The apparent diffusion coef fi cient does not re fl ect cytotoxic edema on the uninjured side after traumatic brain injury
Hong Lu, Xiaoyan Lei
Department of Radiology, Af fi liated Haikou Hospital, Xiangya School of Medicine, Central South University, Haikou, Hainan Province, China
The apparent diffusion coef fi cient does not re fl ect cytotoxic edema on the uninjured side after traumatic brain injury
Hong Lu, Xiaoyan Lei
Department of Radiology, Af fi liated Haikou Hospital, Xiangya School of Medicine, Central South University, Haikou, Hainan Province, China
After traumatic brain injury, vasogenic and cytotoxic edema appear sequentially on the involved side. Neuroimaging investigations of edema on the injured side have employed apparent diffusion coefficient measurements in diffusion tensor imaging. We investigated the changes occurring on the injured and uninjured sides using diffusion tensor imaging/apparent diffusion coef fi cient and histological samples in rats. We found that, on the injured side, that vasogenic edema appeared at 1 hour and intracellular edema appeared at 3 hours. Mixed edema was observed at 6 hours, worsening until 12-24 hours post-injury. Simultaneously, microglial cells proliferated at the trauma site. Apparent diffusion coef fi cient values increased at 1 hour, decreased at 6 hours, and increased at 12 hours. The uninjured side showed no significant pathological change at 1 hour after injury. Cytotoxic edema appeared at 3 hours, and vasogenic edema was visible at 6 hours. Cytotoxic edema persisted, but vasogenic edema tended to decrease after 12-24 hours. Despite this complex edema pattern on the uninjured side with associated pathologic changes, no significant change in apparent diffusion coefficient values was detected over the fi rst 24 hours. Apparent diffusion coef fi cient values accurately detected the changes on the injured side, but did not detect the changes on the uninjured side, giving a false-negative result.
nerve regeneration; brain injuries; blood-brain barrier; magnetic resonance imaging; apparent diffusion coefficient; intracranial edema; cytotoxic edema; vasogenic edema; pathology; NSFC grant; neural regeneration
Funding: This project was supported by the National Natural Science Foundation of China, No. 81160181; the International Cooperation Project of Hainan Province, No. Qiongke (2012) 65.
Lu H, Lei XY. The apparent diffusion coefficient does not reflect cytotoxic edema on the uninjured side after traumatic brain injury. Neural Regen Res. 2014;9(9):973-977.
Introduction
With the rapid development of the transportation and building industries, traf fi c accidents and falls have increased steadily, accompanied by an increased incidence of brain injuries, which account for > 50% of accidental traumas (Sabre et al., 2013). Traumatic brain edema not only induces brain cell (neuron, glial cell) dysfunction, but also causes intracranial hypertension, which can lead to herniation, resulting in death (Suzuki et al., 2006). Using molecular and pathological techniques and MRI, many studies have reported on the complex mechanism of the onset of traumatic brain edema (Donkin et al., 2010; Lescot et al., 2010; Fukuda et al., 2012; Wei et al., 2012). Vasogenic and cytotoxic edema develop over time. Vasogenic edema, characterized by damage to the blood-brain barrier, plays an important role in traumatic brain injury (Donkin et al., 2010; Lescot et al., 2010; Wei et al., 2012). Diffusion-weighted imaging (DWI) shows alterations in apparent diffusion coef fi cient values following the amount, the type, and course of edema (Fukuda et al., 2012). However, studies have mainly focused on brain tissue on the injured side after traumatic brain injury (Kochanek et al., 1995; Stroop et al., 1998; Lescot et al., 2010; Wei et al., 2012). Because the mechanism of traumatic brain injury remains unclear, effective therapeutic interventions are lacking. The cerebrum is a higher integration center, and there are abundant neural network connections and humoral regulation mechanisms between the cerebral hemispheres. Brain tissue on the uninjured side after traumatic brain injury may also undergo abnormal changes, but these changes remain poorly understood. Performing traumatic brain injury studies in rats, we found that brain tissue on the uninjured side did in fact undergo certain changes.
