Combining acellular nerve allografts with brainderived neurotrophic factor transfected bone marrow mesenchymal stem cells restores sciatic nerve injury better than either intervention alone
Yanru Zhang, Hui Zhang, Gechen Zhang, Ka Ka Wenhua Huang
1 School of International Education, Zhengzhou University, Zhengzhou, Henan Province, China
2 Institute of Clinical Anatomy, Southern Medical University, Guangzhou, Guangdong Province, China
3 Department of Orthopedic Surgery, First Af fi liated Hospital of Zhengzhou University, Zhengzhou, Henan Province, China
Combining acellular nerve allografts with brainderived neurotrophic factor transfected bone marrow mesenchymal stem cells restores sciatic nerve injury better than either intervention alone
Yanru Zhang1,2, Hui Zhang3, Gechen Zhang3, Ka Ka1, Wenhua Huang2
1 School of International Education, Zhengzhou University, Zhengzhou, Henan Province, China
2 Institute of Clinical Anatomy, Southern Medical University, Guangzhou, Guangdong Province, China
3 Department of Orthopedic Surgery, First Af fi liated Hospital of Zhengzhou University, Zhengzhou, Henan Province, China
In this study, we chemically extracted acellular nerve allografts from bilateral sciatic nerves, and repaired 10-mm sciatic nerve defects in rats using these grafts and brain-derived neurotrophic factor transfected bone marrow mesenchymal stem cells. Experiments were performed in three groups: the acellular nerve allograft bridging group, acellular nerve allograft + bone marrow mesenchymal stem cells group, and the acellular nerve allograft + brain-derived neurotrophic factor transfected bone marrow mesenchymal stem cells group. Results showed that at 8 weeks after bridging, sciatic functional index, triceps wet weight recovery rate, myelin thickness, and number of myelinated nerve fibers were significantly changed in the three groups. Variations were the largest in the acellular nerve allograft + brain-derived neurotrophic factor transfected bone marrow mesenchymal stem cells group compared with the other two groups. Experimental fi ndings suggest that chemically extracted acellular nerve allograft combined nerve factor and mesenchymal stem cells can promote the restoration of sciatic nerve defects. The repair effect seen is better than the single application of acellular nerve allograft or acellular nerve allograft combined mesenchymal stem cell transplantation.
nerve regeneration; peripheral nerve regeneration; peripheral nerve injury; chemically extracted acellular nerve; brain-derived neurotrophic factor; bone marrow mesenchymal stem cells; nerve tissue engineering; neural regeneration
Zhang YR, Zhang H, Zhang GC, Ka K, Huang WH. Combining acellular nerve allografts with brain-derived neurotrophic factor transfected bone marrow mesenchymal stem cells restores sciatic nerve injury better than either intervention alone. Neural Regen Res. 2014;9(20):1814-1819.
Introduction
Chemically extracted acellular nerve allografts (CEANA) can eliminate Schwann cells, myelin and disintegrating debris that can cause rejection, attenuate immune rejection after transplantation, and also help retain the nerve canal (Tuli et al., 2003; Cao et al., 2012). These canals can provide a good scaffold for the regeneration of nerve defects, and have a similar effect as autologous nerve transplantation in guiding nerve regeneration, offering a local environment for nerve recanalization (Jinbao et al., 2006). Brain-derived neurotrophic factor (BDNF) is an important neurotrophic factor for sensory and motor neurons (Zheng et al., 2009; Sun et al., 2012; Tang et al., 2012; Zhang et al., 2012). Gravel et al. (1997) found that after recombinant adenovirus vector carrying BDNF gene was injected into injured nerve in rats, the vector was expressed and secreted in Schwann cells, thus protecting the damaged motor neurons and promoting the regeneration of nerve axons. Bone marrow mesenchymal stem cells (BMSCs) have abundant sources and strong proliferative capacity (Liu et al., 2013a, b). These cells are accessible and available for culture, and their low immunogenicity help reduce or avoid rejection. Furthermore, BMSCs transplanted into lesion sites can differentiate into neuron-like cells (Gravel et al., 1997).
This study aimed to observe the effect of acellular nerve allograft combined BDNF on the repair of sciatic nerve defects in rats, in a broader attempt to create a favorable microenvironment for nerve regeneration.
Materials and Methods
Experimental animals
A total of 65 healthy male Sprague-Dawley rats, aged 2 months, weighing 200-300 g, were provided by the Experimental Animal Center of Henan Province, China (license No. 410126).
