicebio).The cells were treated with primary antibodies at 4°C overnight and then incubated with relevant secondary antibodies (the antibodies used for cell immunofluorescence staining were the same as those used for immunostaining the spinal cord tissues) for 50 minutes at 25°C (room temperature).Images were captured with a confocal microscope (LSM710, Zeiss, Oberkochen, Baden-Würburg, Germany).
Real-time polymerase chain reaction
After the mice were sacrificed, the spinal cord tissues from the lesion epicenter (around 0.3 cm) were obtained for homogenization and digestion.Approximately 4 × 105cells were added to each well of 12-well plates for RNA isolation.Total RNA was extracted with Trizol reagent (Takara, Shanghai,China), and reverse transcription into complementary DNA was performed(R122-01, Vazyme, Nanjing, China).The real-time polymerase chain reaction(RT-PCR) reactions were performed with AceQ qPCR SYBR Green Master Mix(Q111-02, Vazyme) and an RT-PCR machine (Applied Biosystems, Carlsbad,CA, USA) with the following conditions: 95°C for 30 seconds, 95°C for 10 seconds, and 60°C for 30 seconds for 40 cycles; and then 65°C for 5 seconds.The primer sequences are provided in Table 1.
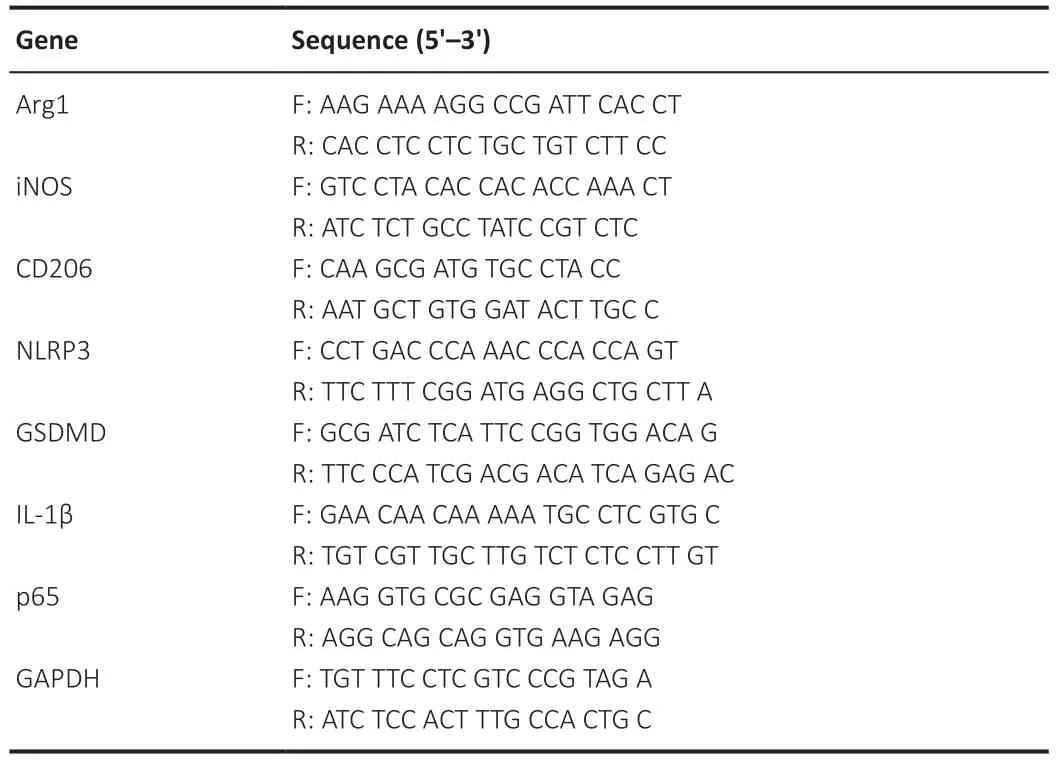
Table 1 |Primers of the genes used in the study
Western blotting
Spinal cord tissue from the lesion epicenter (around 0.3 cm) was harvested,and protein was extracted.Approximately 1 × 106BV2 cells were added to each well of six-well plates for protein isolation.The protein content was quantified using bicinchoninic acid kit (Cat# P0010, Beyotime) according to the manufacturer’s instructions (Sun et al., 2021b).Next, the samples were subjected to electrophoresis and transferred onto polyvinylidene fluoride membranes, which were blocked with milk and then incubated with primary antibodies at 4°C overnight: cleaved caspase-3 (rabbit, 1:500, Cell Signaling Technology, Cat# 9664, RRID: AB_2070042), Bcl-2 (rabbit, 1:500, Zenbio,Chengdu, Sichuan, China, Cat# 381702, RRID: AB_2924433), Bax (mouse,1:500, Zenbio, Cat# 200958, RRID: AB_2924739), glyceraldehyde-3-phosphate dehydrogenase (GAPDH; rabbit, 1:1000, Cell Signaling Technology, Cat# 5174,RRID: AB_10622025), iNOS (rabbit, 1:500, Cell Signaling Technology, Cat#13120S, RRID: AB_2687529), Arg1 (rabbit, 1:500, Cell Signaling Technology,Cat# 93668, RRID: AB_2800207), NLRP3 (rabbit, 1:500, Cell Signaling Technology, Cat#15101, RRID: AB_2722591), ASC (rabbit, 1:500, Zenbio, Cat#340097, RRID: AB_2921364), cleaved caspase-1 (rabbit, 1:500, Cell Signaling Technology, Cat# 89332, RID: AB_2923067), GSDMD (rabbit, 1:500, Cell Signaling Technology, Cat# 46451, RRID: AB_2921367), IL-1β (rabbit, 1:500,Cell Signaling Technology, Cat# 31202, RRID: AB_2799001), inhibitor of NF-κB(IκBα; mouse, 1:500, Cell Signaling Technology, Cat# 4814, RRID: AB_390781),phosphorylated IκBα (p-IκBα; rabbit, 1:500, Cell Signaling Technology, Cat#2859, RRID: AB_561111 ), transcription factor p65 (p65, rabbit, 1:500, Cell Signaling Technology, Cat# 8242, RRID: AB_10859369), phosphorylated p65 (p-p65, rabbit, 1:500, Cell Signaling Technology, Cat# 3033, RRID:AB_331284), and histone H3 (rabbit, 1:500, Cell Signaling Technology, Cat#4499, RRID: AB_10544537).The secondary antibodies, including anti-mouse IgG (horse, 1:1000, Cell Signaling Technology, Cat#7076, RRID: AB_330924)and anti-rabbit IgG (goat, 1:1000, Cell Signaling Technology, Cat#14708, RRID:AB_2798581), were added, and the membranes were incubated at room temperature for 2 hours.The bands were observed with chemiluminescent reagent (PE0010, Solarbio, Beijing, China).Quantification of immunoreactive bands was performed using ImageJ (V 1.8.0, National Institutes of Health,Bethesda, MD, USA) (Schneider et al., 2012).