Materials and Methods
Experimental animals
A total of 60 healthy adult Wistar rats of both genders weighing 220-250 g were provided by the Experimental Animal Center, Sichuan University, China (Animal license No. SCXK (Chuan) 2008-24). Rats were housed in separate cages with a 12-hours light/dark cycle at 22-25°C, and allowed free accessto food and water. The rats were randomly assigned to control and traumatic brain injury groups with fi ve subgroups: 1, 3, 6, 12 and 24 hours according to time points after injury.
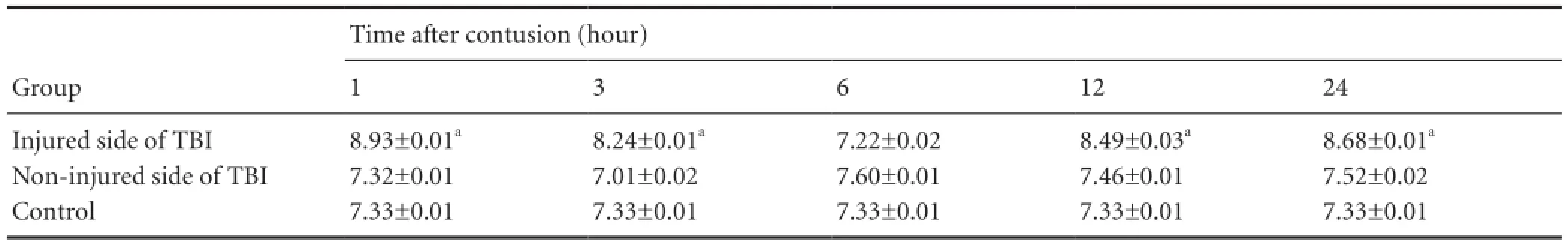
Table 1 Comparison of ADC values (mm2/s ) in each group
Experimental groups and establishment of animal modelsIn accordance with Feeney’s modi fi ed method (Feeney et al., 1981), a Pinpoint? Precision Cortical impactor (Hatteras Instruments, Cary, NC, USA) was used to produce a model of traumatic brain injury. Rats were intraperitoneally injected with 1% sodium pentobarbital (100 mg/kg). In the prone position, the head of the rat was placed in a stereotactic frame (ST-7Setagaya-Ku, Tokyo, Japan). After shaving and sterilizing, a 2-cm incision was made along the midline from left to right. The anterior fontanel was punctured 2.5 cm to the right of the midline between the anterior and posterior fontanelles using a desktop electric dental engine (model 307-2B, Shanghai, China), with a 1.5 mm drill bit at a rotation speed of 4,000 r/min. A 5-mm-diameter round bone window was opened using blood vessel forceps, keeping the dura mater intact. To establish a model of moderate traumatic brain injury, the dura in the bone window was impacted with the impactor at a speed of 2.5 m/s for 0.85 seconds to a depth of 3.5 mm. The impact diameter was 4 mm. Preliminary experiments had concluded that parenchymal injury appeared, but no obvious intracerebral hematoma occurred, under these conditions. The bone window was sealed with bone wax. After achieving local hemostasis, the scalp was sutured. With the exception of impact, the procedures in the control group were identical to the traumatic brain injury group.
Magnetic resonance imaging with DWI
All images were acquired on a 3.0 T (Signa Excite? HDx system, GE Healthcare, Milwaukee WI, USA) with a designated coil (Medical Science and Technology Corp. of Chenguang, Shanghai, China). T1-weighted images (T1WI) were acquired in the coronal plane centered on the optic chiasm with the following parameters: section thickness 2 mm, spacing 0 mm, visual fi eld 4 cm × 4 cm, matrix 128 × 128; DWI: repetition time shortest, echo time shortest, b values of 0 and 1,000 s/mm2,respectively. Data were transmitted to a workstation (AW4.3 GEMdeil system Advanta dow, Chicago, IL, USA). Apparent diffusion coef fi cient values (largest slice; mm2/s) of injured areas with the largest proportion of high signal intensity, their uninjured contralateral counterparts, and the largest layer of the basal ganglia were measured using Function tool software (GEMdeil system Advanta dow).