Preparation of acellular nerve allograft
After 30 rats were anesthetized with 10% chloral hydrate (1 mL/100 g), the skin at the surgical area was shaved and disinfected. Sciatic nerve (8-10 mm) was cut at the level ofthe inferior margin of piriformis muscle (Han et al., 2006), and immersed in distilled water for 13 hours. Subsequently, the harvested sciatic nerve was taken out of distilled water and oscillated in 3.0% Triton X-100 solution (consisting of 48.5 mL distilled water and 1.5 mL Triton X-100 solution) for 12 hours at room temperature (25°C). Then the nerve was transferred to 4.0% sodium deoxycholate solution (consisted of 0.03 L distilled water and 1.2 g sodium deoxycholate powder), and was shaken for 1 day. The above steps were repeated once. Then the nerve was rinsed in distilled water for 0.5 hours, extracted and immersed in 30 mL sterile PBS (pH 7.4), and stored at 4°C. After the above processing, the acellular sciatic nerve exhibited a good appearance and was sterilized for use.
Preparation of BDNF transfected BMSCs
Femoral bone marrow was isolated from five male Sprague-Dawley rats, and BMSCs were cultured to passage 3 and placed in 6-well culture plates precoated with coverslips. When the cells covered the bottom of the culture fl ask, Giemsa staining was performed. In brief, after culture liquid was removed, BMSCs were rinsed with PBS solution, fi xed with 3% methanol for 10 minutes, rinsed three times with PBS solution, and stained with Giemsa for 10 minutes, followed by 5 minutes of washing. The morphology of the stained cells was observed under an inverted phase contrast microscope (type CKX31/41; Olympus, Tokyo, Japan).
Passage 3 BMSCs were randomly divided into two groups (Barbash et al., 2003), six wells for each group. In the transfection group, 1 mL cell suspension, EDTA liquid and 12 μg pcDNA3.1-BDNF plasmid (School of Basic Medical Sciences, Zhengzhou University, Henan Province, China) were pipetted and electrically transferred in an electrical transfer glass (type MINIPACK GPII CUVETTE1652081, 4 mL, Bio-Rad Company, Hercules, CA, USA). Electrical transfer parameters: voltage 300 V, capacitance 25 μF, resistance 200 Ω, shock time 6 ms. The cells were hit by electric shock within 5 minutes and quickly placed in an ice bath, then cultured with DMEM medium containing 15% fetal bovine serum (Dongsheng Biotech Co., Ltd., Guangzhou, China), in 37°C, 5% CO2incubator for 24 hours. Subsequently, the cultured cells were electrically transferred and cultured for 21 days after DMEM medium was replenished. In the non-transfection group, no pcDNA3.1-BDNF plasmid was used and the other steps were the same as those in the transfection group. Cells cultured for 3 weeks in two groups were used for the following experiments. BDNF mRNA and protein expression level in the BMSCs was detected with reverse transcription PCR and western blot assay, respectively (Chen et al., 2004). The expression of surface antigen markers was determined using BD FACS Canto flow cytometer (Becton Dickinson, CA, USA).
Preparation of rat models of sciatic nerve defects and group management
The remaining 30 Sprague-Dawley rats were anesthetized with 10% chloral hydrate (0.4 mL/100 g) for 2.0-2.5 hours. The right sciatic nerve was exposed under sterile conditions, then 7 mm of sciatic nerve was cut 3 mm lateral to the inferior margin of the piriformis muscle, allowing natural sciatic nerve retraction. Therefore, a 10-mm sciatic nerve defect model was established. The rat models were randomly divided into three groups: the CEANA group, CEANA + BMSCs group, and the CEANA + BDNF + BMSCs group. Each group contained 10 rats. In the CEANA group, sciatic nerve defect was repaired with 10-mm acellular nerve allograft through end-to-end anastomosis, and 0.5-mL saline was injected into the tissue around the transplanted site. In the CEANA + BMSCs group, sciatic nerve defect was repaired with 10-mm acellular nerve allograft through end-to-end anastomosis, and 0.5-mL BMSC suspension (1 × 107/mL) was injected into the transplanted site. In the CEANA + BDNF + BMSCs group, sciatic nerve defect was repaired with 10-mm acellular nerve allograft through end-to-end anastomosis, and 0.5-mL suspension (1 × 107/mL) of the BDNF-transfected BMSCs was injected into the transplanted site (Zhao et al., 2010).
General condition of rats after recovery
The mental state, wound healing, muscle atrophy and ulceration at the surgical site, complications, presence of a dragging or crouching walk were observed and recorded every morning for 8 weeks.
Sciatic functional index (SFI) after recovery
At 2, 4, 6, and 8 weeks after repair, the footprints of each rat were collected according to the methods described by Nakazato et al. (2007). Three clear footprints were selected at both sides. The total spreading or toe spread, print length, and distance between intermediary toe spread were measured. SFI was calculated using the Bain formula: SFI = -38.3 (EPL - NPL/NPL) + 109.5 (ETS - NTS/NTS) + 13.3 (EIT - NIT/NIT) - 8.8. 0: normal nerve function; -100:complete loss of nerve function. EPL: Print length of experimental feet. NPL: Print length of normal feet. ETS: Toe spread of experimental feet. NTS: Toe spread of normal feet. EIT: Distance between intermediary toe spread of experimental feet. NIT: Distance between intermediary toe spread of normal feet.
