Sequential expression of cyclooxygenase-2, glutamate receptor-2, and platelet activating factor receptor in rat hippocampal neurons after fl uid percussion injury
Zhiqiang Li, Qingming Shu, Lingzhi Li, Maolin Ge, Yongliang Zhang
1 Second Department of Medicine, Inner Mongolia Corps Hospital, Chinese People’s Armed Police Forces, Huhhot, Inner Mongolia Autonomous Region, China
2 Department of Pathology, General Hospital of Chinese People’s Armed Police Forces, Beijing, China
3 Section of Pharmaceutical Chemistry, Department of Rescue Medicine, Logistics University of Chinese People’s Armed Police Force, Tianjin, China
4 Second Department of Surgery, Inner Mongolia Corps Hospital, Chinese People’s Armed Police Forces, Huhhot, Inner Mongolia Autonomous Region, China
5 Training Department, Logistics University of Chinese People’s Armed Police Force, Tianjin Key Laboratory for Biomarkers of Occupational and Environmental Hazard, Tianjin, China
Sequential expression of cyclooxygenase-2, glutamate receptor-2, and platelet activating factor receptor in rat hippocampal neurons after fl uid percussion injury
Zhiqiang Li1, Qingming Shu2, Lingzhi Li3, Maolin Ge4, Yongliang Zhang5
1 Second Department of Medicine, Inner Mongolia Corps Hospital, Chinese People’s Armed Police Forces, Huhhot, Inner Mongolia Autonomous Region, China
2 Department of Pathology, General Hospital of Chinese People’s Armed Police Forces, Beijing, China
3 Section of Pharmaceutical Chemistry, Department of Rescue Medicine, Logistics University of Chinese People’s Armed Police Force, Tianjin, China
4 Second Department of Surgery, Inner Mongolia Corps Hospital, Chinese People’s Armed Police Forces, Huhhot, Inner Mongolia Autonomous Region, China
5 Training Department, Logistics University of Chinese People’s Armed Police Force, Tianjin Key Laboratory for Biomarkers of Occupational and Environmental Hazard, Tianjin, China
Zhiqiang Li and Qingming Shu
contributed equally to this work.
Yongliang Zhang, M.D., Training
Department, Logistics University of
Chinese People’s Armed Police Force,
Tianjin Key Laboratory for Biomarkers of Occupational and Environmental Hazard, Tianjin 300300, China,
zhang78127@tom.com.
Traumatic brain injury causes gene expression changes in different brain regions. Occurrence and development of traumatic brain injury are closely related, involving expression of three factors, namely cyclooxygenase-2, glutamate receptor-2, and platelet activating factor receptor. However, little is known about the correlation of these three factors and brain neuronal injury. In this study, primary cultured rat hippocampal neurons were subjected to fluid percussion injury according to Scott’s method, with some modi fi cations. RT-PCR and semi-quantitative immunocytochemical staining was used to measure the expression levels of cyclooxygenase-2, glutamate receptor-2, and platelet activating factor receptor. Our results found that cyclooxygenase-2 expression were fi rstly increased post-injury, and then decreased. Both mRNA and protein expression levels reached peaks at 8 and 12 hours post-injury, respectively. Similar sequential changes in glutamate receptor 2 were observed, with highest levels mRNA and protein expression at 8 and 12 hours post-injury respectively. On the contrary, the expressions of platelet activating factor receptor were fi rstly decreased post-injury, and then increased. Both mRNA and protein expression levels reached the lowest levels at 8 and 12 hours post-injury, respectively. Totally, our fi ndings suggest that these three factors are involved in occurrence and development of hippocampal neuronal injury.
nerve regeneration; brain injury; platelet activating factor; cyclooxygenase-2; RT-PCR; immunocytochemistry; hippocampus; platelet activating factor receptor; glutamate receptor 2; NSFC grant; neural regeneration
Funding: This study was supported by the National Natural Science Foundation of China, No. 30471934.
Li ZQ, Shu QM, Li LZ, Ge ML, Zhang YL. Sequential expression of cyclooxygenase-2, glutamate receptor-2, and platelet activating factor receptor in rat hippocampal neurons after fluid percussion injury. Neural Regen Res. 2014;9(9):978-985.
Introduction
Brain injury induces a sequence of gene expression that includes cyclooxygenase-2 (COX-2), glutamate receptor 2 (GluR2), and platelet activating factor receptor (PAFR). These three factors are closely related and play a synergic role (Mukherjee et al., 1999). To date, few investigations have focused directly on the correlation between these three factors and hippocampal neurons. Platelet activating factor is an important active mediator in brain injury pathology, triggering and directly involved in pathological impairment of brain tissue. Increasing evidence suggests that the pathophysiological effects of platelet activating factor are mediated by intracellular signal transduction after binding to PAFR (Pulliam et al., 1998). In addition, platelet activating factor production is accompanied simultaneously by arachidonic acid and its metabolites, especially generation and release of epoxide-dependent thromboxane A2 and prostaglandin I2. Under normal physiological conditions, thromboxane A2 and prostaglandin I2 are well balanced, allowing maintenance of normal vascular tone and blood vessel patency. Disordered balance between thromboxane A2 and prostaglandin I2 causes vascular spasms and occlusion (Bulger et al., 2000). Platelet activating factor also promotes glutamate excitotoxicity (Aihara et al., 2000).
Growing experimental evidence shows that brain injury leads to increased COX-2 mRNA, protein, and reaction products, and COX-2 metabolites may aggravate brain injury and brain neuronal damage via the vasculature (Busija et al., 1996; Domoki et al., 1999). These findings highlight the contribution of COX-2 to brain injury. GluR2 mediates sodium, water, and chloride in fl ux after brain injury, leading to neuronal excitotoxicity and deteriorating brain damage (Iversen et al., 1994; Pellegrini-Giampietro et al., 1994; Essin et al., 2002).
Overall, these studies suggest that PAFR, GluR2, and COX-2 play crucial roles in brain injury pathogenesis, and potentially interact with one another to promote development of brain injury. In this study, we used primary cultured hippocampal neurons to establish a fl uid percussion injury model, and investigated sequential changes in PAFR, COX-2, and GluR2 mRNA and protein expression levels following neuronal injury.