The following formula was used for analysis: Apparent diffusion coef fi cient = In (SI1/SI0)/(b0-b1) (Hui et al., 2012), where b1and b0are the gradient factors of sequences and SI1and SI0are signal intensities from the sequences SI1and SI0, respectively. SI1= region of interest signal intensity with b1(600 s/mm2), SI0= region of interest signal intensity without diffusion gradients b0(b0= 0 s/mm2). To reduce the effect of subjective factors during placement of the region of interest, a mean value was calculated by two experienced physicians.
Hematoxylin-eosin staining
After MRI at the various time points, cerebral perfusion with formalin was performed, and then brain tissue was processed for hematoxylin-eosin staining, electron microscopy, and IgG immunohistochemistry. These studies were conducted in the control group at 6 hours after sham surgery.
In the traumatic brain injury groups, rats were anesthetized with 1% sodium pentobarbital at the corresponding time points of 1, 3, 6, 12, and 24 hours after traumatic brain injury. The left ventricle was perfused with 4% paraformaldehyde until colorless fl uid fl owed from the right atrium. Rats were decapitated and the brain was removed and fi xed in 4% paraformal dehyde for 24 hours. Brain tissues from the injured side and uninjured mirror area were obtained, embedded in paraffin, and sliced into sections. The sections were stained with hematoxylin and eosin, observed and photographed with a light microscope (Olympus, Tokyo, Japan).
Transmission electron microscopy
Brain tissues from the injured area and uninjured mirror area were fixed in glutaral for 24 hours, and then stained with lead and uranium. Transmission electron microscopy (EX-2000 model; PECNAL G2F20, New York NY, USA) was used to observe the morphology of nerve cells, cell organelles, and the microvascular endothelium, i.e., the bloodbrain barrier. Hematoxylin-eosin staining, light, and electron microscopy were performed at 24 hours post sham operation in the control group.
Statistical analysis
All data were expressed as mean ± SD and analyzed using SPSS 18.0 software (SPSS Inc., Chicago IL, USA). Repeated-measures analysis of variance was used for data analysis. A value of P < 0.05 was considered statistically signi fi cant.
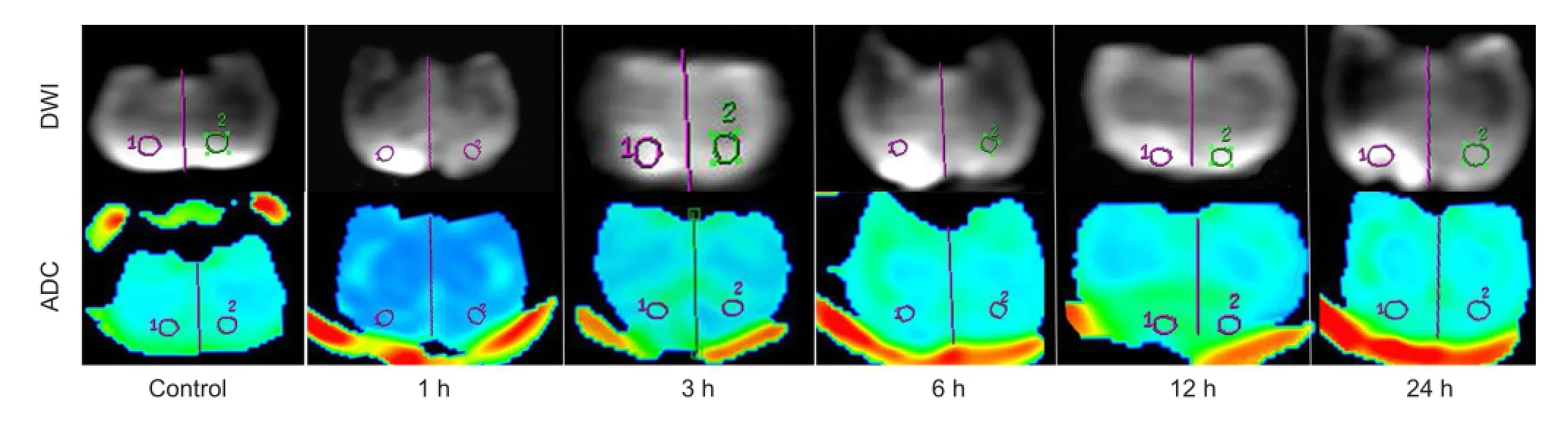
Figure 1 DWI and ADC images in the control and TBI groups on the uninjured (left) and injured (right) sides.