Triceps wet weight recovery rate, total number of myelinated nerve fi bers and myelin thickness after recovery
At 8 weeks after repair, the bilateral triceps surae were harvested and weighed using an electronic balance (type HZT A1000, Shanghai Jia Zhan Instrumentation Equipment, Shanghai, China) after surface blood clot was removed using gauze. Weight was calculated using the following formula:triceps wet weight recovery rate = (triceps wet weight at surgical side/triceps wet weight at control side) × 100%. A 7-mm sciatic nerve was cut at the inferior border of the piriformis muscle of the operated side, 3 mm lateral to the distal anastomosis. The specimen was rinsed, fi xed with glutaraldehyde, and embedded using epoxy 812. Then the specimen was sliced into ultrathin cross-sections and counterstained with uranium-lead. Nerve regeneration was observed under transmission electron microscopy (CM120, Oxoid, Wade,UK). The myelin sheath thickness was measured using Image Pro Plus 5.0 system (Bethesda, Maryland, USA). Semithin sections of sciatic nerve specimens were stained with toluidine blue and observed under transmission electron microscopy (JEM-1200EX, Japanese Electronics Company, Tokyo, Japan), to calculate the total number of myelinated nerve fi bers and the myelin thickness.
Statistical analysis
Data were expressed as the mean ± SD and statistically analyzed using SPSS 18.0 software (SPSS, Chicago, IL, USA). The difference among groups was compared using one-way analysis of variance, and intergroup difference was compared using the Student-Newman-Keuls test. A level ofα= 0.05 was considered signi fi cantly different.
Results
Identi fi cation of subcultured BMSCs
BMSCs adhered to the wall of culture fl asks, and were round in shape with small cell bodies at 1 day after isolation and culture (Figure 1A). Following 10 days in culture, BMSCs clustered into batches and were spindle shaped with large cell bodies (Figure 1B). After 3 weeks of culture, more spindle-shaped BMSCs were seen (Figure 1C). After 4 weeks, several BMSC colonies formed and cells overlapped into clusters (Figure 1D).
The subcultured BMSCs exhibited a sparse distribution and round shape after trypsin digestion, and began to retract. The morphology of cultured cells then changed to a uniform spindled appearance. At a high density, cells were distributed in a swirling or radial arrangement. The subcultured cells had a high adherent rate and began to adhere 4 hours after culture. Subculture was repeated 3-4 days later. Subcultured BMSCs exhibited uniform morphology. Passage 3 cells proliferated and secreted extracellular matrix (Figure 2). Passage 5 cells had the potential to differentiate into fat cells and fi broblasts. Flow cytometry results showed the expression rates of surface antigens on passage 3 subcultured BMSCs to respectively be: CD90 93.25%, CD44 95.42%, C105 89.73%, CD34 0.23%, and CD45 0.31%. Therefore, passage 3 BMSCs were applied for subsequent experiments.
BDNF mRNA and protein expression levels after transfection
At 3 days after transfection, BDNF mRNA expression level (the optical density ratio of BDNF/GAPDH) in the transfected cells was higher than that in the control cells (1.652 ± 0.082, 0.470 ± 0.021;P< 0.01), as detected by RT-PCR test. This evidence suggested the success of BDNF transfection (Figure 3A).
At 1-2 days after transfection, we extracted proteins from the transfected cells. Western blot assay results visualized a 13 kDa BDNF-positive protein band and the band became strongly positive with time, while a weak band was visible in the non-transfection group (Figure 3B). This evidence showed that BDNF-transfected BMSCs ef fi ciently expressed BDNF protein, indicating the success of transfection.
General conditions of rats after acellular nerve allograft combined with BDNF-transfected BMSC transplantation
Rats began eating immediately after waking from anesthesia and had apparent unsteady gait. At 2 weeks after repair, foot muscles of the operated side exhibited atrophy and occasional ulcers. At 4 weeks, muscle atrophy and ulceration of each group were aggravated, and rat feet could not completely straighten when walking. Feet were also unable to support the animals or touch the ground. At 6 weeks, better motor function was observed in the CEANA + BDNF + BMSCs group compared with the other two groups. Rats from the CEANA group showed poor nerve regeneration and restoration, as demonstrated by long-term red swelling of legs and feet, and nonhealing ulcers. Rats achieved neurological functional recovery in the CEANA + BDNF + BMSCs group, with leg muscle strength increasing allowing rats to stand using their right hindlimbs. At 8 weeks, all wounds were healed without infection and ulcers, rats in the CEANA + BDNF + BMSCs group could separate their toes and had no walking disorders.