Separation of cytoplasm and nuclear proteins
BV2 cell nuclear and cytosolic proteins were isolated using a Nuclear Protein Extraction Kit (Cat#R0050, Solarbio).Briefly, after removing the culture medium, the cells were washed once with phosphate-buffered salin, and collected using a cell scraper.Each 20 μL of cell precipitate was mixed with 200 μL of plasma protein extraction reagent.The mixture was vortexed at a high speed for 15 seconds, and then placed in an ice bath for 10 minutes.Next, the samples were centrifuged at 12,000–16,000 ×gfor 10 minutes at 4°C.The resulting supernatants containing the extracted cytoplasmic protein were promptly transferred into pre-cooled sample tubes for subsequent analysis.The pellets, which contained the nuclear proteins, were resuspended in 50–100 μL of nuclear protein extraction reagent.The mixture was vortexed at a high speed for 15 seconds, and then placed in an ice bath for 10 minutes.Next, the samples were centrifuged at 12,000–16,000 ×gfor 10 minutes at 4°C.The resulting supernatants containing the extracted nuclear proteins were promptly transferred to pre-cooled sample tubes.
Enzyme-linked immunosorbent assay
BV2 cells were incubated in Dulbecco’s modified Eagle’s medium(Thermofisher, Shanghai, China, Cat# 11965092) containing fetal bovine serum ( Gibco, Shanghai, China, Cat# 10099141C) and penicillin/streptomycin(Cat# C0222, Beyotime).Approximately 1 × 104BV2 cells were added to each well of 96-well plates, and the supernatants were subjected to enzymelinked immunosorbent assay (ELISA).In addition, spinal cord tissues were homogenized and centrifuged, and the supernatant was collected.ELISA was carried out according to the manufacturer’s instructions (Abcam, Eugene, OR,USA, Cat# ab197742 for IL-1β, Cat# ab216165 for IL-18).
Pyroptosis assay
Propidium iodide (PI) uptake assays and lactate dehydrogenase (LDH) release assays were used to detect pyroptosis.
PI uptake assays
BV2 cells seeded in 24-well plates (about 2 × 105cells/well) were stained with PI (red) and 4′,6-diamidino-2-phenylindole (Cat# G1012, Servicebio) for half an hour.Then, the cells were visualized using confocal microscope.
LDH release assays
BV2 cells were seeded in 96-well plates (about 1 × 104/well), and LDH activity in the supernatants was detected (Servicebio, G1610-100T).The substrate mixture, iodine-nitrotetrazolium substrate solution, dilution buffer,and enzyme solution were mixed together according to the volume ratios specified in the protocol to prepare the working solution, which was then added into the wells.Cells were incubated at 37°C for 30 minutes.Optical density at 490 nm was measured using a microplate reader.
Nissl staining
The samples were embedded in paraffin, and three complete cross-sections at T10 (the injury epicenter) of each spinal cord were obtained.After dewaxing and rehydrating the slices, the samples were stained using Nissl staining solution (Servicebio) for 5 minutes and sealed with neutral resin.Three random visual fields were observed using a light microscope (Olympus,Tokyo, Japan).
Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling assay On day 7 post-injury, three complete cross-sections at T10 were obtained.Apoptosis was identified using a terminal deoxynucleotidyl transferasemediated dUTP nick-end labeling (TUNEL) kit (G1502, Servicebio).The total number of TUNEL-positive cells in the area adjacent to the injury epicenter was determined.Each group comprised three mice, and only one section adjacent to the injury epicenter was assessed.
Molecular docking
To explore the affinity between Lup and p65, molecular docking analysis was performed.The ligand Lup was downloaded from the PubChem compound database (https://pubchem.ncbi.nlm.nih.gov/).The energy was minimized,and the results were saved in mol2 format.PyMOL software (version 1.7.1.0,Schrodinger, New York, NY, USA) was used to remove water and ions.AutodockTools software (version 1.5.6, Scripps, Rahor, CA, USA) was used to convert the PDB files into PDBQT format and locate the binding pockets of core proteins.The binding energies (kcal/mol) were calculated to assess the ligand-binding affinity using the Vina method.Binding energy ≤ -5.0 kcal/mol and an RMSD value < 2.00 indicate that the molecule and protein can form a stable complex.A binding value ≤ -7.0 kcal/mol indicates that the molecule binds strongly to the receptor.Docking visualization was performed using PyMOL software.
Statistical analysis
The investigators responsible for data analysis were blinded to the group assignments.The sample size used for this study was determined based on previous studies related to SCI (Takano et al., 2017; Kong et al., 2020; Patel et al., 2021) and the experimental design of the current study.In addition,during revision of the paper, some experiments were repeated; ultimately, six experimental animals were used for both Nissl and TUNEL staining.Statistical analyses were performed using SPSS (Version 26.0; IBM Corp., Armonk, NY,USA) and GraphPad (Version 8.0.2, GraphPad Software, Boston, MA, USA,www.graphpad.com).Comparisons among multiple groups were carried out using one-way analysis of variance and Tukey’s honest significant differencepost hoctest.Data between two groups were compared using Student’st-test.P< 0.05 indicated a statistically significant difference.
Results
Lup attenuates motor dysfunction after SCI
To determine whether Lup improved locomotor function in mice, BMS (preoperation, 1, 3, 7, 14, 21, and 28 days after SCI), LSS, and footprint analysis were carried out (Figure 1A).All mice were pre-trained and tested before SCI,and no significant difference was observed among the groups prior to injury.On day 1, all mice with SCI received a BMS score of 0, confirming complete hindlimb paralysis.In contrast, on days 7, 14, and 21, the BMS scores of Luptreated mice were markedly higher than those in the control groups (Figure 1C).On day 7, the LSS results showed that the Lup-treated mice had more hindlimb movement and hindlimb alternation, as well as a reduced angle between the trunk and the water surface (Figure 1D).The LSS scores for the SCI + Lup group were markedly improved compared with those for the SCI group (Figure 1E).In the footprint test, the angle of rotation, interlimb coordination, and stride length were measured weekly to quantitatively analyze gait after SCI; representative images from day 7 are shown in Figure 1F.The Lup-treated SCI mice had a more dramatic reduction in angle of rotation than untreated SCI mice at 7, 14, 21, and 28 days after SCI (Figure 1G).The footprint results also demonstrated remarkable improvement in interlimb coordination in the SCI + Lup group at 7, 14, 21, and 28 days after SCI compared with the SCI group (Figure 1H).The Lup-treated SCI mice had a markedly greater stride length at 14 and 21 days after SCI than untreated SCI mice (Figure 1I).Taken together, these results suggest that Lup treatment improved recovery of voluntary locomotive hindlimb function.