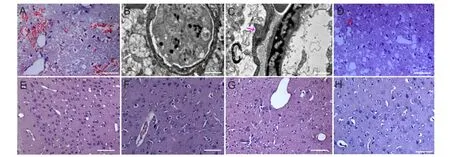
Figure 2 Pathological changes in rat brain tissue on the uninjured and injured sides at 1, 3, 6 and 24 hours after traumatic brain injury (TBI).
Results
Apparent diffusion coef fi cient changes in brain tissue on the injured and uninjured sides following traumatic brain injury
DWI did not show abnormalities in the control group. In the traumatic brain injury groups, DWI revealed high signal intensity at the traumatic brain injury site at 1 hour after injury, which proceeded to enlarge overtime. Apparent diffusion coef fi cient values on the injured side increased at 1 hour, reduced at 3 hours, were lowest at 6 hours, and increased at 12 hours, showing a “V”-shaped pattern. There were signi fi cant differences in apparent diffusion coefficient values between the control group and the 1-, 3-, 12-, and 24-hour groups (P < 0.05), but there was no signi fi cant difference in apparent diffusion coefficient values between the control group and the 6-hour group (P > 0.05;Figure 1, Table 1). On the uninjured side, apparent diffusion coef fi cient values were normal at 1 hour, reduced at 3 hours, increased at 6 hours, and then showed a decreasing trend at 12-24 hours. However, no signi fi cant difference in apparent diffusion coef fi cient values was detected between the uninjured side of the various traumatic brain injury groups and the control group (P > 0.05).
Pathological changes in brain tissue on the uninjured and injured sides after traumatic brain injury
In the control group, there was no abnormal neural cellmorphology and the microvascular endothelium was intact. In the 1-hour traumatic brain injury group, no significant pathological changes in brain tissue on the uninjured side were identi fi ed (Figure 2). Three hours after traumatic brain injury, under light microscopy, swollen glial cells with abundant lightly staining cytoplasm were visible on the uninjured side. Under transmission electron microscopy, there were signs of cytotoxic edema, such as nuclear swelling, nuclear membrane thickening, margination of chromatin, and mitochondrial and endoplasmic reticulum swelling (Figure 2). At 6 hours, cytotoxic edema became more noticeable. Simultaneously, widened tissue spaces, and lightly staining cytoplasm. Electron microscopy revealed manifestations of vasogenic edema, such as vascular endothelial cell swelling, basilar membrane thickening and breakage, and glial cell process swelling (Figure 2). With increasing time, cytotoxic edema gradually increased, and vasogenic edema lessened at 12 hours. Microglial proliferation was noted on the uninjured side at 24 hours after traumatic brain injury.
On the injured side, vasogenic edema appeared 1 hour after injury (Figure 2), showing intercellular space widening, lightly staining cytoplasm, and abundant erythrocytes surrounding the capillaries. Cytotoxic edema appeared at 3 hours. Vasogenic and cytotoxic edema gradually increased over time. Tissue necrosis, in fl ammatory cell in fi ltration and microglial proliferation were visible at 12-24 hours after traumatic brain injury, and most obvious in the 24-hour traumatic brain injury group (Figure 2).
