Susceptibility-weighted imaging is suitable for evaluating signal strength in different brain regions of a rabbit model of acute hemorrhagic anemia
Susceptibility-weighted imaging is suitable for evaluating signal strength in different brain regions of a rabbit model of acute hemorrhagic anemia
Acute hemorrhagic anemia can decrease blood flow and oxygen supply to brain, and affect its physiological function. While detecting changes in brain function in patients with acute hemorrhagic anemia is helpful for preventing neurological complications and evaluating therapeutic effects, clinical changes in the nervous systems of these patients have not received much attention. In part, this is because current techniques can only indirectly detect changes in brain function following onset of anemia, which leads to lags between real changes in brain function and their detection. Some methods such as Transcranial Doppler can detect changes in cerebral blood- fl ow velocity that result from compensatory responses to the de fi cient oxygen delivery seen in anemia (i.e. higher velocity associated with anemia and lower velocity associated with recovery), but cannot re fl ect the actual oxygen supply and metabolism in the brain or organic changes such as stroke (Purkayastha and Sorond, 2012). Conventional CT and MRI can detect early cerebral infarction so as to facilitate early treatment, but cannot monitor physiological changes in the brain that occur because of anemia (Allmendinger et al., 2012). Near-infrared spectroscopy is a non-invasive method capable of measuring total blood-oxygen levels in brain (Murkin and Arango, 2009; Navarro et al., 2012; Yamazaki et al., 2013), but is unable to provide fi ne details concerning alterations in different cerebral tissues. Susceptibility-weighted imaging is a novel, non-invasive method for detecting changes in cerebral oxygen levels that may provide more detailed information regarding cerebral blood fl ow in patients with hemorrhage (Li et al., 2013). In the present study, we used susceptibility-weighted imaging to detect cerebral changes in an animal model of acute hemorrhagic anemia.
Construction of the rabbit model of acute hemorrhagic anemia was based on the method used by Morimoto et al. (2001). A 40-mL blood sample was drawn through an arterial catheter. To compensate for the effects of simple blood-volume loss, the same volume of a 6% hydroxyethyl starch in 0.9% sodium chloride solution was injected through a femoral vein catheter. Following this, a blood sample was drawn again for blood-gas analysis and whole blood tests, and the head of the rabbit was rescanned by MRI. The bloodletting, fl uid infusion, and scanning processes were repeated continuously fi ve times before the animal recovered from anesthesia; on the fi fth occasion, the bloodletting volume and fl uid-infusion volume were both 50 mL. The investigation conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication No. 85-23, revised 1996), and the protocol was approved by the Institutional Animal Care Committee from Second People’s Hospital of Shenzhen City, First Af fi liated Hospital of Shenzhen University, China.
The blood samples obtained from the femoral artery were used for blood-gas analysis, which determined the PaO2, PaCO2, lactic acid, and pH levels. In addition, mean arterial pressure was measured (Figure 1). A catheter was inserted through the femoral vein to the atrium for infusion of fl uid, and red bloodcell count, hemoglobin concentration, hematocrit, and central venous pressure were assessed (Figure 1). There was an approximate halving of the red blood-cell count, hemoglobin concentration, and hematocrit values after the first bloodletting, and these values progressively decreased following each of the four subsequent bloodletting procedures. The values of these parameters after the fifth bloodletting were significantly lower than the pre-bleed values (P < 0.05;Figure 1A). The bloodletting procedures were associated with substantial increases in lactic acid concentration as well as a small, but statistically signi fi cant change in blood pH (Figure 1B). PaO2increased and PaCO2decreased progressively with each bloodletting procedure (P < 0.05, vs. pre-bleed; Figure 1C). Bloodletting was not associated with any changes in central venous pressure or mean arterial pressure (P > 0.05, vs. pre-bleed;Figure 1D).