Materials and Methods
Animals
Forty healthy Sprague-Dawley rats within 24 hours of birth (weight 5-7 g) were provided by the Experimental Animal Center of the Academy of Military Medical Sciences, China (license No. SCXK (Army) 2006-001).
Primary cultures of rat hippocampal neurons
For cell growth observation, coverslips were divided into four small pieces and placed in petri dishes (Falcon 60 mm), with three coverslips per dish. To facilitate cell growth, 24 hours before neuronal harvesting, petri dishes were covered with 10 μg/mL polylysine and incubated overnight at 37°C. The next day, petri dishes were rinsed three times with D-Hank’s solution. Sprague-Dawley rats were immersed in 75% ethanol and disinfected. Brain tissue was harvested by decapitation, and the hippocampus isolated and placed in D-Hank’s solution. Hippocampal tissue was cut into pieces and repeatedly pipetted into tiny blocks before filtering through a 200 mesh filter to prepare cell suspensions. One drop of cell suspension was mixed with one drop of 0.04% trypan blue (Gibco, Grand Island, NY, USA) and counted by light microscopy. The cell suspension was diluted using DMEM-F12 medium to a concentration of 2-4 × 105cells/mL, and then 10% fetal bovine serum (Institute of Hematology, Chinese Academy of Medical Sciences, Tianjin, China) and 1% penicillin-streptomycin added. Cells were incubated at 37°C in a 5% CO2incubator (Forma 3111; Thermo Scienti fi c, CA, USA) for 48 hours. After removing fetal bovine serum, culture medium with 5% N2additives (Gibco), 2.5% glucose and 1% penicillin-streptomycin was added. Half the culture medium was changed every 3-4 days, and cells were cultured for 7-10 days.
Grouping and intervention
Experimental rats were randomly divided into control and fi ve injury (4, 8, 12, 24, and 48 hours after traumatic brain injury) groups. Hippocampal neurons in the control group were cultured normally, without fluid percussion injury. The injury groups were subjected to fl uid percussion injury and RT-PCR or immunocytochemistry performed at corresponding time points post-injury.
Fluid percussion injury model established using a modi fi ed Scott’s method
Based on a previously described method (Shepard et al., 1991), we established a fluid percussion injury model in nerve cells using a modi fi ed injury device, which consists of a stainless steel cell injury chamber, a plexiglass cylinder, a pressure transducer, a pendulum frame, and an oscilloscope (Figure 1). The plexiglass cylinder connects to the piston at one end, and to the injury chamber and pressure transducer at the other end. The cylinder is filled with 37°C saline during the injury process. When the pendulum hammer is lifted to a certain height, the falling weight hits the piston and pushes the fl uid forward, leading to damage to cells cultured in dishes. This model was con fi rmed a success in preliminary studies (He et al., 2010). In brief, after discarding culture medium, cells in petri dishes were digested with 75% ethanol, placed in the injury chamber containing D-Hank’s solution, and tightly covered. Next, the pendulum hammer was lifted to a 25° angle, de fi ning an impact force of 0.1 MPa. Subsequently, the culture medium was replenished and cells cultured in an incubator for RT-PCR or immunohistochemistry analysis.
Immunocytochemistry using the SP method
Neuronal identification
On day 7 after culture, coverslips were removed from petri dishes, placed into 24-well plates, fixed in 4% paraformaldehyde for 4 hours, and rinsed 3 times for 5 minutes with 0.01 mol/L PBS. Coverslips were then incubated with 3% H2O2at room temperature for 20 minutes to eliminate endogenous peroxidases, washed with PBS, and incubated with 0.3% Triton X-100 at 37°C for 30 minutes. Subsequently, cells were rinsed with PBS, blocked with 10% horse serum at room temperature for 30 minutes, and then incubated overnight at 37°C with mouse anti-rat microtubule-associated protein-2 (1:100) and glial fi brillary acidic protein (1:400) monoclonal antibodies (Fuzhou Maxin Biotechnology Company, Fuzhou, Fujian Province, China). After recovery at room temperature for 15-20 minutes and washing with 0.01 mol/L PBS, coverslips were incubated with biotinylated horse anti-mouse IgG (1:200; Beijing Zhongshan Biotechnology Co., Ltd., Beijing, China) at 37°C for 30 minutes, then incubated with SP complex (1:200) for 30 minutes, and developed with DAB for 2-3 minutes. Between steps, cells were rinsed 3 times for 5 minutes with 0.01 mol/L PBS. After terminating DAB coloration with PBS, coverslips were subjected to ethanol dehydration, xylene transparency, and mounting. PBS was used as the primary antibody for negative controls. The number of microtubule-associated protein-2- and glial fibrillary acidic protein-positive cells was calculated, and the percentage of positive cells to total cells was measured as the puri fi cation rate of neuronal cells.Immunocytochemistry detection of PAFR, COX-2, and GluR2 Cells were blocked with 10% rabbit serum at room temperature and incubated overnight at 37°C with the following antibodies: goat anti-PAFR, COX-2, and GluR2 polyclonal antibodies (1:200; Santa Cruz Biotechnology, Santa Cruz, CA, USA), followed by biotinylated goat anti-goat IgG (1:200; Beijing Zhongshan Biotechnology Co., Ltd.) for 30 minutes at 37°C. The rest of the procedure is the same as for microtubule-associated protein-2 and glial fi brillary acidic protein immunocytochemistry.