Lup ameliorates apoptosis in the spinal cord after SCI
Nissl staining of the spinal cord was carried out to assess the neurons 7 days after SCI.No significant difference was observed between the sham and sham+ Lup groups in terms of the number of Nissl bodies (P> 0.05; Figure 2A and B), indicating that Lup had no effect on the spinal cord in the absence of injury.Nissl staining showed that neurons in the SCI group were darker and smaller compared with those in the sham group.There were markedly fewer neurons in the SCI group compared with the sham group.Lup treatment significantly increased the number of neurons (P< 0.01) and restored neuron morphology(Figure 2A and B).Next, a TUNEL assay was performed to assess the number of apoptotic cells 7 days after SCI.There were significantly fewer TUNEL-positive cells in the SCI + Lup group compared with the SCI group (Figure 2C and D).Western blotting showed that expression levels of cleaved caspase-3 and Bax(both key determinants of apoptosis (Gaumer et al., 2000; Abbaszadeh et al.,2020)) were considerably lower in the SCI + Lup group than in the SCI group; in addition, Bcl-2 expression was significantly higher in the SCI + Lup group than in the SCI group (P< 0.001; Figure 2E–H).These results demonstrate that Lup exerted a neuroprotective effect on the injured spinal cord.
Lup-induced microglial polarization in SCI mice
Microglial activation is important for neuroinflammation in SCI (Liu et al., 2020).We investigated whether Lup suppressed microglia-mediated neuroinflammation by regulating microglial polarization.Microglial polarization was evaluated by immunofluorescence staining on 7 days after SCI (Figure 3A).No significant difference was observed in microglia (marked by IBA-1) numbers between the SCI and SCI + Lup groups (P> 0.05; Figure 3A and B).The number of Arg1-positive microglia (M2) was significantly higher in the SCI + Lup group compared with the SCI group (P< 0.001; Figure 3A and C).The number of iNOS-positive microglia (M1) in the SCI + Lup group was significantly lower than in the SCI group (P< 0.05; Figure 3A and D).Western blotting showed that iNOS expression was significantly lower in the SCI + Lup group than in the SCI group (P< 0.01), whereas Arg1 expression in the SCI + Lup group was significantly higher compared with that in the SCI group (P< 0.001; Figure 3E–G).Furthermore, the RT-PCR results showed similar changes (Figure 3H and I).The results demonstrate that Lup promoted a shiftin microglial polarization from a proinflammatory phenotype to an anti-inflammatory phenotype.
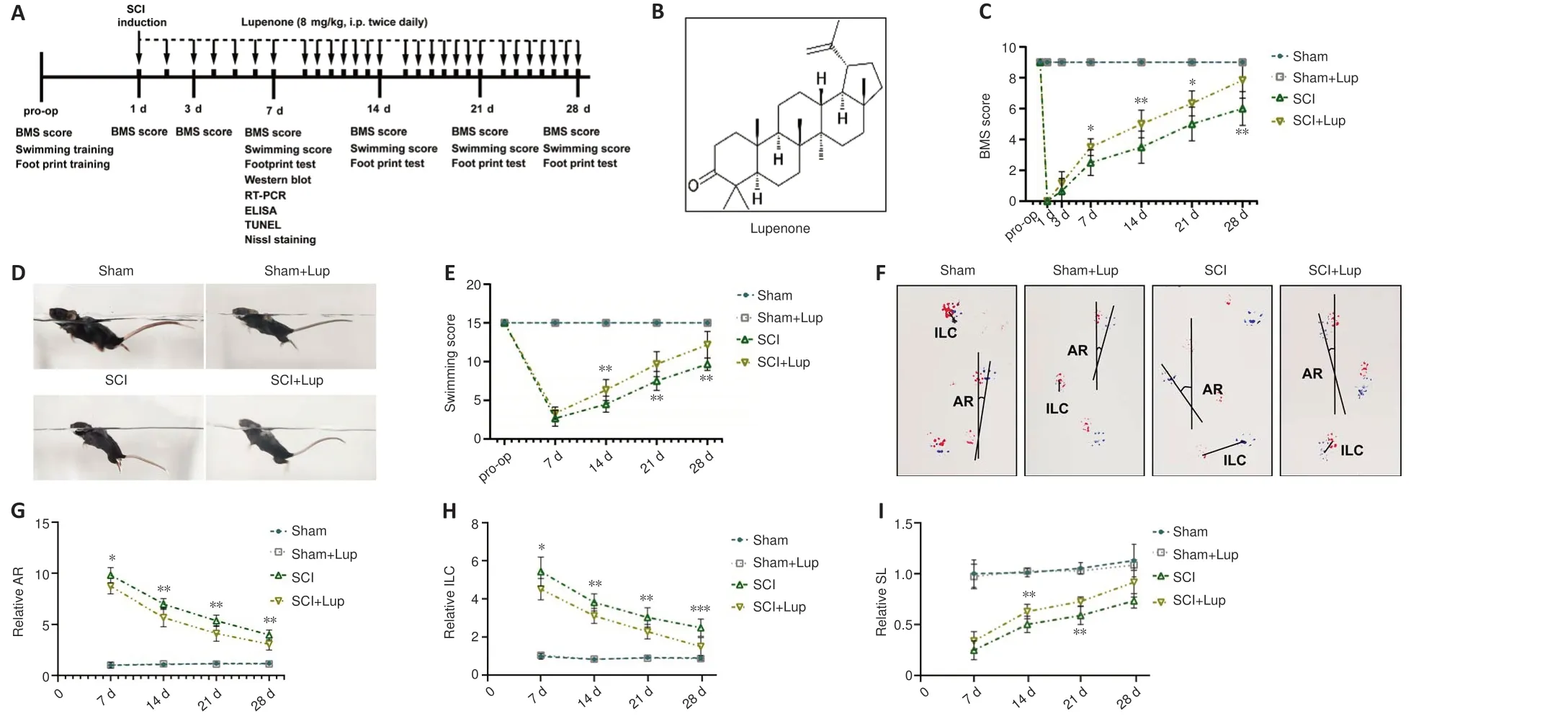
Figure 1 |Lupenone improves neurological function in mice with SCI.