The rabbits were fi xed to a special board in the supine position and scanned by a Siemens Magnetom Avanto 1.5 T MRI Scanner, using a body coil (excitation) and wrap-around surface coil (reception). T2 dual-echo fast spin-echo with fat-suppression (FSET2WI/PD) and susceptibility-weighted imaging 3D sequences were used. The scan extended downward from a plane passing through the superior orbital margin to the medulla oblongata of the rabbit. FSE-T2WI/PD acquisition was conducted using the following parameters: repetition time = 2,800 ms, echo time = 33/78 ms, fi eld of view = 12 cm × 12 cm, matrix size = 256 × 256; acquisition time = 3.09 minutes. Susceptibility-weighted imaging acquisition was performed with a three-dimensional gradient echo sequence, as follows: repetition time = 49 ms; echo time = 40 ms; fl ip angle = 15°; fi eld of view = 15 cm × 15 cm; bandwidth = 80 kHz; IPAT factor = 2. Additional processing was carried out on the phase-corrected susceptibility-weighted imaging sequences. The third ventricle and the olfactory bulb parallel to the corpus callosum were measured. The bilateral frontal cortex, frontal white matter, temporal lobe, and thalamus were selected manually as regions of interest. The control (pre-bleed) susceptibility-weighted imaging-signal value of the frontal white matter was significantly lower than that of the frontal cortex (52.50 ± 20.29 vs. 63.10 ± 22.82; P < 0.05). The fi rst bloodletting was not associated with any signi fi cant changes in the susceptibility-weighted imaging signal of the frontal white matter (P > 0.05). Susceptibility-weighted imaging signals from the frontal cortex, temporal lobe, and thalamus after the second, third, fourth and fi fth bloodletting procedures were signi fi cantly lower compared with the corresponding control (pre-bleed) values (P < 0.05; Figures 2, 3).
We also evaluated the overall cerebral white-gray contrast and vein structure to examine the susceptibility-weighted imaging minimum-intensity projection images. The contrast between cerebral gray and white matter was higher after bloodletting (particularly after the fourth and fi fth procedures) than beforehand, and the venous structure was more abundant and clear (Figure 4).
Hematoxylin and eosin staining showed that degeneration and necrosis of neurons and glial cells were not evident in the brains or cerebellums of our rabbits with acute hemorrhagic anemia (Figure 5). However, spaces had formed around blood vessels and cells, consistent with the occurrence of cerebral edema.
In conclusion, this study has revealed that susceptibility-weighted imaging is an effective tool for detecting PaO2and PaCO2-induced changes in cerebral oxygenation of different brain regions after acute hemorrhagic anemia. Therefore, susceptibility-weighted imaging may be a useful technique for monitoring the pathophysiologic changes and related complications associated with acute anemia.
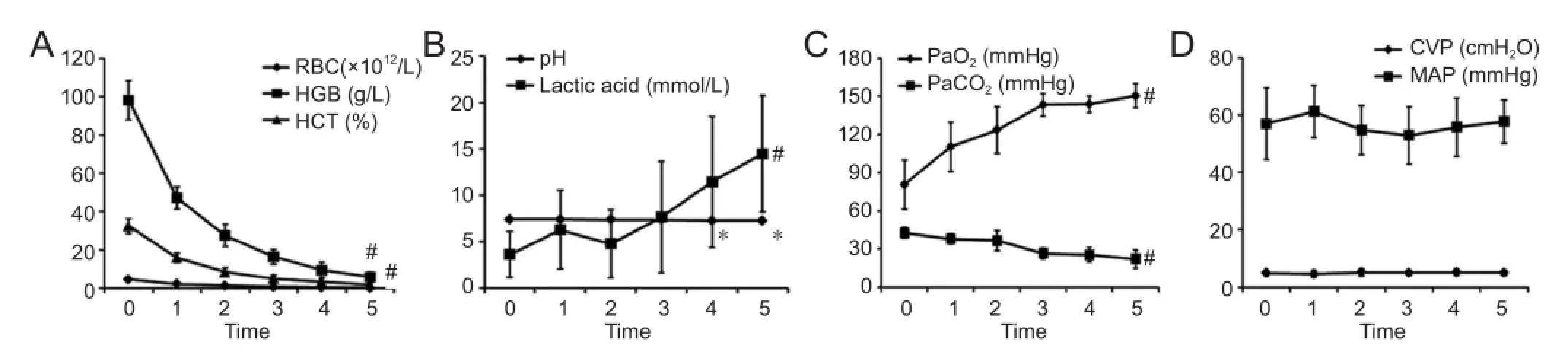
Figure 1 Results of whole blood tests, blood-gas analyses, mean arterial pressure, and central venous pressure after acute hemorrhagic anemia.