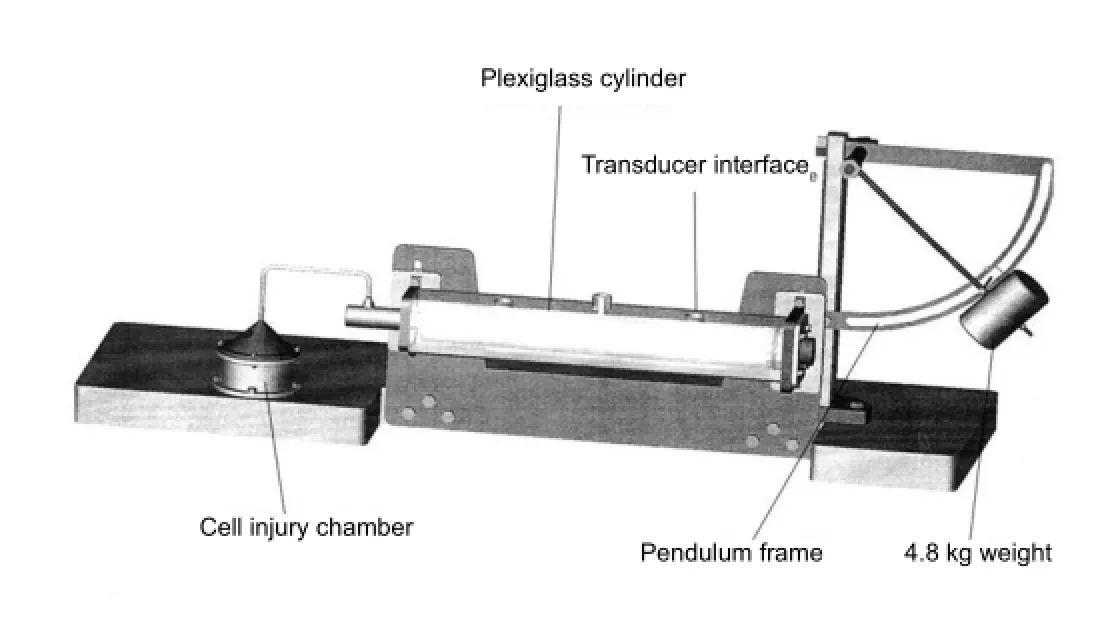
Figure 1 Scott’s model of fl uid pressure injury.
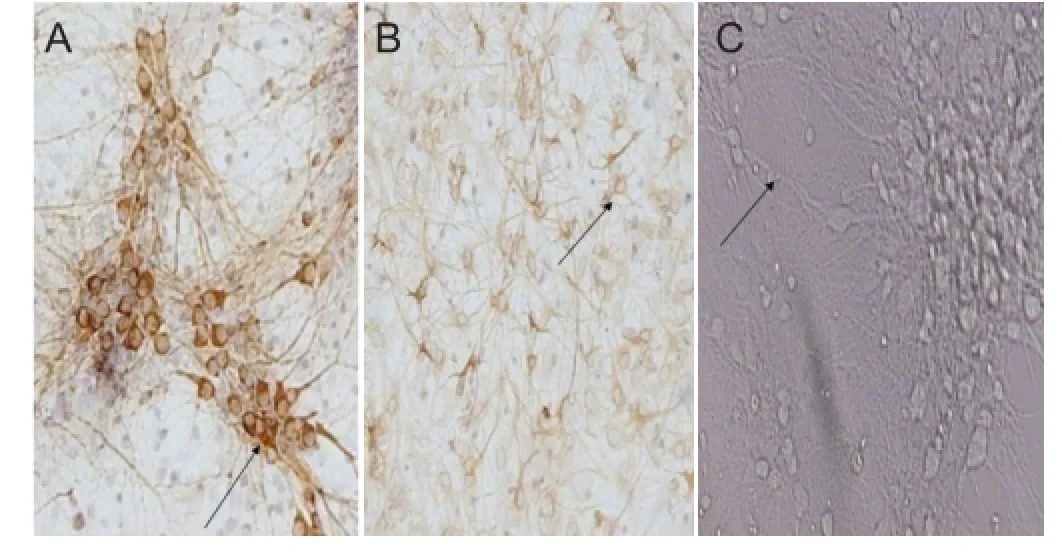
Figure 2 Morphology of rat hippocampal neurons at 7 days of culture.
Quantitative image analysis
Images were quantitatively analyzed using the Mias2000 analysis system (Sichuan University, Chengdu, Sichuan Province, China). Images of hippocampal neurons were collected using a light microscope (Olympus BX-51, Tokyo, Japan) under the same light intensity at 400 × magni fi cation. Immunoreactive areas of 100 cells (μm2) were calculated from each slide.
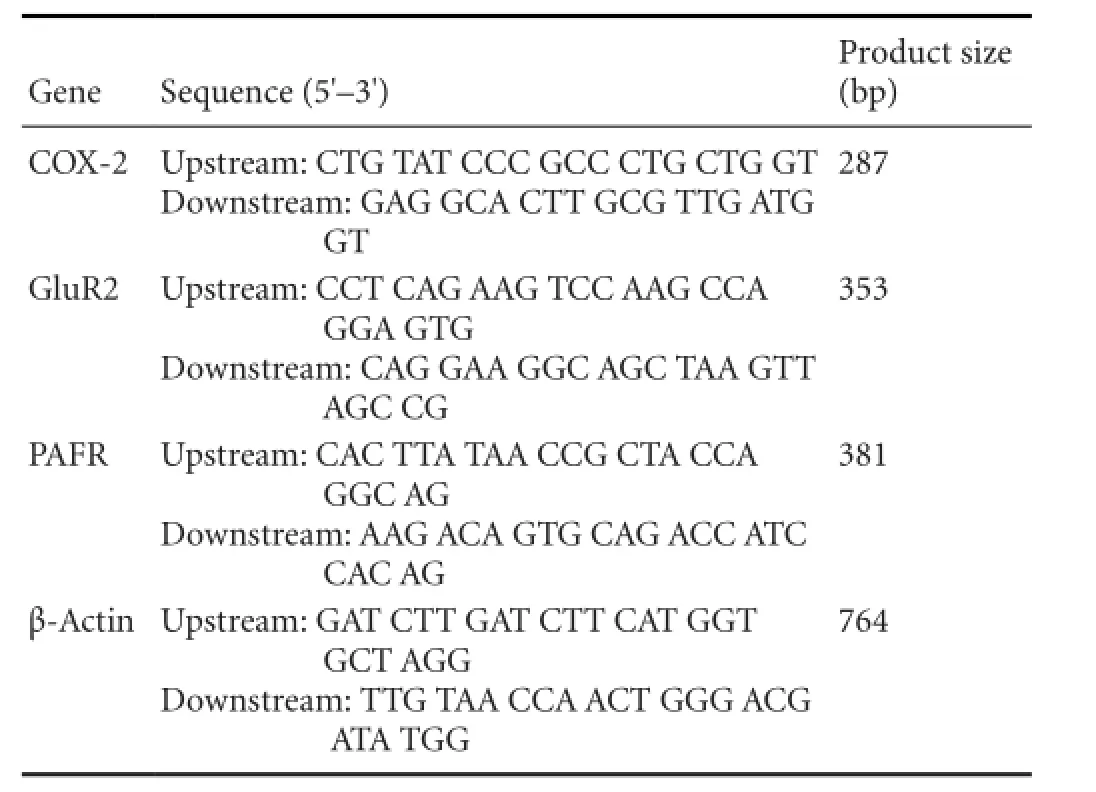
Table 1 PCR primers
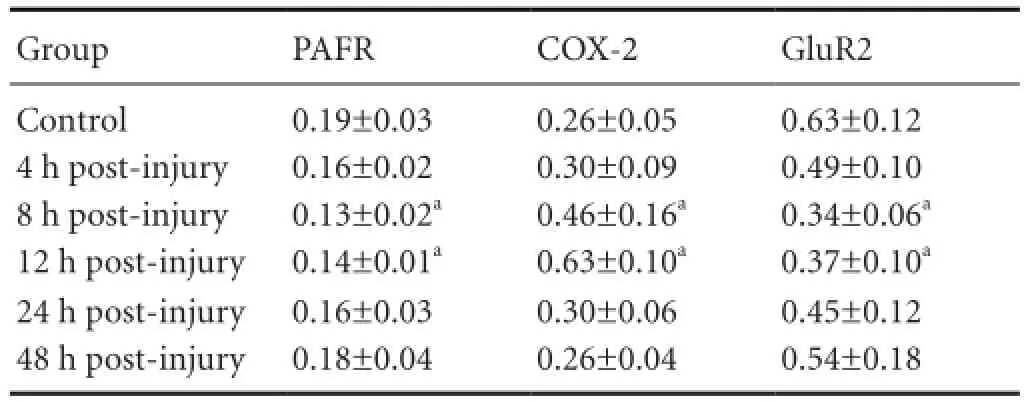
Table 2 Relative expression (/β-actin) of PAFR, COX-2, and GluR2 gene in primary cultured rat hippocampal neurons after injury
RT-PCR detection
RNA extraction
Total RNA was extracted from primary cultured rat hippocampal neurons using TRIzol reagent (Sigma, St. Louis, MO, USA), and stored at -80°C.
Reverse transcription