Effect of acellular nerve allograft combined with BDNF-transfected BMSCs transplantation on sciatic nerve function of rats
At 2, 4, 6, and 8 weeks after the repair, all the incisions were healed well. SFI gradually increased with the time. SFI was the highest in the CEANA + BDNF + BMSCs group, then in the CEANA + BMSCs group, and lowest in the CEANA group at 2, 4, 6, and 8 weeks (P> 0.05; Table 1).
Effect of acellular nerve allograft combined with BDNF-transfected BMSC transplantation on triceps wet weight recovery rate, total number of myelinated nerve fi bers and myelin thickness
At 8 weeks after repair, triceps wet weight recovery rate, myelin sheath thickness and number of myelinated nerve fi bers were measured in the three groups. The variations were the largest in the CEANA + BDNF + BMSCs group compared with the CEANA + BMSCs group, whilst the smallest were seen in the CEANA group (Table 2).
Discussion
Peripheral nerve regeneration has been widely studied in the fi eld of tissue engineering (Friedenstein et al., 1976; Connolly et al., 1995; Pittenger et al., 1999; Wexler et al., 2003; Hu et al., 2009). BMSCs are the preferred seed cells in regenerative medicine studies and show great application prospects in clinical treatment (Friedenstein et al., 1976; Connolly et al., 1995; Pittenger et al., 1999; Wexler et al., 2003; Hu et al., 2009). The development of biotechnology, application of tissue engineering, and genetic engineering technology make gene and cell replacement therapy a hot research topic (Wexler et al., 2003). The long-term or permanent expression of target gene expressionin vivois critically determined by the choice of target cells. The target gene introduced into the target cells should have the ability to self-renew. Stem cells are ideal target cells in cell therapy and gene therapy,due to the potential for self-renewal and multiple differentiation (Devine et al., 2002; Wang et al., 2002). BMSCs are the most studied stem cells because they are easily isolated, amplified, and can multi-directionally differentiate under appropriate conditions. Furthermore, autologous transplantation may eliminate the immunogenicity and complexity caused by allogeneic transplantation. BMSCs can also freely migrate and integrate into adjacent tissue, and are prone to exogenous gene transfection and expression (Murphy et al., 2003; Urdzikova et al., 2006). Extraction of mesenchymal stem cells derived from bone marrow is simple and has an abundant source. Mesenchymal stem cells can greatly proliferate within the short term and the quantity of primitive cells increases a thousand fold within a couple of weeks. In fact, mesenchymal stem cells culturedin vitrostill maintain the proliferation and differentiation capacities up to 19 generations (Jiang et al., 2002; Devine et al., 2003). Meanwhile, cellular immunology studies demonstrated very low immunogenicity of mesenchymal stem cells. A small amount of mesenchymal stem cells express major histocompatibility antigens MHCI molecules, rather than MHCII molecules. Additionally, mesenchymal stem cells differentiatedin vitrocan express Schwann cell markers and promote peripheral nerve regeneration (Jiang et al., 2002; Devine et al., 2003). In summary, mesenchymal stem cells are characterized by an abundant source and easy harvesting, and are more easily cultured compared with other cell types. Furthermore, MSCs have a strong proliferative capacity, low immunogenicity to reduce or avoid rejection, are highly plastic, and differentiate into neuron-like cells after transplantation (Jiang et al., 2002; Devine et al., 2003).
In this study, we successfully transfected BDNF into BMSCs using genetic engineering techniques and repaired peripheral nerve defects with the transfected BMSCs plus acellular nerve allografts. The combination of tissue engineering, genetic engineering, neural tube and microsurgical techniques maximized their advantages, which was conducive to achieving better peripheral nerve regeneration. The results of the present study showed that, SFI, triceps wet weight recovery rate, myelin sheath thickness, and number of myelinated nerve fi bers were changed in the three groups, and the variations were the largest in the CEANA + BDNF + BMSCs group, then in the CEANA + BMSCs group, and finally the CEANA group. BMSCs transfected with BDNF were superior to BMSCs alone in the repair of peripheral nerve. BDNF is not only a crucial nutritional factor of sensory neurons, but is also important for motor neurons. BDNF acts on peripheral and central neurons (Schwartz et al., 2002; Hudson et al., 2004; Caddick et al., 2006). BDNF promoted the survival and neurite outgrowth of chick embryonic sensory neurons culturedin vitro. Local injection of BDNF is a promising strategy to treat facial nerve injury in New Zealand rabbits. BDNF also promoted the regeneration and myelination of the injured sciatic nerve (Schwartz et al., 2002; Hudson et al., 2004; Caddick et al., 2006). After sciatic nerve transection, BDNF mRNA expression at the distal end of the sciatic nerve was upregulated, then BDNF was transfected into the affected nerveviaosmotic pumps. The diameter of regenerating axons was increased signi fi cantly and the myelin became thicker (Zhang et al., 2006; Chen et al., 2007).