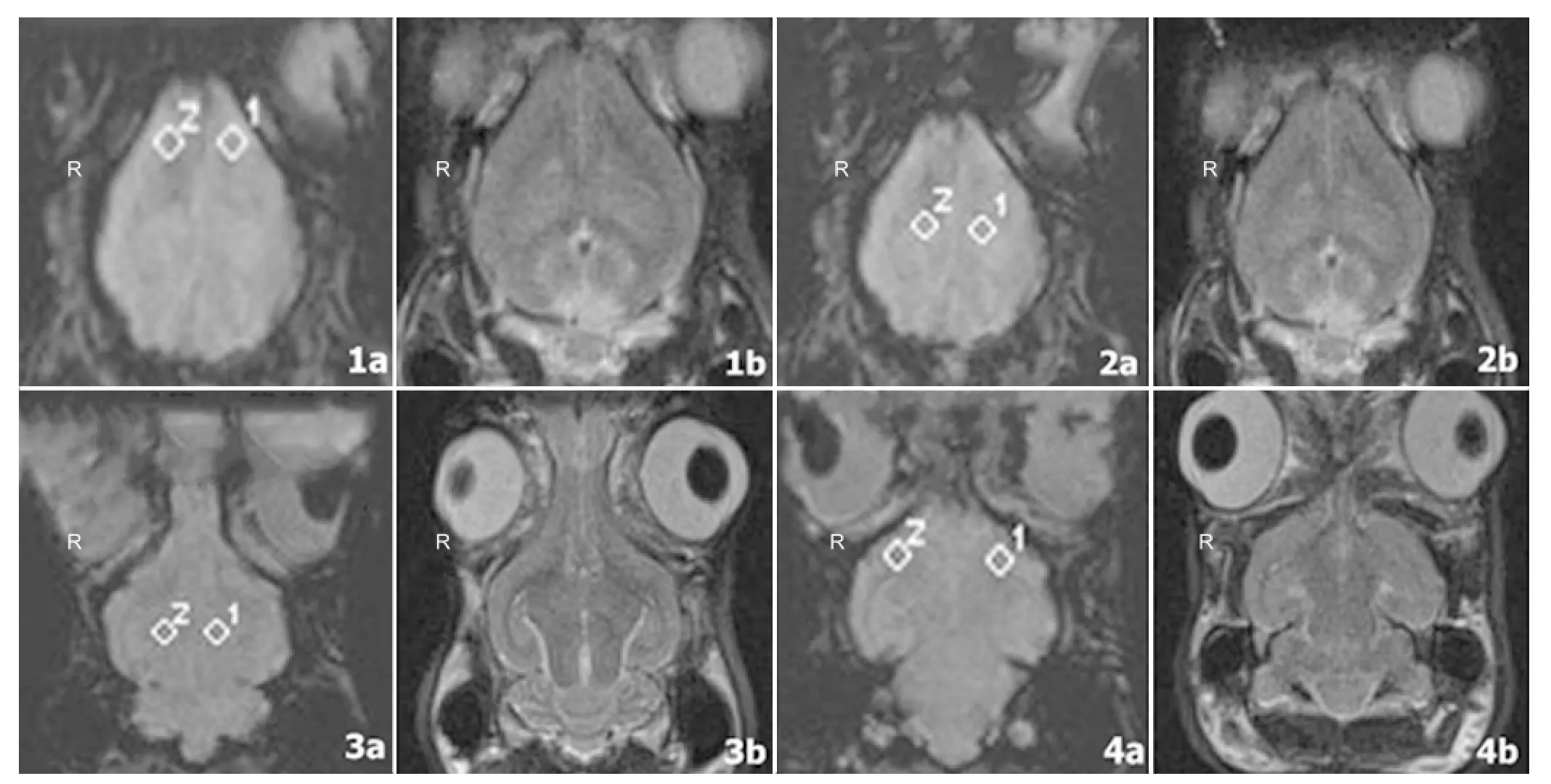
Figure 2 Representative susceptibility-weighted imaging (SWI) and corresponding T2-weighted images of a rabbit brain that was obtained at the superior aspect of the olfactory bulb (1a, 1b), the border of the olfactory bulb (2a, 2b), the thalamus (3a, 3b), and the cerebellum (4a, 4b) after the fi fth bloodletting.