Extracted RNA was transcribed to cDNA using a RT-PCR kit (Promega, Madison, WI, USA).
PCR amplification
PCR master mix (50 μL) consisted of 5 × buffer (10 μL), TaKaRa Taq (0.25 μL), sterile distilled water (28.75 μL), primers (Table 1; 0.25 μL), and cDNA template (5 μL). Ampli fi cation was performed using a PCR instrument (PT-0200; MJ Research, Waltham, MA, USA), with the following conditions: an initial denaturation at 94°C for 2 minutes, then 30 cycles of 94°C for 30 seconds, primer annealing at appropriate temperatures (PAFR, 60°C; COX-2, 55°C; and GluR2, 58°C) for 30 seconds, and extension at 72°C for 30 seconds, with a fi nal extension at 72°C for 7 minutes.
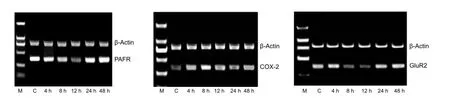
Figure 3 PAFR (381 bp), COX-2 (287 bp), and GluR2 (352 bp) mRNA expression detected by RT-PCR in hippocampal neurons at different injury time points.
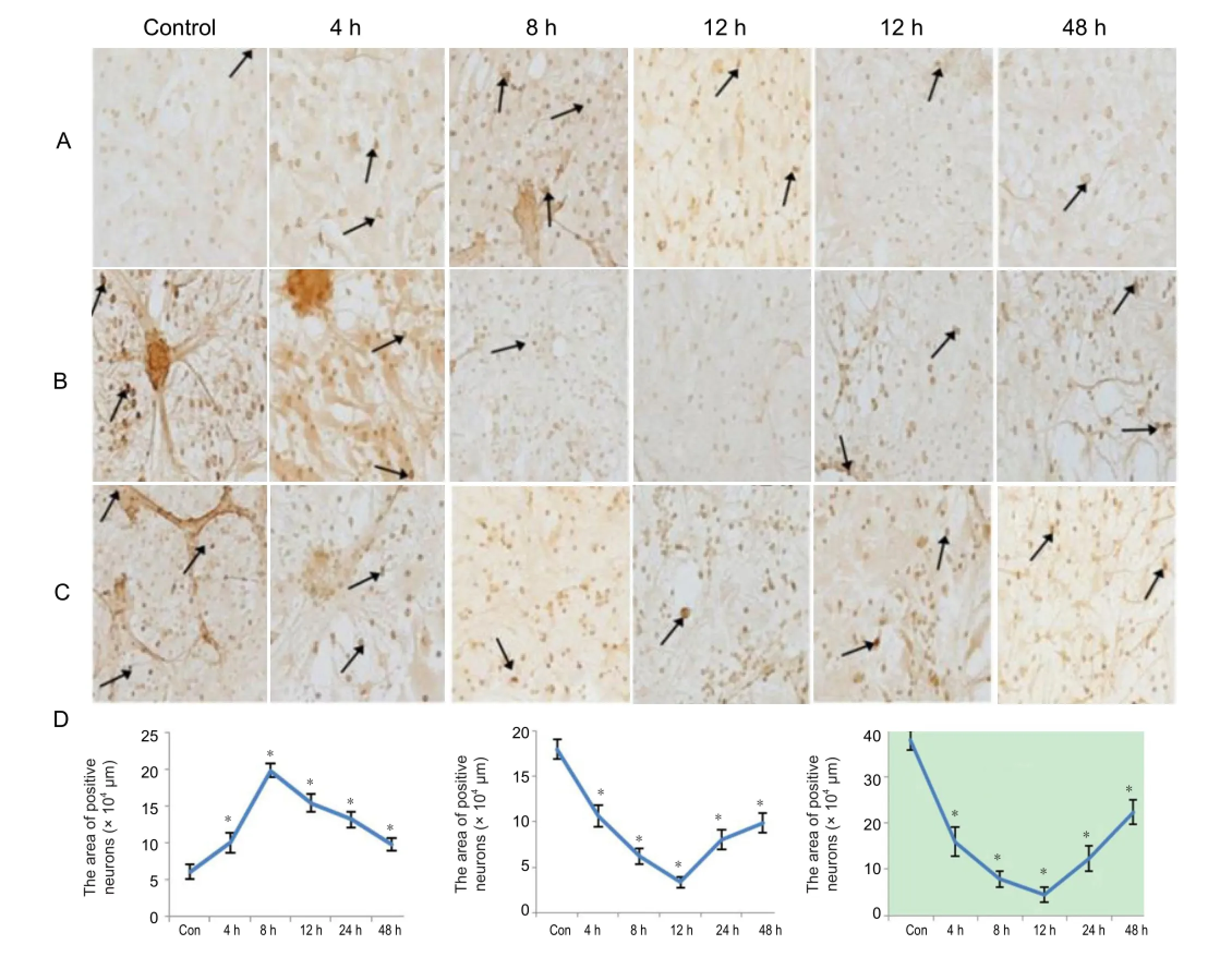
Figure 4 Immunohistochemical staining of COX-2, GluR2, and PAFR in hippocampal neurons 4-48 hours after injury.