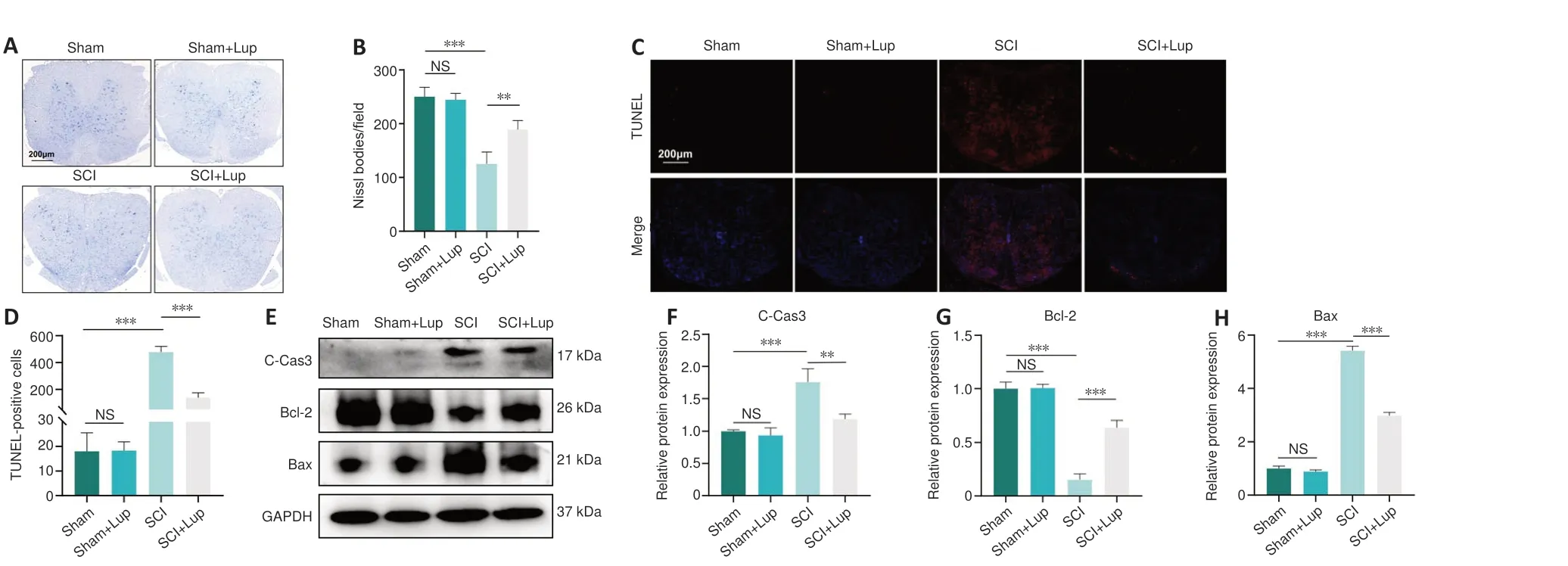
Figure 2 |SCI-induced apoptosis can be alleviated by treatment with Lup.
Lup inhibits the NLRP3 inflammasome and pyroptosis in SCI mice
Western blotting showed that NLRP3, ASC, cleaved caspase-1, IL-1β, and N-GSDMD expression levels were markedly upregulated after SCI; in contrast,treatment with Lup markedly reduced the expression of these marker proteins(Figure 4A–F).Furthermore, similar changes were observed for NLRP3 and GSDMD at the mRNA level (Figure 4G and H).Taken together, these results indicate activation of the NLRP3 inflammasome.Considerably lower levels of inflammatory factors including IL-1β and IL-18 were released from the pyroptosis-related pore in the SCI + Lup group compared with the SCI group(Figure 4I and J).These results also indicate that Lup treatment ameliorated over-activation of the NLRP3 inflammasome and NLRP3-induced pyroptosis in SCI mice.
Lup regulates BV2 cell polarization
To further explore the effects of Lup on microglia, we performedin vitroexperiments in BV2 cells, an alternative to investigating microgliain vitro(Hou et al., 2019).Lup did not cause significant cell death in BV2 cells at concentrations less than or equal to 20 μM (P> 0.05; Figure 5A).Thus, 20μM of Lup was used to treat BV2 cells in subsequent experiments.In the LPS + ATP-stimulated BV2 cells, Lup significantly reduced expression of the proinflammatory protein iNOS (P< 0.001) and increased expression of the anti-inflammatory protein Arg1 (P< 0.01; Figure 5B–D).Similar changes were observed at the mRNA level: IL-1β and iNOS mRNA levels were markedly lower, and Arg1 and CD206 mRNA levels were higher, in the LPS + ATP + Lup group than in the LPS + ATP group (Figure 5E–H).Next, immunofluorescence analysis was performed to explore the polarization phenotype.Weaker iNOS fluorescence intensity and stronger Arg1 fluorescence intensity were observed in the LPS + ATP + Lup group than in the LPS + ATP group(Figure 5I and J).These results indicate that Lup promoted polarization to the anti-inflammatory M2 phenotype and suppressed polarization to the proinflammatory M1 phenotype in BV2 cells, confirming that Lup treatment improves motor function by altering microglia polarization.
Lup decreases inflammasome activation-related inflammation in BV2 cells
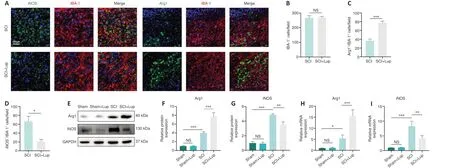
Figure 3 |Lup improves microglial polarization in SCI mice.
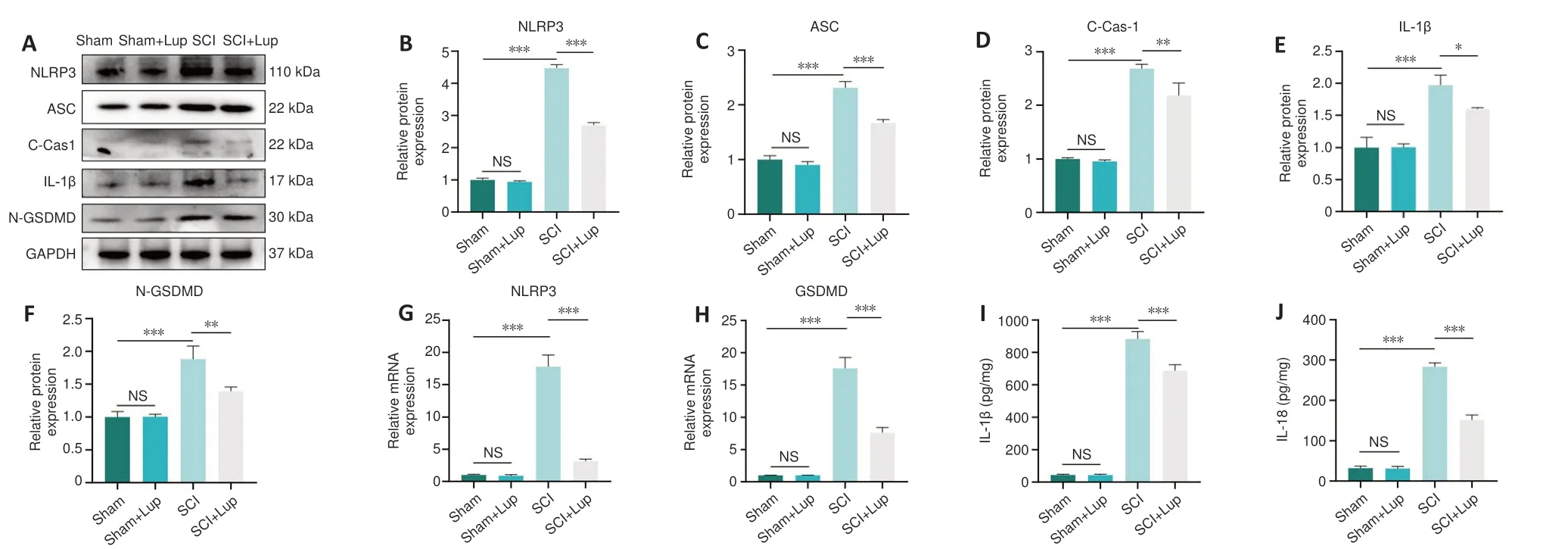
Figure 4 |Lup inhibits over-activation of the NLRP3 inflammasome and pyroptosis in SCI mice.