BDNF may promote nerve regeneration by regulating the expression of calcium channel proteins on the cell membrane and affecting calcium ion flow. BDNF also regulates the expression of neuropeptides to maintain intracellular calcium ion homeostasis. BDNF prevents free radical damage, initiating cell repair, and promoting intracellular antioxidant enzyme activity. BDNF functions to regulate gene expression within neurons, which is achieved by transcription factors and other upstream components (Tolwani et al., 2004; Yetiser et al., 2008). BDNF maintains normal signal transfer and promotes the secretion of molecules that are responsible for normal neuronal functions (Tolwani et al., 2004; Yetiser et al., 2008). This study is limited in that no further investigations on the synergic role of BDNF and BMSCs, and no detailed study on underlying mechanisms of action were performed. Further studies are needed to verify the repair effect on peripheral nerve injury using genetic engineering and cell engineering techniques.
In summary, acellular nerve allograft combined with BDNF-transfected BMSCs can improve the repair effect of nerve grafts and the morphology of injured nerves. This combination is better than nerve allograft alone.
Author contributions:All authors were responsible for the acquisition and integration of experimental data, the study concept and design, and approved the final version of the manuscript.
Con fl icts of interest:None declared.
Barbash IM, Chouraqui P, Baron J, Feinberg MS, Etzion S, Tessone A, Miller L, Guetta E, Zipori D, Kedes LH, Kloner RA, Leor J (2003) Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution. Circulation 108:863-868.
Caddick J, Kingham PJ, Gardiner NJ, Wiberg M, Terenghi G (2006) Phenotypic and functional characteristics of mesenchymal stem cells differentiated along a Schwann cell lineage. Glia 54:840-849.
Cao RL, Li CY, Zhang XT (2012) Component analysis of a natural acellular sciatic nerve scaffold. Zhongguo Zuzhi Gongcheng Yanjiu 16:1367-1370.
Chen DP, Zhang ZJ, Wu XL (2007) Distribution of intravenously grafted bone marrow mesenchymal stem cells in the viscera tissues of rats before and after cerebral ischemia. Zhongguo Zuzhi Gongcheng Yanjiu yu Linchuang Kangfu 11:10160-10164.
Chen SL, Fang WW, Ye F, Liu YH, Qian J, Shan SJ, Zhang JJ, Chunhua RZ, Liao LM, Lin S, Sun JP (2004) Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am J Cardiol 94:92-95.
Connolly JF (1995) Injectable bone marrow preparations to stimulate osteogenic repair. Clin Orthop Relat Res 313:8-18.
Devine SM (2002) Mesenchymal stem cells: will they have a role in the clinic? J Cell Biochem Suppl 38:73-79.
Devine SM, Cobbs C, Jennings M, Bartholomew A, Hoffman R (2003) Mesenchymal stem cells distribute to a wide range of tissues following systemic infusion into nonhuman primates. Blood 101:2999-3001.
Ferrari G, Cusella-De Angelis G, Coletta M, Paolucci E, Stornaiuolo A, Cossu G, Mavilio F (1998) Muscle regeneration by bone marrow-derived myogenic progenitors. Science 279:1528-1530.

Figure 1 Morphology of rat bone marrow mesenchymal stem cells after subculture (inverted phase contrast microscope, × 400).
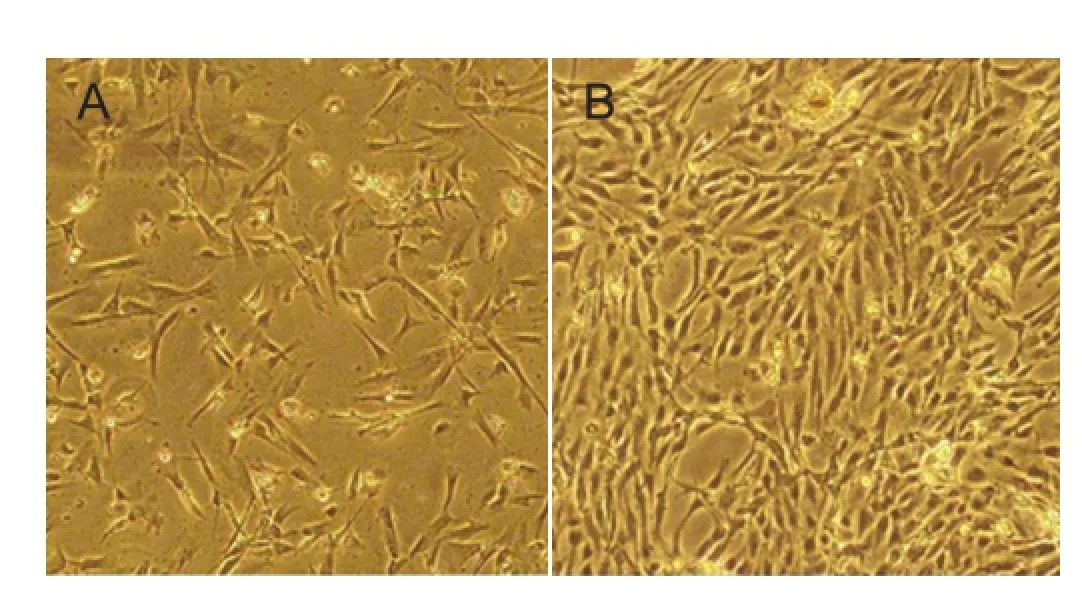
Figure 2 Morphology of subcultured bone marrow mesenchymal stem cells at passage 3 (inverted phase contrast microscope, × 100).
