Ganglioside promotes the bridging of sciatic nerve defects in cryopreserved peripheral nerve allografts
Yaodong Wang, Yuguang Liu, Qiang Liu,
1 Department of Neurosurgery, Qilu Hospital of Shandong University, Jinan, Shandong Province, China
2 Brain Science Research Institute, Shandong University, Jinan, Shandong Province, China
3 Department of Neurology, Third Af fi liated Hospital of Liaoning Medical University, Jinzhou, Liaoning Province, China
Ganglioside promotes the bridging of sciatic nerve defects in cryopreserved peripheral nerve allografts
Yaodong Wang1, Yuguang Liu1, Qiang Liu1,2,3
1 Department of Neurosurgery, Qilu Hospital of Shandong University, Jinan, Shandong Province, China
2 Brain Science Research Institute, Shandong University, Jinan, Shandong Province, China
3 Department of Neurology, Third Af fi liated Hospital of Liaoning Medical University, Jinzhou, Liaoning Province, China
Previous studies have shown that exogenous gangliosides promote nervous system regeneration and synapse formation. In this study, 10 mm sciatic nerve segments from New Zealand rabbits were thawed from cryopreservation and were used for the repair of left sciatic nerve defects through allograft bridging. Three days later, 1 mL ganglioside solution (1 g/L) was subcutaneously injected into the right hind leg of rabbits. Compared with non-injected rats, muscle wet weight ratio was increased at 2-12 weeks after modeling. The quantity of myelinated fi bers in regenerated sciatic nerve, myelin thickness and fiber diameter were elevated at 4-12 weeks after modeling. Sciatic nerve potential amplitude and conduction velocity were raised at 8 and 12 weeks, while conduction latencies were decreased at 12 weeks. Experimental fi ndings indicate that ganglioside can promote the regeneration of sciatic nerve defects after repair with cryopreserved peripheral nerve allografts.
nerve regeneration; ganglioside; peripheral nerve; bridge; repair; nerve graft; cryopreservation; nerve allograft; sciatic nerve; neural regeneration
Wang YD, Liu YG, Liu Q. Ganglioside promotes the bridging of sciatic nerve defects in cryopreserved peripheral nerve allografts. Neural Regen Res. 2014;9(20):1820-1823.
Introduction
Transplantation of autologous nerves is regarded as the optimal method for restoring peripheral nerve defects (Han, 2011; Yuksel et al., 2014). However, the application of autologous nerves is limited due to loss of neurological function, restriction of donor tissue, and deterioration of donor injury (Chen et al., 2012; Li et al., 2012; Luo et al., 2013). Rapid development of tissue engineering technology allows the use of a variety of arti fi cial nerves in the repair of peripheral nerve defects, and these nerve grafts are shown to promote nerve regeneration in animals (Huang et al., 2012; Wang et al., 2012; Xiang et al., 2012; Tang et al., 2013; Krause et al., 2014). Artificial nerve, skeletal muscle, autologous veins, amnion and other materials contribute to the repair of peripheral nerve injury (den Dunnen and Meek, 2001). Nerve allograft transplantation has been widely studied along with the progress and development of transplantation technology. Cryopreservation of nerve grafts is an area of research in neurosurgery that is intensely focused on neurological function and post-transplantation effects.
Gangliosides belong to a class of sialic acid-containing glycosphingolipid that are widely present in the cell membrane of vertebrates, especially in the brain of the fetus and newborn infant. Ganglioside therefore plays a crucial role in neuronal differentiation, neurite outgrowth, and synapse formation (Ning and Chen, 2009). This study aimed to observe the effect of ganglioside on the regeneration of sciatic nerve defects after repair with cryopreserved nerve allograft.
Materials and Methods
Experimental animals
Sixty healthy adult New Zealand rabbits, irrespective of sex, at 45-90 days old, weighing 1.0-2.0 kg (mean 1.5 kg), were purchased from the Experimental Animal Center, Qilu Hospital of Shandong University, China (license No. SCXK (Lu) 2004-0013). Experimental disposals were approved by the Ethics Committee, Qilu Hospital of Shandong University, China. Among the 60 rabbits, six were randomly selected as donors and the remaining 54 were the recipients. Recipient animals were randomly divided into ganglioside + nerve allograft group and nerve allograft group.