To verify the role of Lup in inflammasome activation-related inflammation,we detected the expression levels of relevant indicators.NLRP3, GSDMD, and N-GSDMD expression levels increased dramatically in LPS + ATP-stimulated BV2 cells but decreased markedly after Lup treatment (Figure 6A–D).In addition, pro-IL-1β and IL-1β expression levels were markedly lower in the LPS+ ATP + Lup group than in the LPS + ATP group (Figure 6A, E, and F).Next, we assessed two indicators of pyroptosis, PI uptake and LDH release (Liu et al.,2020).A significantly greater number of PI-positive cells were observed in LPS+ ATP–treated BV2 cells compared with the LPS + ATP + Lup group (P< 0.001;Figure 6G and H).Similarly, as shown in Figure 6I, cells incubated with LPS +ATP released significantly higher levels of LDH (P< 0.001) than cells incubated with LPS + ATP + Lup (P< 0.05; Figure 6I).ELISA assays demonstrated that IL-1β and IL-18 expression levels were markedly lower in the LPS + ATP + Lup group than in the LPS + ATP group (Figure 6J and K).These results indicate that Lup suppressed LPS + ATP-induced NLRP3 activation and alleviated subsequent inflammation in BV2 cells.
Inhibiting NLRP3 with CY-09 alters microglial polarization and reduces pyroptosis in BV2 cells
To explore the upstream and downstream relationships between NLRP3 and microglia polarization, CY-09 was used to specifically inhibit NLRP3, and microglial polarization was assessed.NLRP3 expression was markedly lower in the LPS + ATP + CY-09 group compared with the LPS + ATP group (Figure 7A and B), indicating that NLRP3 activation was inhibited by CY-09.Next, we assessed expression levels of indicators of microglia polarization, including the anti-inflammatory factor Arg1 and the proinflammatory factor iNOS.Arg1 was expressed at markedly higher levels in the LPS + ATP + CY-09 group than in the LPS + ATP group (Figure 7A and C); and iNOS expression was markedly lower in the LPS + ATP + CY-09 group compared with the LPS + ATP group (Figure 7A and D).These results demonstrate that inhibiting NLRP3 suppresses proinflammatory M1 polarization and enhances anti-inflammatory M2 polarization in BV2 cells.RT-qPCR analysis of NLRP3, Arg1, and iNOS mRNA expression levels yielded similar results (Figure 7E–G).Furthermore,the expression levels of inflammatory cytokines such as IL-1β and IL-18 were markedly lower in the LPS + ATP + CY-09 group compared with those in the LPS+ATP group (Figure 7H and I).
Finally, we confirmed the relationship between NLRP3 and pyroptosis in BV2 cells.The data demonstrated that LDH levels were markedly higher in the LPS + ATP group compared with the LPS+ATP+CY-09 group (Figure 7J).Furthermore, the number of PI-positive cells in the LPS + ATP group was markedly higher than that in the LPS + ATP + CY-09 group (Figure 7K and L).The results demonstrated that inhibiting activation of the NLRP3 inflammasome alters microglial polarization and alleviates pyroptosis in BV2 cells.
The NF-κB pathway can be regulated by Lup in BV2 cells
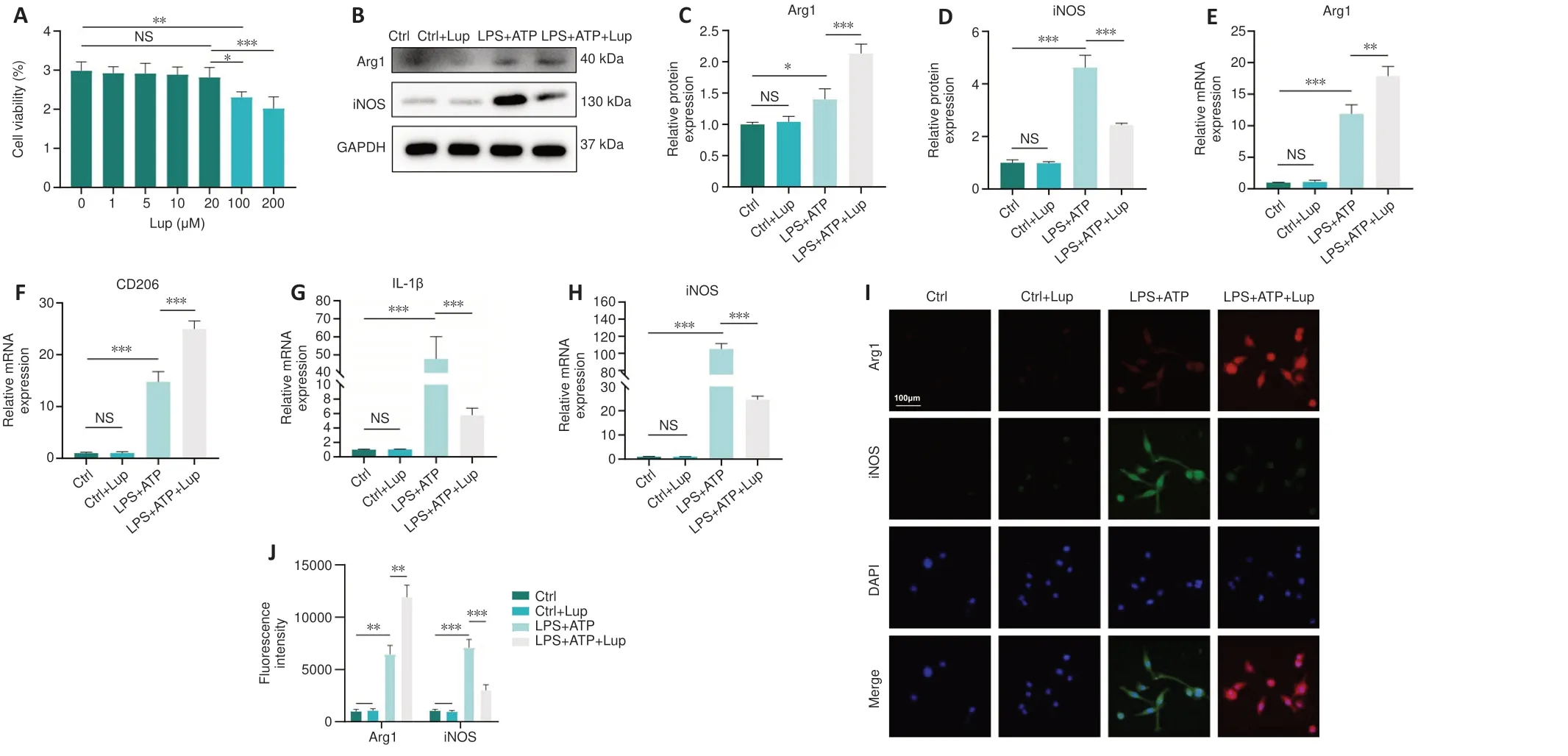
Figure 5 |Lup regulates BV2 cell polarization.