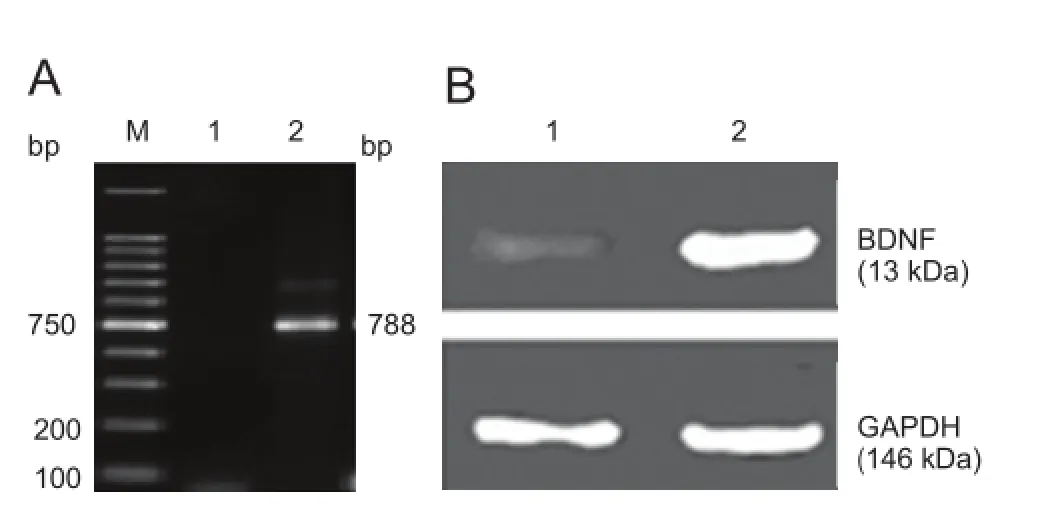
Figure 3 Brain-derived neurotrophic factor (BDNF) mRNA and protein expression levels in transfected bone marrow mesenchymal stem cells.
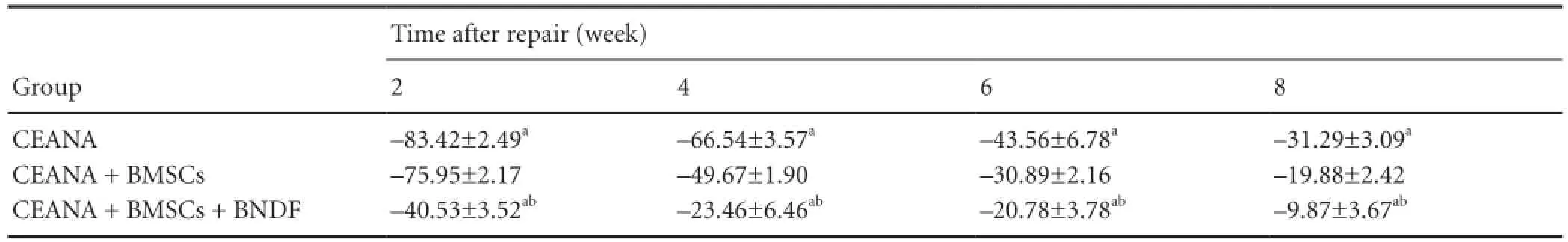
Table 1 Effects of CEANA combined with BDNF-transfected BMSCs on sciatic functional index in rats
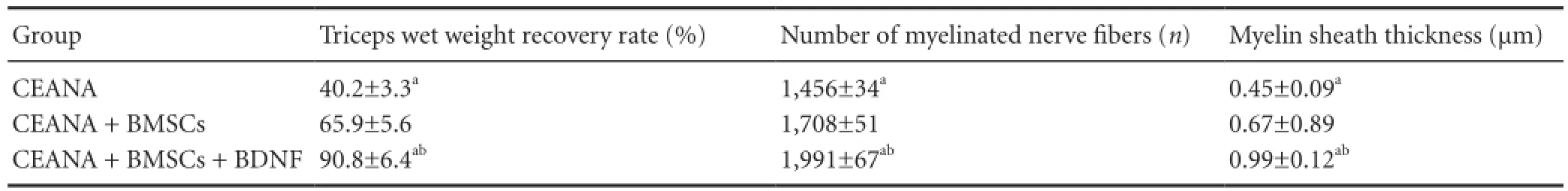
Table 2 Effects of CEANA combined with BDNF-transfected BMSCs on triceps wet weight recovery rate, total number of myelinated nerve fi bers and myelin sheath thickness in rats with sciatic nerve injury
Friedenstein AJ, Gorskaja JF, Kulagina NN (1976) Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol 4:267-274.
