一種新的流變相法制備鋰離子電池納米-LiVOPO4正極材料
熊利芝 何則強,*
(1吉首大學(xué)生物資源與環(huán)境科學(xué)學(xué)院,湖南吉首 416000;2中南大學(xué)化學(xué)化工學(xué)院,長沙 410083)
Recently,performance of mobile electronic devices,such as mobile phone or laptop computer,is drastically improving and so,the demands for battery become more severe.Due to its large power density and cycle stability,lithium ion battery is now widely used for the electric source of mobile equipment.The current most important requirement for lithium ion rechargeable battery is to decrease cost and increase the power density.In the current battery,LiCoO2and graphitic carbon are commonly used for cathode and anode,respectively.However,natural abundance of Co is limited and this element is expensive.Therefore, development of cathode material without containing Co is strongly required.At present,great attentions are paid for tansition metal phosphates,such as LiMPO4(M=Fe,Mn,Co)[1-4], Li3V2(PO4)3[5-10],and LiVPO4F[11-12],as a new class of cathode materials for lithium ion batteries.These materials contain both mobile lithium ions and redox-active transition metals within a rigid phosphate network,and display remarkable electrochemical,and thermal stabilities as well as comparable energy density.Among these materials,LiFePO4is of great interest for the replacement of LiCoO2in Li ion batteries due to its low cost,nontoxicity and good electrochemical properties since 1997[1,13-17].However,compared with LiFePO4,LiVOPO4has an advantage of higher potential(4.0 and 3.7 V(versus Li/Li+))for charge and discharge, and this phosphate is highly interesting from the viewpoint of the alternative cathode[18-21].Kerr et al.[22]presented that the triclinic phase LiVOPO4synthesized from ε-VOPO4showed the capacity of 100 mAh·g-1up to 100 cycles at C/10 of current rate. Azmi et al.[19,23]reported that orthorhombic phase of LiVOPO4could be synthesized by impregnation method and exhibited fairly good cycle stability for Li de-intercalation and intercalation.
For all functional materials,their properties were greatly influenced by the synthesis methods.Many preparation methods have been investigated with an aim to achieve high capacity LiVOPO4,however,the capacity of the products ever reported is usually unsatisfactory in particular when discharged at a high current rate.To meet high power demands of lithium ion batteries in new applications,the rate capability of LiVOPO4has to be sig-nificantly improved.There are two main frequently employed strategies:one is to increase the intrinsic electronic conductivity by microstructure controlling,the other is to enhance lithium ion transport by reducing the bulk diffusion length,which can be achieved by utilization of nanostructured materials.
The rheological phase method is the process of preparing compounds or materials from a solid-liquid rheological mixture. That is,the solid reactants are fully mixed in a proper molar ratio, and made up by a proper amount of water or other solvents to form a Bingham body in which the solid particles and liquid substance are uniformly distributed,so that the product can be obtained under suitable experiment conditions[24].Because of its low temperature,short calcination time,and products with small particle with uniform distribution,rheological method has been used to synthesize cathode and anode materials for lithium ion batteries[25-26].In the present study,rheological technique is used to synthesize nano-LiVOPO4.The microstructure and electrochemical properties of LiVOPO4as cathode material for lithium ion batteries were studied.
1 Experimental
Analytical grade powders of LiOH·2H2O(AR),NH4VO3(AR), (NH4)2HPO4(AR)and citric acid(AR)with equal amount of substance were mixed uniformly to get a mixture.Then 1.5 mL distilled water per gram mixture was added to the mixture under magnetic force stirring to obtain a mash.The mash was dried in vacuum at 80℃for 4 h to form the precursor.The precursor was calcined in Ar atmosphere at 650℃for 6 h to obtain blue LiVOPO4powders.
Phase identification and surface morphology studies of the samples were carried out by an X-ray diffractometer(XRD;D/ MAX-gA,Rigaku Corporation,Japan)with Cu Kαradiation and scanning electron microscope(SEM;JSM 5600LV,JEOL Ltd., Japan,accelerating voltage of 20 kV).Elemental analyses for lithium,vanadium,and phosphorus were determined by atomic absorption spectroscopy(AAS;SP-3530AA)and inductively coupled plasma-atomic emission spectrometer(ICP;TY9900).