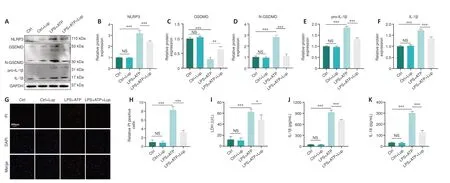
Figure 6 |Lup decreases NLRP3 activation and subsequent pyroptosis in BV2 cells.
The NF-κB pathway can trigger NLRP3 inflammasome activation and regulate microglial polarization (Ye et al., 2019).To determine whether Lup ameliorated inflammation in BV2 cells via the NF-κB pathway, we assessed the interaction between Lup and the NF-κB pathway in the BV2 cells.The molecular docking results (Figure 8A) showed that hydrogen bonds formed between Lup and PHE239, and hydrophobic interactions were observed between Lup and hydrophobic amino acid residues, including LYS28, GLU222,ARG236, and PRO275.In addition, the molecular docking results revealed that the binding energy between Lup and p65 was –8.0 kcal/mol, indicating a stable binding interaction (Figure 8A).We then assessed the activation status of the NF-κB signaling pathway in BV2 cells treated with LPS + ATP or LPS + ATP + Lup.Whole-cell western blotting demonstrated that Lup did not affect p65, p-p65, IκBα, and p-IκBα expression levels in the absence of LPS + ATP (Figure 8B–G).However, p-p65 and p-IκBα expression levels in the LPS + ATP group were markedly higher than those in the control group(Figure 8B–G), and the levels of both p-p65 and p-IκBα in the LPS + ATP + Lup group were markedly lower than those in the LPS + ATP group (Figure 8B–D).Furthermore, p-p65 expression in the nucleus was markedly lower in the LPS + ATP + Lup group than in the LPS + ATP group; and p65 expression in the cytoplasm was markedly higher in the LPS + ATP + Lup group than in the LPS +ATP group (Figure 8E–G).Therefore, Lup may suppress over-activation of the NF-κB pathway.To confirm this, we overexpressed p65, the key protein in the NF-κB pathway (Jiang et al., 2013; Liu and Su, 2023) in BV2 cells and validated effective overexpression by detecting p65 mRNA levels (Figure 8H).Both p65 and p-p65 levels were substantially higher in the LPS + ATP + OE group than in the LPS + ATP + vector group (Figure 8I–K).In addition, p-p65 expression was substantially lower in the LPS + ATP + OE + Lup group than in the LPS + ATP +OE group (Figure 8I and J), indicating that p65 activation was inhibited by Lup.Nuclear translocation of the p65 subunit is regarded as an indicator of NF-κB activation (Zusso et al., 2019).Immunofluorescence analysis showed that Lup treatment substantially reduced the increase in p65 translocation caused by LPS + ATP stimulation (Figure 8L and M).Furthermore, p65 overexpression further increased p65 translocation.However, the increased p65 translocation caused by p65 overexpression was markedly reversed by Lup treatment (Figure 8N and O).These findings suggest that Lup inhibits overactivation of the NFκB pathway by inhibiting the function of the p65 subunit.
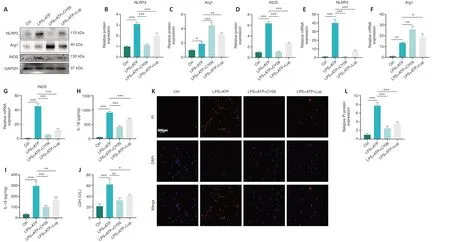
Figure 7 |CY-09 helps alter microglial polarization and ameliorate pyroptosis in BV2 cells.
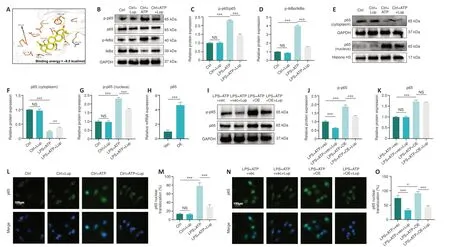
Figure 8 |Activation status of the NF-κB signaling pathway is regulated by Lup.
Lup ameliorates NLRP3 inflammasome overactivation and NLRP3-induced microglia polarization and pyroptosis through the NF-κB pathway in BV2 cells
Next, we evaluated the effects of Lup on NLRP3 activation, microglial polarization, and pyroptosis in the BV2 cells overexpressing p65.NLRP3 expression was markedly higher in the LPS + ATP + OE group compared with the LPS + ATP + vector group and the LPS + ATP + OE + Lup group (Figure 9A and B), indicating that the NLRP3 inflammasome is regulated by p65.Then, microglial polarization was assessed by western blot, which showed that p65 overexpression increased the levels of both Arg1 (a marker of anti-inflammatory M2 polarization) and iNOS (a marker of proinflammatory M1 polarization; Xie et al., 2021; Li et al., 2023) (Figure 9A, C, and D).Treatment with Lup intervention further increased expression of the anti-inflammatory factor Arg1 and decreased expression of the proinflammatory factor iNOS(Figure 9A, C, and D).RT-PCR analysis yielded similar results (Figure 9E–G).These results demonstrate that Lup inhibited M1 polarization and promoted M2 polarization by suppressing NF-κB pathway-mediated activation of the NLRP3 inflammasome.
Next, we assessed the expression of pyroptosis indicators.The number of PI-positive cells increased in cells overexpressing p65, and this effect was markedly reversed by Lup treatment (Figure 9H and I).Similarly, the elevation in LDH levels caused by p65 overexpression was markedly reversed by treatment with Lup (Figure 9J).Furthermore, the expression levels of inflammatory cytokines such as IL-1β and IL-18 were increased in the LPS +ATP + OE group and markedly suppressed by Lup (Figure 9K and L).Taken together, these findings suggest that Lup ameliorates overactivation of the NLRP3 inflammasome and NLRP3 activation-induced microglia polarization and pyroptosis by inhibiting the NF-κB pathway.