Agarose gel electrophoresis
PCR products (5 μL) were electrophoresed and photographed under ultraviolet light. Absorbance was measured with β-actin serving as a reference. Absorbance values of ampli fi ed bands were obtained using a gel imaging analysis system (GelPro 4.5; Media Cybernetics, Silver Spring, MD, USA). Ratios of absorbance values for PAFR, COX-2, and GluR2 ampli fi ed bands (Af) to the absorbance value for the β-actin ampli fi ed band (Ab) were calculated as the relative content of RT-PCR products.
Statistical analysis
Measurement data were expressed as mean ± SD and analyzed using SPSS 11.5 software (SPSS, Chicago, IL, USA). Differences between groups were compared using one-way analysis of variance and least significant difference tests. A P < 0.05 value was considered statistically significant.
Results
Morphology of hippocampal neurons
After in vitro culture for 2-4 hours, hippocampal neurons began to adhere and were scattered or grew in clusters. Adherent cells were round, small, and stereoscopic, with a visible halo. At 24 hours, some neurons extended processes. At 48 hours, N2was added to the culture medium and non-adherent cells removed. The cultured neurons became larger, with granular substances in cell bodies, a clearly visible halo around cells, and ingrowth of processes. At 3 days, the majority of neurons extended processes and adjacent cells also exhibited fiber connections. Cultured neurons in clusters began to migrate towards peripheral tissue. At 5-7 days, the cells were most vital, exhibiting strong refraction and nuclei located in the center or to one side of the cells, with the nucleus and nucleolus clearly visible. Neurites were thick and formed a network.
Identi fi cation of rat hippocampal neurons
Microtubule-associated protein-2 is a neuronal specific marker, and after 7 days of culture was used to identify neurons. Rat hippocampal neurons were microtubule-associated protein-2 positive, with positive substance stained brown and located in the cytoplasm and dendrites. No microtubule-associated protein-2 expression was found in glial cells. The percentage of positive cells (puri fi cation rate of cultured neurons) was ≤ 75%. Glial fibrillary acidic protein is an astrocyte specific marker, and was ubiquitously expressed in glial cells, with positive substance stained brownish yellow and located in the cytoskeleton. Minimal glial fi brillary acidic protein expression was found in neurons, speci fi cally, primitive immature neurons (Figure 2).
COX-2, GluR2, and PAFR mRNA expression in rat hippocampal neurons
COX-2 mRNA expression
COX-2 mRNA expression was relatively low in the control group, although expression levels gradually increased along with time post-injury and reached a peak at 12 hours before decreasing. COX-2 mRNA expression in the post-injury 8 and 12 hour groups was higher than controls (P < 0.05;Table 2, Figure 3).
GluR2 and PAFR mRNA expression
GluR2 and PAFR mRNA expression was relatively high in the control group, with expression levels gradually decreasing at 4 hours post-injury, reaching a lowest level at 8 hours before increasing. GluR2 and PAFR mRNA expression in the post-injury 8 and 12 hour groups was signi fi cantly lower than in controls (P < 0.05;Table 2, Figure 3).
COX-2, GluR2, and PAFR protein expression in rat hippocampal neurons
COX-2 immunocytochemical staining results
In the control group, a small number of COX-2 positive cells were hippocampal neurons with brown positive substance located in the cytoplasm and nuclear membrane. COX-2 was strongly expressed in the nuclear membrane, but negatively expressed within nuclei. The cytoplasm was slightly stained, and twisted and spiral dendrites and axons grew in some cells. Staining was weakest in the control group and COX-2 immunoreactivity increased in the injury groups, reaching a peak at 8 hours. Neuronal swelling and rupture, cytoplasmic leakage, neurite breakage, and cell shrinkage were visible after injury. Subsequently, darkly stained cytoplasm, nuclear condensation, and increased cell debris were observed. The degree of staining was attenuated with reduced COX-2 positive cells, albeit with greater numbers than in the control group.
A small number of COX-2 immunoreactive cells were expressed in the control group, with cell numbers increasing after injury. The number of COX-2 immunoreactive cells in hippocampal neurons after injury was higher than the control group (P < 0.01), reaching a peak at 8 hours (Figure 4A, D).
GluR2 immunocytochemical staining results
GluR2 immunoreactive cells were strongly and widely expressed in the control group. Positive substance was brown and located in the cytoplasm, which showed strong expression, while no nuclear expression was found. Some neurons extended positive, short processes. In the injury groups, immunoreactive expression of neurons decreased, and GluR2 content in positive cells also decreased. At 4 hours post-injury, neuronal cell bodies were swollen and connections between neuronal processes interrupted. Subsequently, cell bodies ruptured, a large amount of cell debris were visible, and neuronal processes exhibited fracture. Residual cells began to shrink, with darkly stained cytoplasm and pyknotic nuclei. The number of GluR2 positive cells was signi fi cantly reduced at 48 hours because of neuronal death.
A large number of GluR2 immunoreactive cells were expressed in the control group, with a reduced number after injury. The number of neuronal GluR2 immunoreactive cells after injury was significantly lower than the control group (P < 0.01), with a lowest level at 12 hours (Figure 4B, D).