Preparation of frozen sciatic nerve allograft
Donor rabbits were anesthetized with intravenous injection of 30 g/L sodium pentobarbital (1 mg/kg)viathe ear vein, and bilateral sciatic nerves were cut. A midline incision was then made along the thigh skin and subcutaneous tissue was resected. Blunt dissection was then used to expose the sciatic nerve between the biceps femoris and semitendinosus muscles. A segment of 10 mm sciatic nerve was prepared, dried and cryopreserved in the refrigerator (Haier Group, Qingdao, Shandong Province, China). When the freezing temperature was -40°C and drying chamber temperature was -30°C, both the drying rack and nerve tissue were placed in the drying chamber for about 4 hours to allow precooled drying. Cryopreservation was implemented when the vacuum degree reached 1.33 Pa, then the vacuum system was closed and specimens were stored at room tem-perature for further use.
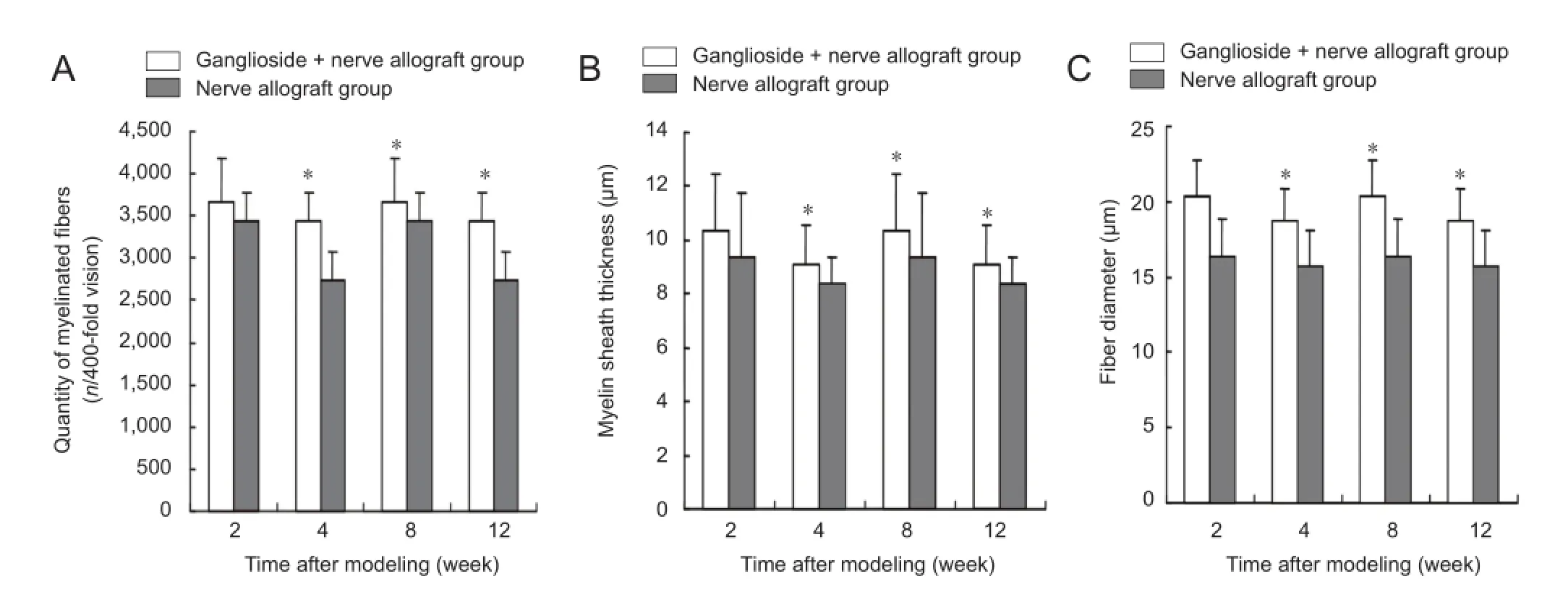
Figure 4 Effect of ganglioside on nerve fi bers in rabbit sciatic nerve repaired with cryopreserved peripheral nerve allograft.