Discussion
Although a certain degree of inflammation is a vital defense mechanism against noxious stimuli in SCI, persistent and exacerbated inflammatory responses can damage neurons in the central nervous system (Saavedra,2012).Finding effective ways to prevent or suppress aggravated inflammation has become an urgent need in SCI therapy.Lup is a triterpenoid found in many plants (Na et al., 2009; Lee et al., 2020).Several studies have demonstrated that Lup exerts therapeutic effects through its potent anti-inflammatory activity (Na et al., 2009; Xu et al., 2014, 2018, 2020, 2022; Lee et al., 2020).Xu et al.(2022) found that excessive inflammatory responses and oxidative stress in the pancreas of diabetic rats were suppressed by Lup.In the present study, we explored the role of Lup in treating SCI and the potential underlying mechanisms.
Using a mouse model of SCI, we found that Lup significantly facilitated locomotor recovery and ameliorated neuronal death after SCI.These results are consistent with previous studies showing that Lup exerted therapeutic effects in various disorders such as diabetes, edema, and chronic inflammation (Xu et al., 2014, 2020).Meanwhile, in line with previous studies indicating an anti-inflammatory role for Lup, ourin vitroandin vivoimmunofluorescence assays demonstrated that Lup promoted anti-inflammatory M2 polarization and inhibited proinflammatory M1 polarization.Microglia, the resident macrophages of the central nervous system, play crucial roles in neuronal development, homeostatic function,and neurodegenerative disease (Benveniste, 1997; Perry et al., 2010; Niraula et al., 2017; McQuade et al., 2018).Under normal conditions, microglia tend to remain in a relatively quiescent state while they monitor changes in the spinal cord (Davalos et al., 2005).However, when injury occurs, microglia can play both pathogenic and protective roles.To cope with stimuli generated by injuries, resident microglial cells polarize to the proinflammatory M1 phenotype (Orihuela et al., 2016).Many proinflammatory cytokines are then produced and released to clear pathogenic factors (Orihuela et al.,2016).The results from ourin vivoandin vitroexperiments showed that the expression levels of iNOS and other inflammatory indicators such as IL-1β were dramatically increased after SCI or treatment with LPS + ATP,which indicated that the microglia had polarized to a proinflammatory M1 phenotype to mitigate mechanical damage to tissues and cells.However, an excessive inflammatory response results in tissue damage that is detrimental to the functional recovery of neuronal cells and is closely related to neuron death.Therefore, treatment is mainly directed at controlling the inflammatory response after SCI.M2 polarization of microglia, characterized by increased expression of anti-inflammatory factors such as Arg1 and IL-10, plays a pivotal role in reestablishing microenvironmental homeostasis through inflammatory dampening and immunoregulation (Orihuela et al., 2016).Our results showed that expression of an indicator of the anti-inflammatory M2 phenotype was increased after SCI or treatment with LPS + ATP, indicating that the antiinflammatory M2 phenotype was activated.These results demonstrated that SCI triggered microglial polarization to both the proinflammatory M1 phenotype and the anti-inflammatory M2 phenotype, which is in line with the complex microenvironment of SCI.Maintaining a balance between proand anti-inflammatory responses can prevent the potential adverse effects of inflammation.In treating SCI, promoting M2 polarization and inhibiting M1 polarization may be essential for modulation of the inflammatory response.Consistent with this, our study revealed that Lup-induced inhibition of microglial polarization to the M1 phenotype and promotion of microglial polarization to the M2 phenotype can favor recovery of neurological function.
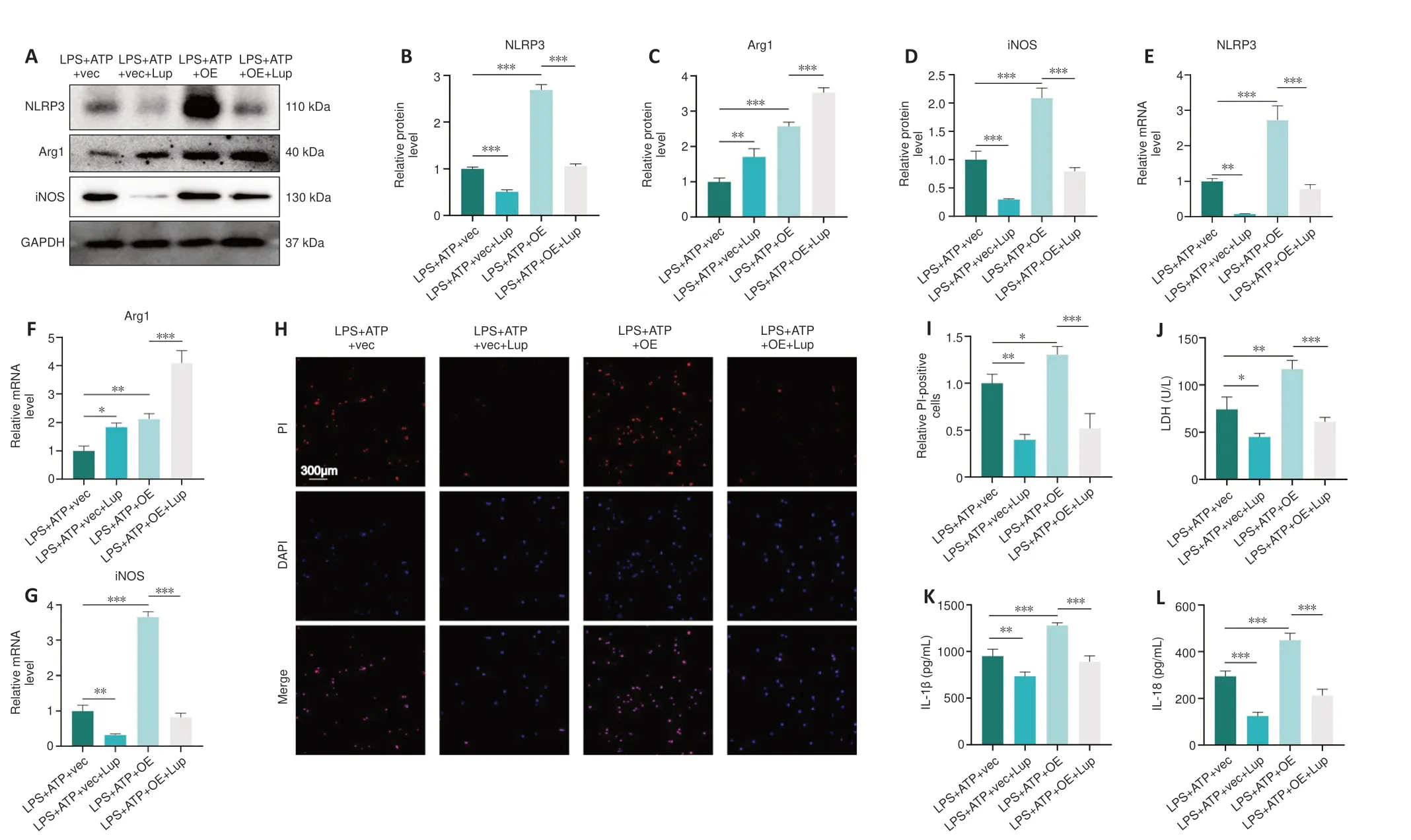
Figure 9 |Lup ameliorates overactivation of the NLRP3 inflammasome and NLRP3 activation-induced microglial polarization and pyroptosis through the NF-κB pathway in BV2 cells.