PAFR immunocytochemical staining results
In the control group, PAFR was widely expressed in hippo-campal neurons, with brown positive substance located in the cytoplasm and nucleus. After injury, neuronal cell bodies were swollen, intercellular connections interrupted, and the cytoplasm irregularly and lightly stained, showing nuclear enlargement or disappearance, cell body rupture, and cell debris. Subsequently, cell shrinkage, darkly stained cytoplasm, pyknotic nuclei, and cell debris were clearly visible. The number of PAFR positive cells was reduced at 48 hours because of neuronal death.
PAFR immunoreactive cells were widely expressed in the control group, while the number reduced after injury. Expression of PAFR immunoreactive cells in hippocampal neurons after injury was lower than the control group (P < 0.01), with a lowest level at 12 hours post-injury (Figure 4C, D).
Discussion
Increasing evidence from proteomic studies of traumatic brain injury (Blain et al., 2004; Conti et al., 2004; Siman et al., 2004; Sironi et al., 2004; Ding et al., 2006; Glanzer et al., 2007; Crecelius et al., 2008) has provided novel pathways for clinical treatment, and been bene fi cial for improving the quality of life among patients. Preliminary studies from our research group using the SELDI-TOF MS technique, have detected protein expression profiles in serum and hippocampus of rats with closed traumatic brain injury (Shu et al., 2005; Zhan et al., 2007; Shu et al., 2010). Our results indicate that brain injury simultaneously causes alterations of protein expression profiling in serum and hippocampus. Although the serum and hippocampal protein expression pro fi les are different, it is still possible to detect the same protein peaks in two samples using the same chip. We speculate that during occurrence and development of brain injury, cellular protein changes can re fl ect serum changes. After injury, some hippocampal protein changes precede those in serum, and vice versa. A possible explanation for this discrepancy is destruction of the blood-brain barrier, and then increased permeability, inflammation, immune response, and oxidation reactions (Hellal et al., 2004; Nimmo et al., 2004; Grond-Ginsbach et al., 2008).
Establishment of a brain injury model allows further investigation of organ functions and related treatments (Wasinger et al., 1995; Tao et al., 2004). The cellular mechanism of brain injury has been the subject of intense investigations. In this study, we applied Scott’s method, with some modi fi cations, for producing fl uid percussion injury in nerve cells, to examine changes and the underlying cellular mechanisms after moderate neuronal injury caused by a 0.2 MPa impact.
COX-2 cannot be detected in most normal tissues and its expression is induced by a variety of physiological stimuli (Subbaramaiah et al., 1997). In the adult rat brain, COX-2 is mainly distributed in the hippocampus and temporal cortex, which are related because of the high densities of excitatory amino acid receptors in both (Kulmacz et al., 1995). After traumatic brain injury caused by fluid percussion, Dash et al. (2000) found increased COX-2 protein levels in the rat hippocampus at 3 hours, which reached a peak at 24 hours. Similarly, using RNA probe hybridization studies, Strauss et al. (2000) found that hippocampal COX-2 mRNA expression reached a peak at 6 hours before decreasing after brain injury caused by local impact. In previous studies from our research group, we duplicated Marmarou’s closed head injury model in rats (Xiong et al., 2013) and using RT-PCR found that hippocampal COX-2 mRNA expression begins to increase at 4 hours after brain injury, reaching a peak at 8 hours before gradually declining, but is still higher than the control group at 48 hours (Yu et al., 2008). These experimental findings suggest that COX-2 gene transcription and protein expression levels sharply increase and then gradually decrease in the rat hippocampus after fl uid percussion injury.
Glutamate is the major excitatory neurotransmitter of the central nervous system. AMPA receptors mediate fast excitatory synaptic transmission, and are composed of a central hydrophilic channel surrounded by GluR1, GluR2, GluR3, and GluR4 subunits that form a dimer structure. AMPA receptor permeability to Ca2+is controlled by GluR2 (Bettler et al., 1995; Seeburg et al., 1998). Once injury occurs, GluR2 is reduced, leading to formation of GluR1- or GluR3-dominant ion channels that mediate quick Ca2+in fl ux and thereby cause nerve cell damage. Using Marmarou’s closed head injury model in rats, we found by RT-PCR that hippocampal GluR2 mRNA expression begins to decrease at 4 hours after brain injury, reaching a lowest level at 12 hours before gradually increasing, yet is still lower than the control group at 48 hours (Strauss et al., 2000). These experimental fi ndings suggest that GluR2 gene transcription and protein expression levels in hippocampal neurons decrease rapidly and then gradually increase, consistent with our current results. Moreover, changes in protein expression levels occurred after changes in gene transcription.
Platelet activating factor is a membrane phospholipid metabolite. Platelet activating factor generation increases during various wounds, and nerve cells may release platelet activating factor after stimulation. Cerebral ischemia causes a sharp increase in platelet activating factor and decreased cortical PAFR gene expression levels, suggesting PAFR synthesis within the cerebral ischemic area gradually reduces, resulting in decreased receptor levels. Using a rat model of focal ischemia-reperfusion injury, Zhang et al. (1995) found that PAFR gene expression levels significantly decreased, reaching lowest levels at 48 hours for 5 days. Following cerebral ischemia, reduced PAFR mRNA levels may be a reactive negative feedback regulation (Stengel et al., 1997). The main mechanism of decreased PAFR expression is mediated by intracellular migration of PAFR, which are gradually degraded by lysosomes. Using Marmarou’s closed head injury model, we found by RT-PCR that hippocampal PAFR mRNA expression begins to decrease at 4 hours post-injury, reaching a lowest value at 8 hours before gradually increasing, but is still lower than normal levels at 48 hours (Zhang et al., 1995). The present study shows that PAFR mRNA expression is high in the control group, but sharply decreases and then gradually increases after injury.