Discussion
Pathological changes in brain tissue on injured and uninjured sides after traumatic brain injury
The main pathological change after traumatic brain injury was brain edema, showing that excessive water accumulated in the intracellular and extracellular spaces, which induces an increase in brain volume, weight, and intracranial pressure, potentially resulting in brain herniation and even death. Brain edema can be divided into four categories: cytotoxic, vasogenic, interstitial, and osmotic (Papadopoulos et al., 2007). The occurrence of cytotoxic edema and vasogenic edema is characteristic of traumatic brain injury. Vasogenic edema is associated with interruption of the blood-brain barrier (Unterberg et al., 2004), allowing fl uid and protein to leak out of blood vessels and enter the intercellular space, widening it (Tait et al., 2008). After an insult, if the bloodbrain barrier is intact, intracellular fl uid accumulates excessively, causing cytotoxic edema. The extracellular space becomes narrowed (Tait et al., 2008). Previous studies showed that pathologically, vasogenic edema first occurred in the early stages of traumatic brain injury (< 24 hours), and was a factor in inducing cytotoxic edema (Ferrier et al., 2007; Donkin et al., 2010). Edema mainly occurs in the center of the injured area, with cytotoxic edema caused by ischemia/ hypoxia of brain tissue. The distribution of cytotoxic edema is more extensive, and is predominant in traumatic brain injury (Zhang et al., 2010). Results of this study revealed that vasogenic edema in brain tissue on the injured side appeared 1 hour after traumatic brain injury, and cytotoxic edema was visible at 3 hours. With increasing time, vasogenic edema and intracellular edema gradually increased. Tissue necrosis, inflammatory cell infiltration, and microglial proliferation were detected at 12-24 hours, which was identical to a previously published study (Lescot et al., 2010). Other previous studies have indicated that no pathological or imaging changes were observed on the uninjured side after traumatic brain injury (Stroop et al., 1998; Lescot et al., 2010). Results from this study revealed that no abnormality was observed on the uninjured side 1 hour after traumatic brain injury, but at 3 hours after injury, cytotoxic edema was visible, and gradually increased. Six hours post-traumatic brain injury, significant vasogenic edema had appeared, decreasing by 12-24 hours, while cytotoxic edema persisted. At this point, microglial proliferation was observed. These pathological changes on the uninjured side were later compared with those on the injured side. Cytotoxic edema appeared first on the injured side, followed by vasogenic edema, which decreased more slowly than on the uninjured side.
Few reports have been concerned with the molecular mechanisms on the uninjured side of a traumatic brain injury. The brain is a higher integration center. It may be assumed that when one side is injured, corresponding pathological changes on the uninjured side can be induced by neural network connections between the cerebral hemispheres and humoral regulation. Recent studies demonstrated that aquaporin-4 is strongly associated with the occurrence and development of traumatic cerebral edema, and has a complex role in the formation of cerebral edema (Shenaq et al., 2012; Lo Pizzo et al., 2013; Ren et al., 2013). Fukuda et al. (2012) used a model of traumatic brain injury, and found that the degree of movement of water molecules in brain tissue was reduced, and brain water content was increased, at 24 hours after traumatic brain injury, but aquaporin-4 expression was increased in the injured area and normal in the uninjured area. Kiening et al. (2002) believed that aquaporin-4 expression in cerebral tissues diminishes after traumatic brain injury, especially on the injured side. In combination with the results from this study, whether the types and evolution of cerebral edema on the uninjured side were associated with aquaporin-4 expression requires further investigation.
Apparent diffusion coef fi cient value changes and pathological processes in brain tissue on the injured and uninjured side after traumatic brain injury
DWI is dependent on Brownian motion of water molecules and intracellular and extracellular transmembrane water transport. Imaging time is short. DWI is an effective method to examine water molecule diffusion in vivo. DWI images are obtained by assessment of the apparent diffusion coef fi cient. DWI converts morphologic changes in intracellular and extracellular spaces after the onset of edema into visible images (Xu et al., 2010). Decreased apparent diffusion coefficient values, apparent diffusion coefficient maps with low signal intensity (black), and corresponding DWI images with high signal intensity (bright) indicate cytotoxic edema. In contrast, high signal on both apparent diffusion coef fi cient and DWI images represents vasogenic edema (T2 shine-through effect on DWI). DWI has been extensively used in the earlydiagnosis of cerebral ischemia (Lu et al., 2011). Newcombe et al. (2013) veri fi ed that diffusion of water molecules is limited in the injured area in the early stages of traumatic brain injury. A penumbral area of brain parenchyma around the injured region exhibited high signal on DWI images and low signal on apparent diffusion coef fi cient maps. This pathological change was interpreted as cytotoxic edema induced by microvascular injury surrounding the injured region. Thus, DWI and apparent diffusion coef fi cient have signi fi cant value in the assessment of early brain injury. Our results demonstrate that cytotoxic and transient vasogenic edema occurs on the uninjured side after traumatic brain injury; however, no significant difference in apparent diffusion coefficient values was detectable between the uninjured side and control group, which was identical to results from previous studies. We observed edema and blood-brain barrier damage in brain tissue on the uninjured side, and these pathological fi ndings changed over time. During our observation period, cytotoxic edema and vasogenic edema briefly coexisted. Cytotoxic edema can increase apparent diffusion coef fi cient values, but vasogenic edema can diminish apparent diffusion coef fi cient values (Lu et al., 2011). These two effects may have counteracted each other. Overall apparent diffusion coef fi cient values were altered, but no signi fi cant difference in apparent diffusion coefficient values was observed between the uninjured side of the traumatic brain injury groups and the control group, revealing a false-negative result.