A slurry containing 80%(mass fraction,similarly hereinafter) LiVOPO4,10%acetylene black,and 10%PVDF(polyvinylidene fluoride)was made using N-methylprrolidinone(NMP)as the solvent.The electrodes with an area of 1 cm2were prepared by coating the slurry(about 100 μm in thickness)on aluminum foils followed by drying in vacuum at 60℃for 12 h.Electrochemical tests were performed using a conventional cointype cell,employing lithium foil as a counter electrode and 1.0 mol· L-1LiPF6in ethylene carbonate/dimethyl carbonate(EC/DMC) (with EC and DMC volume ratio of 1∶1)as the electrolyte.The assembly was carried out in an Ar-filled glove box.The electrochemical tests were carried out with an electrochemical work station(CHI660B,CHI Instruments Inc.,Shanghai,China).
2 Results and discussion
Fig.1 shows the XRD pattern of LiVOPO4derived from rheological phase method.As shown in Fig.1(a),All the reflections from the LiVOPO4could be indexed reliably using a standard structural refinement program.XRD peaks in Fig.1 agree well withthoseofthestandardJCPDScard No.72-2253.The LiVOPO4compound possesses an orthorhombic symmetry,space group Pnma,characterized by the unit cell parameters a=0.7446(4) nm,b=0.6278(4)nm,and c=0.7165(4)nm.Except for peaks corresponding to LiVOPO4,no other peaks can be found,suggesting that the rheologically synthsized LiVOPO4is very pure. The LiVOPO4framework structure is closely related to that found in VOPO4and comprises infinite chains of corner-shared VO6octahedra,cross-linked by corner-sharing PO4tetrahedron[27-28]. The cell parameters for the rheologically prepared material compare favorably with literature values reported by Lii et al.[28]for a hydrothermallypreparedsample,i.e.,a=0.7446(3)nm,b=0.6292(2) nm,and c=0.7177(2)nm.Elemental analysis results confirmed the expected stoichiometry of LiVOPO4.

Fig.1 XRD pattern(a)and SEM image(b)of LiVOPO4
AsseenfromFig.1(b),thescanningelectronmicroscopy(SEM) examination indicated that the rheologically synthsized LiVOPO4consists of particles with average primary size in the range of 10-60 nm,which agrees well with the average crystal size of around 35 nm calculated from the XRD profile.They also showed the presence of considerable material agglomeration. The agglomerates averaged around 50 nm in size.
The lithium extraction/insertion behavior for the LiVOPO4active material relies on the reversibility of the V4+/V5+redox couple:

Fig.2 shows the initial charge-discharge curve of the rheologically synthesized LiVOPO4material.These data were collected at 25℃at an approximate 0.1C(16 mA·g-1)rate using voltage limits of 3.0 and 4.3 V(vs Li/Li+).As shown in Fig.2,at low current density,orthorhombic LiVOPO4prepared by rheological phase method is highly attractive as the alternative cathode for lithium ion rechargeable battery.This is because LiVOPO4exhibits high discharge potential of 3.85 V and reasonably large capacity.The initial oxidation process equates to a material spe-cific capacity of 145.8 mAh·g-1during this lithium extraction. Based on a theoretical specific capacity for LiVOPO4of 166 mAh·g-1[20]and assuming no side reactions,the fully charged material corresponds to Li0.12VOPO4.Excursions to higher oxidation potentials(ultimately up to 5.0 V(vs Li/Li+))resulted in the increased irreversibility as well as active material degradation evidenced by electrolyte discoloration.The reinsertion process amounts to 135.7 mAh·g-1,indicating a higher first-cycle charge reversibility of 93%than the literature value(85%)reported by Barker et al.[29].

Fig.2 Electrochemical performance data for a typical Li/ LiVOPO4cell cycled between 3.0 and 4.3 V at approximate 0.1C(16 mA·g-1)rate for charge and dischargeThe inset figure in Fig.2 is the cycling performance curve.