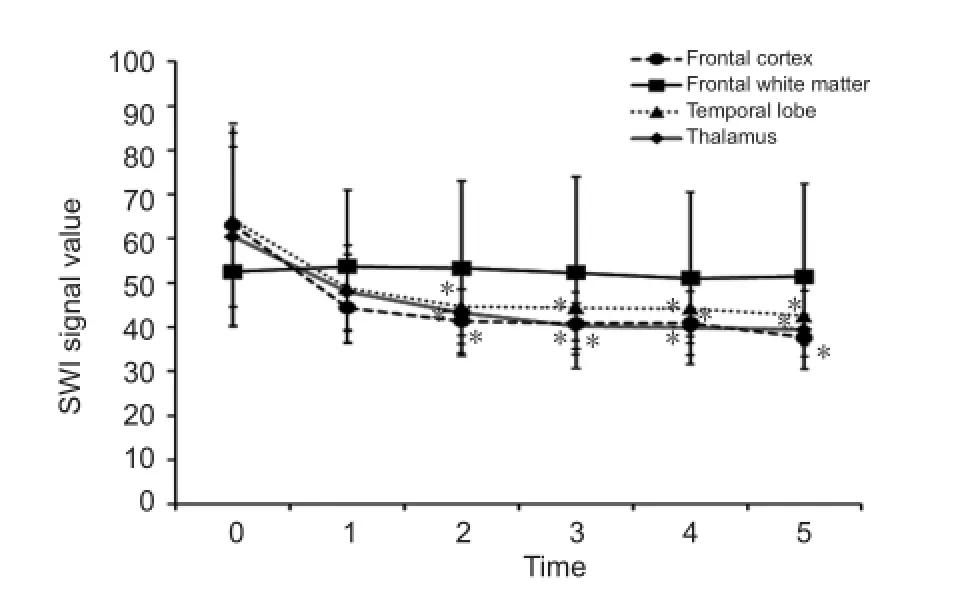
Figure 3 Effects of repeated bloodletting on the susceptibility-weighted imaging (SWI) signal values of selected regions of the rabbit brain.
Jun Xia1, Ni Xie2, Anyu Yin1, Guozhao Teng3, Fan Lin1, Yi Lei1
1 Department of Radiology, Second People’s Hospital of Shenzhen City, First Affiliated Hospital of Shenzhen University, Shenzhen, Guangdong Province, China
2 Biobank, Second People’s Hospital of Shenzhen City, First A ffi liated Hospital of Shenzhen University, Shenzhen, Guangdong Province, China
3 Medical Record and Statistics Room, Second People’s Hospital of Shenzhen City, First A ffi liated Hospital of Shenzhen University, Shenzhen, Guangdong Province, China
Jun Xia and Ni Xie contributed equally to this article.
Shenzhen University, Shenzhen 518000, Guangdong Province, China,
xiajun2003sz@aliyun.com.
Funding: This study was supported by the Science and Technology Project of Shenzhen, No. JCY20120613170958482; the First Affiliated Hospital of Shenzhen University Breeding Program, No. 2012015.
Author contributions: Xia J wrote the manuscript. All authors participated in experimental design, performance and evaluation and approvedthe final version of this paper.