Platelet activating factor transduced by its receptor can ac-tivate cytosolic PLA2 in nerve cells, and cytosolic PLA2 is the mediator for synthesis between platelet activating factor and arachidonic acid, with the metabolism of all three closely related (Farooqui et al., 1997). arachidonic acid eicosanoids are generated during platelet activating factor reproduction and synthesis, which in turn produce prostaglandins, thromboxanes, leukotrienes, and other active substances. These active substances are important factors for brain injury deterioration (Shindon et al., 2000). In nerve cells, platelet activating factor binds to high-af fi nity sites on intracellular receptors, activating the FOS/JUN/AP-1 transcription signaling system and the COX-2 gene, thereby promoting arachidonic acid metabolism (Musto et al., 2011). COX-2 can enhance the toxic effects of excitatory amino acids, and prostaglandin E2, its reaction product, is responsible for NMDA receptor-mediated neurotoxic effects that cause brain damage, potentially by increasing microglial Ca2+influx and promoting glutamate release (Li et al., 2008). Following brain injury, there is enhanced synaptic activity, GluR2 activation, increased COX-2 expression, and increased reaction products, suggesting that glutamate promotes COX-2 expression. In primary cultured neurons and microglia cells, glutamate induces platelet activating factor synthesis and release, enhancing chemotaxis of microglia cells via PAFR, and leading to brain injury deterioration (Aihara et al., 2000). Platelet activating factor also functions to promote presynaptic and postsynaptic glutamate release, and facilitates glutamate-mediated neurotransmission. The latter acts on postsynaptic NMDA and AMPA/kainate receptors, thus increasing cytosolic free Ca2+and activating the cytosolic PLA2 system, which in turn generates platelet activating factor and arachidonic acid.
In summary, PAFR, COX-2, and GluR2 jointly promote occurrence and development of hippocampal neuronal injury following brain injury.
Acknowledgments:We would like to thank Yu YY and Liu X from the Disaster Medicine Department, Logistics University of Chinese People’s Armed Police Force, China for providing technical support.
Author contributions:Zhang YL was responsible for the study design and the funds. Li ZQ completed animal modeling, preliminary experiment, cytoimmunohistochamical staining, data processing and writing the manuscript. Shu QM conducted cell culture experiment. Ge ML completed RT-PCR experiment. Li LZ evaluated and supervised the study. All authors approved the final version of the manuscript.
Con fl icts of interest:None declared.
Aihara M, Ishii S, Kume K, Shimizu T (2000) Interaction between neuron and microglia mediated by platelet-activating factor. Genes Cells 5:397-406.
Bettler B, Mulle C (1995) Neurotransmitter receptors AMPA and kainate receptors. Neuropharmacology 34:123-139.
Blain JF, Paradis E, Gaudreault SB, Champagne D, Richard D, Poirier J (2004) A role for lipoprotein lipase during synaptic remodeling in the adult mouse brain. Neurobiol Dis 15:510-519.
Bulger E, Maier R (2000) Lipid mediators in the pathophysiology of critical illness. Crit Care Med 28:27-36.
Busija DW, Thore C, Beasley T, Bari F (1996) Induction of cyclooxygenase-2 following anoxic stress in piglet cerebral arteries. Microcirculation 3:379-386.
Conti A, Sanchez-Ruiz Y, Bachi A, Beretta L, Grandi E, Beltramo M, Alessio M (2004) Proteome study of human cerebrospinal fl uid following traumatic brain injury indicates fibrin (ogen) degradation products as trauma-associated markers. J Neurotrauma 21:854-863.
Crecelius A, G?tz A, Arzberger T, Fr?hlich T, Arnold GJ, Ferrer I, Kretzschmar HA (2008) Assessing quantitative post-mortem changes in the gray matter of the human frontal cortex p roteome by 2-D DIGE. Proteomics 8:1276-1291.
Dash PK, Mach SA, and Moore AN (2000) Regional expression and role of cyclooxygenase-2 following experimental traumatic brain injury. J Neurotrauma 1:69-81.
Ding Q, Vaynman S, Souda P, Whitelegge JP, Gomez-Pinilla F (2006) Exercise affects energy metabolism and neural plasticity-related proteins in the hippocampus as revealed by proteomic analysis. Eur J Neurosci 24:1265-1276.
Domoki F, Veltkamp R, Thrikawala N, Robins G, Bari F, Louis TM, Busija DW (1999) Ischemia-reperfvsion rapidly increases Cox-2 expression in piglet cerebral arteries. Am J Physiol 277:H1207-1214.
Essin K, Nistri A, Magazanik L (2002) Evaluation of GluR2 subunit involement in AMPA receptor function neonatal rat hypoglossal motoneurons. Eur J Neurosci 15:1899-1906.
Farooqui AA, Yang HC, Rosenberger TA, Horrocks LA (1997) Phospholipase A2 and its role in brain tissue. J Neurochem 69:889-901.
Glanzer JG, Enose Y, Wang T, Kadiu I, Gong N, Rozek W, Liu J, Schlautman JD, Ciborowski PS, Thomas MP, Gendelman HE (2007) Genomic and proteomic microglial pro fi ling: Pathways for neuroprotective in fl ammatory responses following nerve fragment clearance and activation. J Neurochem 102:627-645.
Grond-Ginsbach C, Hummel M, Wiest T, Horstmann S, P fl eger K, Hergenhahn M, Hollstein M, Mansmann U, Grau AJ, Wagner S (2008) Gene expression in human peripheral blood mononuclear cells upon acute ischemic stroke. J Neurol 255:723-731.
He T, Yang SW, Gup Y, Zhao ZK, Chen XY, Zhang YL (2010) The study in primary cultured astrocytes following fluid percussion injury. Chin J Appl Physiol 26:46-50.
Hellal F, Bonnefont-Rousselot D, Croci N, Palmier B, Plotkine M, Marchand-Verrecchia C (2004) Pattern of cerebral edema and hemorrhage in a mice model of diffuse brain injury. Neurosci Lett 357: 21-24.
Iversen L, Mulvihill E, Haldeman B, Diemer NH, Kaiser F, Sheardown M, Kristensen P (1994) Changes in metabotroptic glutamate receptor mRNA levels following global ischemia: increase of a putative presynaptic subtype (mGluR4) in highly vnlnerable rat brain areas. J Neurochem 63:625-633.
Kulmacz RJ, Wang LH (1995) Comparision of hydriperoxide inhibitor requirements for the cyclooxygenase activities of prostaglandin H syntheses-1 and -2. J Biol chem 270:24019-240231.