In summary, brain tissue on the uninjured side was not completely normal after traumatic brain injury. Some pathological changes were observed, which occurred later than on the injured side. Cytotoxic edema appeared fi rst, followed by transient vasogenic edema. We presume that this is associated with neuroregulation and humoral regulation. Significant apparent diffusion coef fi cient value changes were not observed, demonstrating the possibility of a false-negative result if used to assess the contralateral side of a traumatic brain injury. Aquaporin-4 expression may exert an effect on the uninjured side; further investigation is needed to identify the precise mechanism.
Acknowledgments:We thank Xia QJ from the Molecular Genetics Laboratory, West China Medical Center, Sichuan University, China, for the support on western blot experiment.
Author contributions:Lu H had full access to the study concept and design, and was responsible for obtaining funds. Lei XY was responsible for manuscript authorization and animal experiments. Both authors approved the final version of the paper.
Con fl icts of interest:None declared.
Akiguchi I, Tomimoto H, Suenaga T, Wakita H, Budka H (1998) Bloodbrain barrier dysfunction in Binswanger’s disease: an immunohistochemical study. Acta Neuropathol 95:78-84.
Donkin JJ, Vink R (2010) Mechanisms of cerebral edema in traumatic brain injury: therapeutic developments. Curr Opin Neurol 23:293-299.
Feeney DM, Boyeson MG, Linn RT, Murray HM, Dail WG (1981) Responses to cortical injury: I. Methodology and local effects of contusions in the rat. Brain Res 211:67-77.
Ferrier MC, Sarin H, Fung SH (2007) Validation of dynamic contrast-enhanced magnetic resonance imaging-derived vascular permeability measurements using quantitative autoradiography in the RG2 rat brain tumor model. Neoplasia 9:546-555.
Fukuda AM, Pop V, Spagnoli D, Ashwal S, Obenaus A, Badaut J (2012) Delayed increase of astrocytic aquaporin 4 after juvenile traumatic brain injury: possible role in edema resolution? Neuroscience 222:366-378.
Hu H, Lu H, He ZP, Han XJ, Chen J, Tu R (2012) Gene interference regulates aquaporin-4 expression in swollen tissue of rats with cerebral ischemic edema: correlation with variation in apparent diffusion coef fi cient. Neural Regen Res 7:1659-1666.
Kiening KL, van Landeghem FK, Schreiber S, Thomale UW, von Deimling A, Unterberg AW, Stover JF (2002) Decreased hemispheric Aquaporin-4 is linked to evolving brain edema following controlled cortical impact injury in rats. Neurosci Lett 324:105-108.
Kochanek PM, Marion DW, Zhang W, Schiding JK, White M, Palmer AM (1995) Severe controlled cortical impact in rats: assessment of cerebral edema, blood fl ow, and contusion volume. J Neurotrauma 12:1015-1025.
Lescot T, Fulla-Oller L, Po C, Chen XR, Puybasset L, Gillet B, Plotkine M, Meric P, Marchand-Leroux C (2010) Temporal and regional changes after focal traumatic brain injury. J Neurotrauma 27:85-94.
Lo Pizzo M, Schiera G, Di Liegro I, Di Liegro CM, Pál J, Czeiter E, Sulyok E, Dóczi T (2013) Aquaporin-4 distribution in control and stressed astrocytes in culture and in the cerebrospinal fluid of patients with traumatic brain injuries. Neurol Sci 34:1309-1314.
Lu H, Hu H, He ZP (2011) Reperfusion of the rat brain tissues following acute ischemia: the correlation among diffusion-weighted imaging, histopathology, and aquaporin-4 expression. Chin Med J (Engl) 124:3148-3153.