In addition to microglial polarization, we assessed the role of the NLRP3 inflammasome in SCI because of the complexity and diversity of inflammatory mechanisms.Our western blot and RT-PCR results revealed that NLRP3,ASC, cleaved caspase-1, and N-GSDMD expression levels were increased in SCI mice, indicating that the NLRP3 inflammasome was activated after SCI.Furthermore, the elevation in NLRP3 inflammasome-related protein expression levels was reversed by Lup treatment.These results demonstrate that Lup attenuates the NLRP3 inflammasome-induced inflammatory cascade after SCI.This is consistent with previous reports showing that the NLRP3 inflammasome is activated in SCI and could be a potential target for treatment(Jiang et al., 2017; Al Mamun et al., 2021).Pyroptosis involves the formation of pores in the cell membrane and can lead to cell rupture, and leakage of cytosolic contents, including IL-18 and IL-1 (Liu et al., 2020).Activation of the NLRP3 inflammasome in endothelial cells has been reported to result in cellular pyroptosis (GSDMD-mediated programmed necrosis) characterized by the release of proinflammatory cytokines, including IL-1β and IL-18 (Wu et al., 2018; Al Mamun et al., 2021).Consistent with this, we confirmed the relationship between inflammasome activation and pyroptosis by specifically blocking the NLRP3 inflammasome using CY-09.
We also explored the upstream and downstream relationships between the NLRP3 inflammasome and microglial polarization in our study.Inhibiting NLRP3 inhibitor not only decreased the expression levels of inflammasomerelated proteins but also increased the levels of an M2 marker and decreased the expression of an M1 indicator, demonstrating that the NLRP3 inflammasome may trigger microglial polarization.The findings from our study provide support for the relationship between the NLRP3 inflammasome and microglial polarization that has been explored in previous studies.In a study of graft-versus-host disease in mice, Wu et al.(2020) found that trimethylamine N-oxide promoted M1 polarization by activating the NLRP3 inflammasome.In addition, Xia et al.(2021) found that inhibiting the NLRP3 inflammasome promoted microglial M2 polarization.Furthermore, Liu et al.(2021b) reported that ubiquitin-specific protease-19 promoted M2-like macrophage polarization by modulating the function of the NLRP3 inflammasome.Therefore, the two inflammation-associated procedures––microglial polarization and pyroptosis––can be ameliorated by suppressing activation of the NLRP3 inflammasome.Lup exerted an anti-inflammatory effect by inhibiting the NLRP3 inflammasome and alleviating NLRP3-activated microglia polarization and pyroptosis.Ultimately, locomotor function in SCI mice was improved by the anti-inflammatory effects of Lup treatment.
The NF-κB signaling pathway regulates a variety of cellular functions (Sen and Baltimore, 1986).This family of transcription factors comprises five members:p65 (RelA), RelB, c-Rel, NF-κB1, and NF-κB2.Under unstimulated conditions,the DNA-binding domain of p65, a marker of NF-kB activity, binds to IκBα(a p65 inhibitor) in most quiescent cells.Phosphorylation in response to a stimulus activates p65 and causes it to translocate to the nucleus, where it exerts its regulatory function.The NF-κB pathway is central in regulating inflammatory responses in various cells (Kang et al., 2018; Liu et al., 2021a;Sun et al., 2021a).Nuclear translocation of p65 promotes assembly of the transcriptional complex, leading to proinflammatory gene activation.Here we found that p65 expression was increased by stimulation with LPS + ATP and reduced by treatment with Lup, indicating that the NF-κB pathway is involved in regulating inflammation in BV2 cells and can be suppressed by Lup.This is consistent with previous studies revealing the role of NF-κB in inflammation(Kang et al., 2018; Liu et al., 2021a; Sun et al., 2021a).The results from the p65 overexpression experiment not only confirmed that p65 was suppressed by Lup but also showed that p65 overexpression-enhanced activation of the NLRP3 inflammasome and the subsequent pyroptosis and microglia polarization were ameliorated by Lup-induced modulation of the NF-κB signaling pathway.
This study had several limitations.First, although there are multiple types of inflammasomes, we have only explored the role of NLRP3, the most studied inflammasome, in SCI.Second, we only investigated the NF-κB signaling pathway, but various pathways could regulate inflammatory responses.Third, we did not perform a detailed analysis of the potential mechanisms underlying crosstalk among the NLRP3 inflammasome, pyroptosis, and microglial polarization.These are important research topics that we intend to pursue in the future.This study focused on investigating the effects of Lup on one type of neural cell.Further studies are required to explore the role of Lup in other cells, such as astrocytes and neurons.
In summary, our study demonstrated that Lup improves the local inflammatory microenvironment by inhibiting neuroinflammation via suppression of the NF-κB signaling pathway.The results indicated that Lup alleviates neuroinflammation by modulating activation of inflammasome and subsequent microglial polarization and pyroptosis.To our knowledge,this study is the first to report that Lup could be an effective tool for treating SCI and explore the relevant mechanisms.These findings provide a novel potential therapeutic approach for restoring motor function after SCI.
Acknowledgments:We would like to all the frontline workers for their dedication and effort during this COVID-19 epidemic.
Author contributions:Study design:QK and FL;experimental implementation:FL,QK,KS,XS,FK;data analysis:FL,QK,KS,XS,FK;manuscript draft:FL,XJ.All authors read and approved the final version of the manuscript.
Conflicts of interest:There are no conflicts of interest.
Data availability statement:No additional data are available.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
 中國(guó)神經(jīng)再生研究(英文版)2024年8期
中國(guó)神經(jīng)再生研究(英文版)2024年8期
- 中國(guó)神經(jīng)再生研究(英文版)的其它文章
- The big data challenge – and how polypharmacology supports the translation from pre-clinical research into clinical use against neurodegenerative diseases and beyond
- P-aminobenzoic acid promotes retinal regeneration through activation of Ascl1a in zebrafish
- Two-photon live imaging of direct glia-to-neuron conversion in the mouse cortex
- Ferroptosis mechanism and Alzheimer’s disease
- Neutrophil extracellular traps mediate neuroimmunothrombosis
- Impact of increasing one-carbon metabolites on traumatic brain injury outcome using pre-clinical models