Li W, Wu S, Hickey RW, Rose ME, Chen J, Graham SH (2008) Neuronal cyclooxygenase-2 activity and prostaglandins PGE2, PGD2, and PGF2 alpha exacerbate hypoxic neuronal injury in neuron-enriched primary culture. Neurochem Res 33:490-499.
Mukherjee PK, DeCoster MA, Campbell FZ, Davis RJ, Bazan NG (1999) Glutamate receptor signaling interplay modulates stress-sensitive mitogen-activated protein kinases and neuronal cell death. J Biol Chem 274:649-658.
Musto AE, Samii M (2011) Platelet-activating factor receptor antagonism targets neuroin fl ammation in experimental epilepsy. Epilepsia 52:551-561.
Nimmo AJ, Cernak I, Heath DL, Hu X, Bennett CJ, Vink R (2004) Neurogenic in fl ammation is associated with development of edema and functional de fi cits following traumatic brain injury in rats. Neuropeptides 38:40-47.
Pellegrini-Giampietro DE, Pulsinelli WA, Zukin RS (1994) NMDA and non-NMDA receptor gene expression following global brain ischemia in rat:effect of NMDA and non-NMDA receptor antagonists. J Neurochem 62:1067-1073.
Pulliam L, Zhou M, Stubblebine M, Bitler CM (1998) Differential modulation of cell death proteins in human brain cells by tumor necrosis factor alpha and platelet activating factor. J Neurosci Res 54:530-538.
Seeburg PH, Higuchi M, Sprengel R (1998) RNA editing of brain glutamatereceptor channels: mechanism and physiology. Brain Res Brain Res Rev 26:217-229.
Shepard SR, Ghajar JB, Giannuzzi R, Kupferman S, Hariri RJ (1991) Fluid percussion barotrauma chamber: a new in vitro model for traumatic brain injury. J Surgical Res 51:417-424.
Shindon H, Ishii S, Uozumi N, Shimizu T (2000) Roles of cytosolic phospholipase A2 and platelet-activating factor receptor in the Ca-induced biosynthesis of PAF. Biochem Biophys Res Common 271:812-817.
Shu QM, Li ZQ,Yang SW, Li LZ, Bai X, Zhang YL (2010) Two-dimensional gel electrophoresis and surface-enhanced laser desorption ionization-time of fl ight-mass spectrometry for detection of protein expression pro fi les in the hippocampus following closed brain injury. Neural Regen Res 5:1795-1801.
Shu QM, Zhang YL, Li LZ, He B, Li ZQ (2005) Detection of distinct proteins for brain injury rats serum using a protein chip. Zhongguo Yingyong Shenglixue Zazhi 21(3):270-273.
Siman R, McIntosh TK, Soltesz KM, Chen Z, Neumar RW, Roberts VL (2004) Proteins released from degenerating neurons are surrogate markers for acute brain damage. Neurobiol Dis 16:311-320.
Sironi L, Guerrini U, Tremoli E, Miller I, Gelosa P, Lascialfari A, Zucca I, Eberini I, Gemeiner M, Paoletti R, Gianazza E (2004) Analysis of pathological events at the onset of brain damage in stroke-prone rats: a proteomics and magnetic resonance imaging approach. J Neurosci Res 78(1):115-122.
Stengel D, Antonucci M, Arborati M, Hourton D, Griglio S, Chapman MJ, Ninio E (1997) Expression of the PAFreceptor in human monocyte - derived macrophages is downregulated byoxidized LDL: relevance to the in fl ammatory phase of atherogenesis. Artetrioscler Thromb Vasc Biol 17:954-962.
Strauss KI, Barbe MF, Marshall RM, Raghupathi R, Mehta S, Narayan RK (2000) Prolorged cyclooxygenase-2 induction in neurons and glia following traumatic brain injury in the rat. J Neurotrauma 8:695-711.
Subbaramaiah K, Zakim D, Weksler BB, Dannenberg AJ (1997) Inhibition of cyclooxygenase: a novel approach to cancer prevention. Proc Soc Exp Biol Med 216:201-210.
Tao W, Wang M, Voss ED, Cocklin RR, Smith JA, Cooper SH, Broxmeyer HE (2004) Comparative proteomic analysis of human CD34+ stem/progenitor cells and mature CD15+ myeloid cells. Stem Cells 22:1003-1014.
Wasinger VC, Cordwell SJ, Cerpa-Poljak A, Yan JX, Gooley AA, Wilkins MR, Duncan MW, Harris R, Williams KL, Humphery-Smith I (1995) Progress with gene-product mapping of the Mollicutes: Mycoplasma genitalium. Electrophoresis 16:1090-1094.
Xiong Y, Mahmood A, Chopp M (2013) Animal models of traumatic brain injury. Nat Rev Neurosci 14:128-142.
Yu YY, Yang SW, Bai J,Liu CR, Zhang YL (2008) The expression and signi fi cance of platelet activating factor receptor and correlated factors following traumatic brain injury in rats. Wujing Yixueyuan Xuebao 17:361-364.
Zhan L, Liang L, Shu QM, Yang SW, Zhang YL (2007) Distinct proteins in cortex of rats with closed traumatic brain injury detected by a WCX-2 protein chip. Neural Regen Res 2:339-343.
Zhang X, Wang W, Ruan XZ (1995) The expression of platelet activating factor receptor gene during reperfusion after focal cerebral ischemia in rats. Zhonghua Shenjingke Zazhi 5:22-24.
Copyedited by James R, Norman C, Wang J, Yang Y, Li CH, Song LP, Zhao M
10.4103/1673-5374.133151
http://www.nrronline.org/
Accepted: 2014-03-10
- 中國神經(jīng)再生研究(英文版)的其它文章
- Susceptibility-weighted imaging is suitable for evaluating signal strength in different brain regions of a rabbit model of acute hemorrhagic anemia
- Age-related changes of lateral ventricular width and periventricular white matter in the human brain: a diffusion tensor imaging study
- The apparent diffusion coef fi cient does not re fl ect cytotoxic edema on the uninjured side after traumatic brain injury
- Acupuncture and moxibustion reduces neuronal edema in Alzheimer’s disease rats
- Neuropeptide Y protects cerebral cortical neurons by regulating microglial immune function
- Protective effect of alpha-synuclein knockdown on methamphetamine-induced neurotoxicity in dopaminergic neurons