Newcombe VF, Williams GB, Outtrim JG, Chatfield D, Gulia Abate M, Geeraerts T, Manktelow A, Room H, Mariappen L, Hutchinson PJ, Coles JP, Menon DK (2013) Microstructural basis of contusion expansion in traumatic brain injury: insights from diffusion tensor imaging. J Cereb Blood Flow Metab 33:855-862.
Papadopoulos MC, Verkman AS (2007) Aquaporin-4 and brain edema. Pediatr Nephrol 22:778-784.
Ren Z, Iliff JJ, Yang L, Yang J, Chen X, Chen MJ, Giese RN, Wang B, Shi X, Nedergaard M (2013) Hit & Run’ model of closed-skull traumatic brain injury (TBI) reveals complex patterns of post-traumatic AQP4 dysregulation. J Cereb Blood Flow Metab 33:834-845.
Sabre L, Tomberg T, K?rv J, Kepler J, Kepler K, Linnam?gi U, Asser T (2013) Brain activation in the acute phase of traumatic spinal cord injury. Spinal Cord 51:623-629.
Shenaq M, Kassem H, Peng C, Schafer S, Ding JY, Fredrickson V, Guthikonda M, Kreipke CW, Rafols JA, Ding Y (2012) Neuronal damage and functional de fi cits are ameliorated by inhibition of aquaporin and HIF1alpha after traumatic brain injury (TBI). J Neurol Sci 323:134-140.
Stroop R, Thomale UW, P?user S, Bernarding J, Vollmann W, Wolf KJ, Lanksch WR, Unterberg AW (1998) Magnetic resonance imaging studies with cluster algorithm for characterization of brain edema after controlled cortical impact injury (CCII). Acta Neurochir Suppl 71:303-305.
Suzuki R, Okuda M, Asai J, Nagashima G, Itokawa H, Matsunaga A, Fujimoto T, Suzuki T (2006) Astrocytes co-express aquaporin-1, -4, and vascular endothelial growth factor in brain edema tissue associated with brain contusion. Acta Neurochir Suppl 96:398-401.
Tait MJ, Saadoun S, Bell BA (2008) Water movements in the brain: role of aquaporins. Trends Neurosci 31:37-43.
Unterberg AW, Stover J, Kress B (2004) Edema and brain trauma. Neuroscience 129:1021-1029.
Wei XE, Zhang YZ, Li YH, Li MH, Li WB (2012) Dynamics of rabbit brain edema in focal lesion and perilesion area after traumatic brain injury: a MRI study. J Neurotrauma 29: 2413-2420.
Xu FJ, He QY, Han HB (2010) Measurement of brain extracellular space and its physiological and pathophysiological signi fi cance. Beijing Da Xue Xue Bao 42:234-237.
Zhang SG, Pan TH, He AL, Gong WY, Shi L, Zhou JF (2010) Expression of aquaporin-4 in the brain tissues from patients with severe brain injuries and its signi fi cance. Zhonghua Chuangshang Zazhi 26:589-591.
Copyedited by Cone L, Raye W, Wang J, Qiu Y, Li CH, Song LP, Zhao M
10.4103/1673-5374.133150
Hong Lu, Department of Radiology, Affiliated Haikou Hospital, Xiangya School of Medicine, Central South University, Haikou 570208, Hainan Province, China, cqluh@sohu.com.
http://www.nrronline.org/
Accepted: 2014-03-20
- 中國神經(jīng)再生研究(英文版)的其它文章
- Susceptibility-weighted imaging is suitable for evaluating signal strength in different brain regions of a rabbit model of acute hemorrhagic anemia
- Age-related changes of lateral ventricular width and periventricular white matter in the human brain: a diffusion tensor imaging study
- Sequential expression of cyclooxygenase-2, glutamate receptor-2, and platelet activating factor receptor in rat hippocampal neurons after fl uid percussion injury
- Acupuncture and moxibustion reduces neuronal edema in Alzheimer’s disease rats
- Neuropeptide Y protects cerebral cortical neurons by regulating microglial immune function
- Protective effect of alpha-synuclein knockdown on methamphetamine-induced neurotoxicity in dopaminergic neurons