The cycling performance was tested at 0.1C(16 mA·g-1)in the range of 3.0-4.3 V as shown in the insert figure in Fig.2.After cycling 60 times,the discharge capacity of LiVOPO4is sustainedat134.2mAh·g-1,whichis98.9%oftheinitialcapacity,and the capacity loss per cycle is only 0.018%,suggesting LiVOPO4derived by rheological phase method is promising as alternative cathode material for lithium ion batteries with high capacity and good cycling performance.
Fig.3 shows the discharge capacity of LiVOPO4as a function of current rate.As shown in Fig.3,discharge capacity of LiVOPO4drastically decreased with increasing current rate due to the increase of the polarization of electrode.The discharge capacity of LiVOPO4at 0.1C(16 mA·g-1),1.0C(160 mA·g-1),and 2.0C(320 mA·g-1)is 135.7,130.9,and 124.3 mAh·g-1,respectively.More than 96.5%and 91.6%of the discharge capacity at 0.1C are sustained at 1.0C and 2.0C,respectively.This result is better than that of the LiVOPO4reported by Azmi et al.[19],indicating good current rate capability of LiVOPO4synthesized by rheological phase method.The good current rate capability may result mainly from the small particle size and large surface area of LiVOPO4nanoparticles.The smaller the particle size,the larger the surface area and the lower the current density,which results in less polarization of electrode and better current rate capability of LiVOPO4.Further work is underway to find out if there are any other reasons leading to good current rate capability of LiVOPO4.
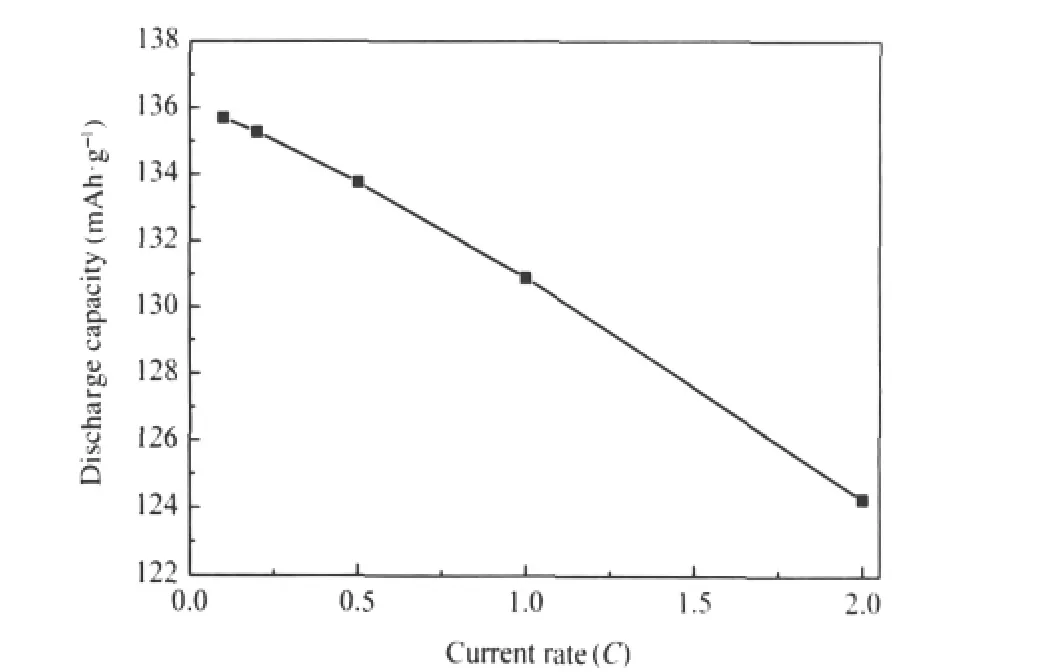
Fig.3 Discharge capacity of LiVOPO4as a function of current ratepotential window:3.0-4.3 V(vs Li/Li+);1C=160 mA·g-1
The chemical diffusion coefficient was measured with the potential step technique.In this method,the current generated due to an applied voltage step,is measured as a function of time. The measured current decays as the lithium ion diffuses through the electrode.The step ends when the current becomes less than 1%of the maximum current at the onset of the applied potential. The i-t and i-t-1/2curves for the two powders at the applied potential step of 0.1 V(vs Li/Li+)(3.94→4.04 V)are shown in Fig. 4.By assuming that the semi-finite diffusion of lithium ion in the electrode is the rate-determining procedure,the diffusion coefficient(D)of lithium ion in the electrode can be determined by the following Cottrell equation[30]:

where,n is the number of the redox reactions,F is the Faradayconstant,and c0is the lithium ion concentration in the solid electrode,which can be calculated from the open circuit voltage. According to Fig.4 and Cottrell equation,the diffusion coefficient of lithium ion in the electrode can be calculated to be 5.52×10-11cm2·s-1,which is as same magnitude again as the value(2.79×10-11cm2·s-1)reported by Ren et al.[20].The experiment results show that the current rate capability of LiVOPO4by rheological phase method is better than that reported by Azmi et al.[19],while the diffusion coefficient of lithium ion in the electrode is in the same order.This may be due to the difference in preparation methods of materials and testing means of diffusion coefficient.