Gravel C, Gotz R, Lorrain A, Sendiner M. Adenoviral gene transfer of ciliary neurotrophic factor and brain-derived neurotrophic factor leads to long-term survival of axotomized motor neurons. Nat Med 1997;3:765-770.
Han JB, Chen JW, Zhao BH, Zhang JH, Tian DH, Han JH (2006) Preparation of acellular nerve grafts with triton X-100. Neural Regen Res 7:645-648.
Hu P, Kalb RG, Walton KD (2003) BDNF heitens the sensitivity of motor neurons to excitotoxic insults through activation of TrkB. J Neurochem 84:1421-1430.
Hudson TW, Zawko S, Deister C, Lundy S, Hu CY, Lee K, Schmidt CE (2004) Optimized acellular nerve graft is immunologically tolerated and supports regeneration. Tissue Eng 10:1641-1651.
Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM (2002) Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 418:41-49.
Kurozumi K, Nakamura K, Tamiya T, Kawano Y, Kobune M, Hirai S, Uchida H, Sasaki K, Ito Y, Kato K, Honmou O, Houkin K, Date I, Hamada H (2004) BDNF gene-modified mesenchymal stem cells promote functional recovery and reduce infarct size in the rat middle cerebral artery occlusion. Mol Ther 9:189-197.
Li Y, Chen J, Chen XG, Wang L, Gautam SC, Xu YX, Katakowski M, Zhang LJ, Lu M, Janakiraman N, Chopp M (2002) Human marrow stromal cell therapy for stroke in rat: neurotrophins and functional recovery. Neurology 59:514-523.
Liechty KW, MacKenzie TC, Shaaban AF, Radu A, Moseley AM, Deans R, Marshak DR, Flake AW (2000) Human mesenchymal stem cells engraft and demonstrate site-specific differentiation after in utero transplantation in sheep. Nat Med 6:1282-1286.
Liu XG, Deng YB, Cai H (2013a) Glial cell line-derived neurotrophic factor promotes neuron-like cell differentiation of mesenchymal stem cells. Zhongguo Zuzhi Gongcheng Yanjiu 17:1856-1861.
Liu XG, Deng YB, Cai H, Wang LN, Ma YL, Zhang XP, Wei KX (2013b) Transplantation of controlled release glial cell line-derived neurotrophic factor and bone marrow mesenchymal stem cells-derived neuron-like cells reduces myelosyringosis after spinal cord injury. Zhongguo Zuzhi Gongcheng Yanjiu 17:68-73.
Menovsky T, van der Bergh Weerman M, Kubista OL, Bartels RH, van Overbeeke JJ (1999) Peripheral nerve graft repair of the oculomotor nerve in rats: a and morphometric study. Microsurgery 19:392-400.
Mezey E, Chandross KJ, Harta G, Maki RA, McKercher SR (2000) Turning blood into brain: cells bearing neuronal antigens generated in vivo from bone marrow. Science 290:1779-1782.
Muraglia A, Cancedda R, Quarto R (2000) Clonal mesenchymal progenitors from human bone marrow differentiate in vitro according to a hierarchical model. J Cell Sci 113:1161-1166.
Murphy JM, Fink DJ, Hunziker EB, Barry FP (2003) Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum 48:3464-3474.
Orgogozo JM, Gilman S, Dartigues JF, Laurent B, Puel M, Kirby LC, Jouanny P, Dubois B, Eisner L, Flitman S, Michel BF, Boada M, Frank A, Hock C (2003) Subacute meningoencephalitis in a subset of patients with AD after Abeta42 immunization. Neurology 61:46-54.
Palermo AT, Labarge MA, Doyonnas R, Pomerantz J, Blau HM (2005) Bone marrow contribution to skeletal muscle: a physiological response to stress. Dev Biol 279:336-344.
Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, Boggs SS, Greenberger JS, Goff JP (1999) Bone marrow as a potential source of hepatic oval cells. Science 284:1168-1170.
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284:143-147.
Schwartz RE, Reyes M, Koodie L, Jiang Y, Blackstad M, Lund T, Lenvik T, Johnson S, Hu WS, Verfaillie CM (2002) Multipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte-like cells. J Clin Invest 109:1291-1302.