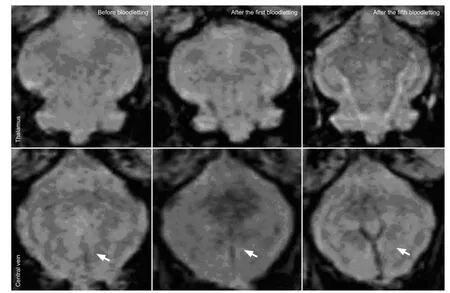
Figure 4 Susceptibility-weighted imaging (SWI) minimum-intensity projection images of the rabbit thalamus and cerebral veins obtained by magnetically-sensitive scanning after bloodletting.
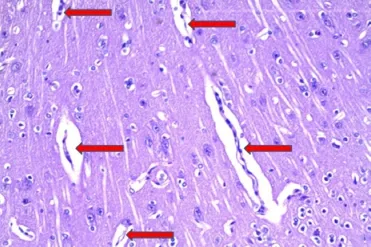
Figure 5 Pathological changes in rabbit brain tissue after the fi fth bloodletting (hematoxylin-eosin staining, × 100).
Allmendinger AM, Tang ER, Lui YW, Spektor V (2012) Imaging of stroke: Part 1, Perfusion CT--overview of imaging technique, interpretation pearls, and common pitfalls. AJR Am J Roentgenol 198:52-62.
Li M, Hu J, Miao Y, Shen H, Tao D, Yang Z, Li Q, Xuan SY, Raza W, Alzubaidi S, Haacke EM (2013) In vivo measurement of oxygenation changes after stroke using susceptibility weighted imaging fi ltered phase data. PloS One 8:e63013.
Morimoto Y, Mathru M, Martinez-Tica JF, Zornow MH (2001) Effects of profound anemia on brain tissue oxygen tension, carbon dioxide tension, and pH in rabbits. J Neurosurg Anesthesiol 13:33-39.
Murkin JM, Arango M (2009) Near-infrared spectroscopy as an index of brain and tissue oxygenation. Br J Anaesth 103 Suppl 1:i3-13.
Navarro LH, Lima RM, Khan M, Dominguez WG, Voigt RB, Kinsky MP, Mileski WJ, Kramer GC (2012) Continuous measurement of cerebral oxygen saturation (rSO(2)) for assessment of cardiovascular status during hemorrhagic shock in a swine model. J Trauma Acute Care Surg 73:S140-146.
Purkayastha S, Sorond F (2012) Transcranial Doppler ultrasound: technique and application. Semin Neurol 32:411-420.
Yamazaki K, Suzuki K, Itoh H, Muramatsu K, Nagahashi K, Tamura N, Uchida T, Sugihara K, Maeda H, Kanayama N (2013) Cerebral oxygen saturation evaluated by near-infrared time-resolved spectroscopy (TRS) in pregnant women during caesarean section-a promising new method of maternal monitoring. Clin Physiol Funct Imaging 33:109-116.
Copyedited by Phillips A, Yu J, Qiu Y, Li CH, Song LP, Zhao M
Jun Xia, M.D., Department of Radiology, Second People’s Hospital of Shenzhen City, First Affiliated Hospital of
10.4103/1673-5374.133153 http://www.nrronline.org/
Accepted: 2014-04-14
Xia J, Xie N, Yin AY, Teng GZ, Lin F, Lei Y. Susceptibility-weighted imaging is suitable for evaluating signal strength in different brain regions of a rabbit model of acute hemorrhagic anemia. Neural Regen Res. 2014;9(9):990-992.
- 中國神經再生研究(英文版)的其它文章
- Age-related changes of lateral ventricular width and periventricular white matter in the human brain: a diffusion tensor imaging study
- Sequential expression of cyclooxygenase-2, glutamate receptor-2, and platelet activating factor receptor in rat hippocampal neurons after fl uid percussion injury
- The apparent diffusion coef fi cient does not re fl ect cytotoxic edema on the uninjured side after traumatic brain injury
- Acupuncture and moxibustion reduces neuronal edema in Alzheimer’s disease rats
- Neuropeptide Y protects cerebral cortical neurons by regulating microglial immune function
- Protective effect of alpha-synuclein knockdown on methamphetamine-induced neurotoxicity in dopaminergic neurons