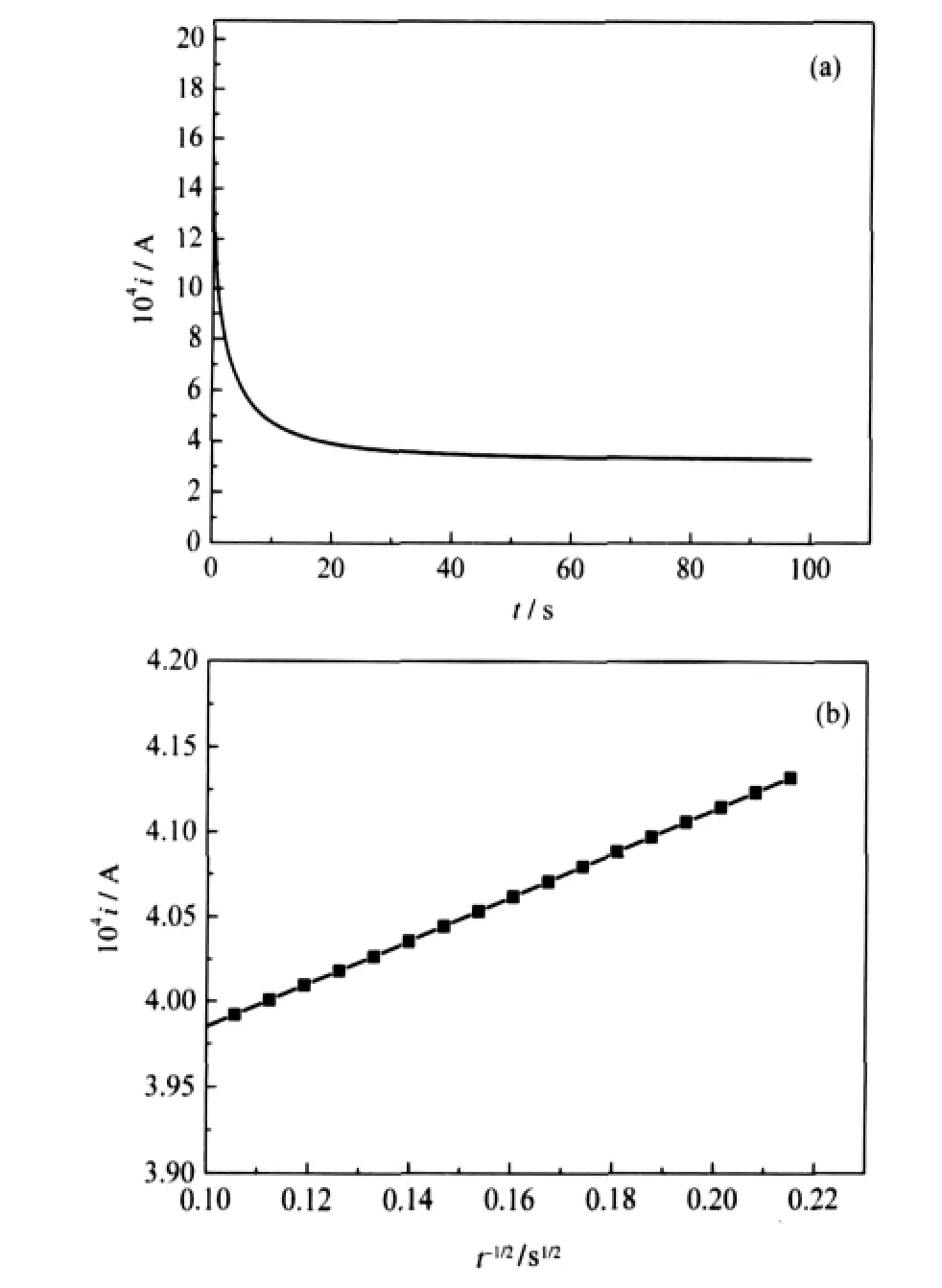
Fig.4 i-t(a)and i-t-1/2(b)curves of nano-LiVOPO4electrode
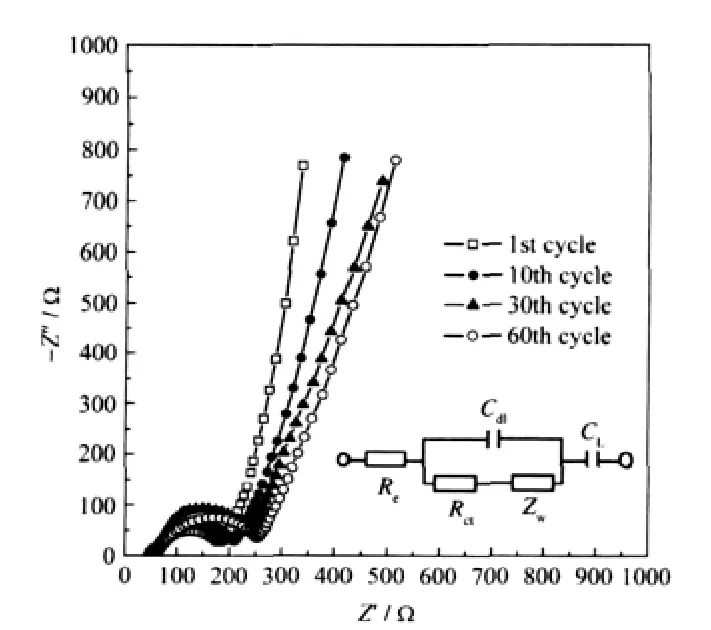
Fig.5 Electrochemical impedance spectroscopy of nano-LiVOPO4electrode at various cycling timesIn the equivalent circuit,Reis the electrolyte resistance,Rctis the charge-transfer resistance,Cdlis the double layer capacitance,Zwis the Warburg impedance,and CLis the intercalation capacitance.
The electrochemicalimpedance spectroscopy ofnano-LiVOPO4and the equivalent circuit are displayed in Fig.5.
All the spectra show a semicircle in the high frequency range and an inclined line in the low frequency range.The semicircle in the high frequency range is associated with the“charge transferreactions”attheinterfaceofelectrolyte/oxideelectrode,which corresponds to the charge transfer resistance.The inclined line in the low frequency range is attributable to“Warburg impedance”that is associated with lithium ion diffusion through the oxide electrode.The semicircle increases with the increase of cycle number.This indicates that the“charge transfer”resistance becomes larger with the increase of cycle number.The figure also shows that the slope of the inclined line varies with the cycle number.The slope of the inclined line at the first cycle is the biggest and after cycling 10 times it gets smaller.However, when the cycle number reaches 60,the slope of the inclined line becomes stable.
3 Conclusions
(1)Orthorhombic nano-LiVOPO4with particle size in the range of 10-60 nm was synthesized by a new rheological phase method.
(2)The first discharge of LiVOPO4is 135.7 mAh·g-1and 98.9%of that is kept after 60 cycles.More than 96.5%and 91.6%of the discharge capacity at 0.1C are sustained at 1.0C and 2.0C,respectively.The chemical diffusion coefficient of lithium ion in the nano-LiVOPO4was measured with the potential step technique and the value is in the order of 10-11cm2·s-1.
(3)Rheological phase method is a good route to synthesize LiVOPO4cathode material with high capacity,good cycling performance,and good current rate capability for lithium ion batteries.