Sun ZM, Yuan Y, Pan SM, Zhang JH, Wang YL, Tang GT, Guan MW, Chen HG (2012) Transfection of an eukaryotic expression vector expressing brain derived neurotrophic factor into human umbilical cord mesenchymal stem cells. Zhongguo Zuzhi Gongcheng Yanjiu 16:8413-8418.
Tang YY, Li ZY, Yin YY, Ji LL, Wang ZY (2012) Brain-derived neurotrophic factor promotes the directional differentiation of hypoglycemia neonatal rat hippocampal neural stem cells. Zhongguo Zuzhi Gongcheng Yanjiu 16:7694-7697.
Tolwani RJ, Cosgaya JM, Varma S, Jacob R, Kuo LE, Shooter EM (2004) BDNF overexpression produces a long-term increase in myelin formation in the peripheral nervous system. J Neurosci Res 77:662-669.
Tuli R, Seghatoleslami MR, Tuli S, Wang ML, Hozack WJ, Manner PA, Danielson KG, Tuan RS (2003) A simple, high-yield method for obtaining multipotential mesenchymal progenitor cells from trabecular bone. Mol Biotechnol 23:37-49.
Urdzíková L, Jendelová P, Glogarová K, Burian M, Hájek M, Syková E (2006) Transplantation of bone marrow stem cells as well as mobilization by granulocyte-colony stimulating factor promotes recovery after spinal cord injury in rats. J Neurotrauma 23:1379-1391.
Wang L, Li Y, Chen X, Chen J, Gautam SC, Xu Y, Chopp M (2002) Ischemic cerebral tissue and MCP-1 enhance rat bone marrow stromal cell migration in interface culture. Exp Hematol 30:831-836.
Wexler SA, Donaldson C, Denning-Kendall P, Rice C, Bradley B, Hows JM (2003) Adult bone marrow is a rich source of human mesenchymal ‘stem’ cells but umbilical cord and mobilized adult blood are not. Br J Haematol 121:368-374.
Woodbury D, Schwarz EJ, Prockop DJ, Black IB (2000) Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res 61:364-370.
Yetiser S, Kahraman E (2008) An analysis of time-dependent changes of neurotrophic factors (BDNF, CNTF) in traumatic facial nerve injury of a nerve-cut and nerve-crush model in rats. Otol Neurotol 29:392-396.
Zhang MH, Hu YM, Zhang H, Hu YP, Liu QZ, Wang HC, Li WY (2012) Brain-derived neurotrophic factor expression in the spinal dorsal horn and dorsal root ganglion in a rat model of sciatic nerve injury after intrathecal transplantation of neural stem cells. Zhongguo Zuzhi Gongcheng Yanjiu 16:1851-1855.
Zhang Y, Pardridge WM (2006) Blood-brain barrier targeting of BDNF improves motor function in rats with middle cerebral artery occlusion. Brain Res 1111:227-229.
Zhao Z, Zhao B, Wang Y, Peng J, Zhang L, Chen J, Zhao Q, Ren Z, Liu Y, Xu W, Lu S (2010) Functional evaluation of chemically extracted acellular nerve allograft supplement with different tissues of Schwann cells for peripheral nerve regeneration. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 24:1281-1287.
Zheng MH, Zhang ZJ, Huang DY (2009) Brain-derived neurotrophic factor gene-modi fi ed bone marrow mesenchymal stem cells in treatment of sciatic nerve injury. Zhongguo Zuzhi Gongcheng Yanjiu 13:120-124.
Copyedited by Apricò K, Norman C, Wang J, Yang Y, Li CH, Song LP, Zhao M
Wenhua Huang, Ph.D., Institute of Clinical Anatomy, Southern Medical University, Guangzhou 510515, Guangdong Province, China, jiaoxueban2010@126.com.
10.4103/1673-5374.143427
http://www.nrronline.org/
Accepted: 2014-08-06
 中國(guó)神經(jīng)再生研究(英文版)2014年20期
中國(guó)神經(jīng)再生研究(英文版)2014年20期
- 中國(guó)神經(jīng)再生研究(英文版)的其它文章
- Meta analysis of olfactory ensheathing cell transplantation promoting functional recovery of motor nerves in rats with complete spinal cord transection
- Ultrasonographic reference values for assessing normal radial nerve ultrasonography in the normal population
- Penile erectile dysfunction after brachial plexus root avulsion injury in rats
- Synergistic actions of olomoucine and bone morphogenetic protein-4 in axonal repair after acute spinal cord contusion
- Three-dimensional conformal intensity-modulated radiation therapy of left femur foci does not damage the sciatic nerve
- Ganglioside promotes the bridging of sciatic nerve defects in cryopreserved peripheral nerve allografts