1 Padhi,A.K.;Najundaswamy,K.S.;Goodenough,J.B. J.Electrochem.Soc.,1997,144:1188
2 Yamada,A.;Chung,S.C.J.Electrochem.Soc.,2001,148:A960
3 Amine,K.;Yasuda,H.;Yamachi,M.Electrochem.Solid State Lett.,2000,3:178
4 Azuma,G.;Li,H.;Tohdam,M.Electrochem.Solid State Lett., 2002,5:A135
5 Saidi,M.Y.;Barker,J.;Huang,H.;Sowyer,J.L.;Adamson,G.J. J.Power Sources,2003,119-112:266
6 Yin,S.C.;Grond,H.;Strobel,P.;Huang,H.;Nazar,L.F.J.Am. Chem.Soc.,2003,125:326
7 Hung,H.;Yin,S.C.;Kerr,T.;Taylor,N.;Nazar,L.F.Adv.Mater., 2002,14:1525
8 Ren,M.M.;Zhou,Z.;Li,Y.Z.;Gao,X.P.;Yan,J.J.Power Sources,2006,162:1357
9 Li,Y.Z.;Zhou,Z.;Ren,M.M.;Gao,X.P.;Yan,J.Electrochim. Acta,2006,51:6498
10 Ren,M.M.;Zhou,Z.;Gao,X.P.;Peng,W.X.J.Phys.Chem.C, 2008,112:5689
11 Barker,J.;Saidi,M.Y.;Swoyer,J.L.J.Electrochem.Soc.,2003, 150:A1394
12 Li,Y.Z.;Zhou,Z.;Gao,X.P.;Yan,J.J.Power Sources,2006, 160:633
13 Yamada,A.;Chung,S.C.;Hinokuma,K.J.Electrochem.Soc., 2001,148:A224
14 Andersson,A.S.;Thomas,J.O.;Kalska,B.;Haggstrom,L. Electrochem.Solid State Lett.,2000,3:66
15 Konarova,M.;Taniguchi,I.J.Power Sources,2009,194:1029
16 Kuwahara,A.;Suzuki,S.;Miyayama,M.Ceramics International, 2008,34:863
17 Li,J.;Suzuki,T.;Naga,K.;Ohzawa,Y.;Nakajima,T.Mater.Sci. Eng.B-Solid State Mater.Adv.Technol.,2007,142:86
18 Azmi,B.M.;Ishihara,T.;Nishiguchi,H.;Takita,Y. Electrochim.Acta,2002,48:165
19 Azmi,B.M.;Ishihara,T.;Nishiguchi,H.;Takita,Y.J.Power Sources,2005,146:525
20 Ren,M.M.;Zhou,Z.;Su,L.W.;Gao,X.P.J.Power Sources, 2009,189:786
21 Yang,Y.;Fang,H.;Zheng,J.;Li,L.;Li,G.;Yan,G.Solid State Sciences,2008,10:1292
22 Kerr,T.A.;Gaubicher,J.;Nazar,L.F.Electrochem.Solid State Lett.,2000,3:460
23 Azmi,B.M.;Ishihara,T.;Nishiguchi,H.;Takita,Y. Electrochemistry,2003,71:1108
24 Sun,J.;Xie,W.;Yuan,L.;Zhang,K.;Wang,Q.Mater.Sci.Eng. B-Solid State Mater.Adv.Technol.,1999,64:157
25 He,Z.Q.;Li,X.H.;Xiong,L.Z.;Wu,X.M.;Xiao,Z.B.;Ma,M. Y.Materials Chemistry and Physics,2005,93:516
26 He,B.L.;Zhou,W.J.;Bao,S.J.;Liang,Y.Y.;Li,H.L. Electrochim.Acta,2007,52:3286
27 Gaubicher,J.;Orsini,F.;Le Mercier,T.;Llorente,S.;Villesuzanne, A.;Angenault,J.;Quarton,M.J.Solid State Chem.,2000,150: 250
28 Lii,K.H.;Li,C.H.;Cheng,C.Y.;Wang,S.L.J.Solid State Chem.,1991,95:352
29 Barker,J.;Saidi,M.Y.;Swoyer,J.L.J.Electrochem.Soc.,2004, 151:A796
30 Bard,A.J.;Faulkner,L.R.Electrochemical methods: fundamentals and applications.2nd ed.New York:Wiley,2001
——水墨的維度