Figure 3 Effect of ganglioside on gastrocnemius wet weight in rabbit sciatic nerve repaired with cryopreserved peripheral nerve allograft.
Cryopreserved nerve allograft bridging of sciatic nerve defects
All recipient rabbits were anesthetized with intravenous injection of 30 g/L sodium pentobarbital (1 mg/kg)viathe ear vein, and a midline incision was made along the skin of the left thigh and subcutaneous tissue was resected, exposing the sciatic nerve between the biceps femoris and semitendinosus through blunt dissection. A segment of 10 mm sciatic nerve was cut, as previously in the donor, and a sciatic nerve defect was produced at 5 mm above the sciatic nerve bifurcation. Subsequently, the donor tissue was immersed in saline for 3-4 hours and was used to repair sciatic nerve defects through bridging (Yang et al., 2011). Rabbits were given oral antibiotics to prevent infection post-operation.
Ganglioside injection
Three days after modeling, rabbits in the ganglioside + nerve allograft group were subcutaneously injected with 1 mL ganglioside solution into the right hind legs every day. Ganglioside injection (1 g/L) was provided from Qilu Hospital of Shandong University in China.
Electrophysiological detection
At 2, 4, 8, and 12 weeks after modeling, rabbits in the two groups were detected using EMG evoked potentials (Nanjing Repson Biotechnology Co., Ltd., Nanjing, Jiangsu Province, China). Stimulation electrodes were placed on the proximal and distal ends of the left sciatic nerve allograft, and recording electrodes were placed in the tibialis anterior muscle belly. Sciatic nerve was stimulated at 60 mV, 200 Hz, to determine potential amplitude, conduction velocity and conduction latencies.
Ultrastructural observation
Nerve segments in each group were sutured and fi xed in glutaraldehyde solution, and sliced into ultrathin sections (0.2 mm thick) for electron microscopy observation. Paraffin sections were observed under transmission electron microscopy (JEOL), and the quantity of myelinated fi bers, myelin thickness and fi ber diameter were determined.
Muscle wet weight
After all rabbits were killed, bilateral gastrocnemius were harvested and wet weight was measured using an EL204 electronic balance (Mettler-Toledo Instruments, Greifensee, Switzerland). The wet mass ratio was calculated according to the formula: the ratio = wet weight of gastrocnemius from the injury side/wet weight of gastrocnemius from the contralateral side.
Figure 1 Effect of ganglioside on the morphology of sciatic nerve defects repaired with cryopreserved peripheral nerve allograft (electron microscopy, × 10,000).
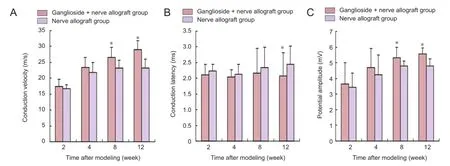
Figure 2 Effect of ganglioside on the functions of sciatic nerve defects repaired with cryopreserved peripheral nerve allograft.
Statistical analysis
Data were analyzed using SPSS 18.0 software (SPSS, Chicago, IL, USA) and were expressed as the mean ± SD. The differences were compared using paired samplet-test and aP<0.05 level was considered a signi fi cant difference.
Results
Ganglioside improved the morphology of sciatic nerve defects repaired with cryopreserved peripheral nerve allograft
At 2 weeks after modeling, myelinated nerve fiber began to disintegrate and Schwann cell proliferation was not obvious in the nerve allograft group. The ganglioside + nerve allograft group showed a slower disintegration of myelinated nerve fi bers and more obvious proliferation of Schwann cells compared with the nerve allograft group. At 4 and 8 weeks after modeling, the transplanted sciatic nerve was not changed when compared with 2 weeks. At 12 weeks, a small amount of myelinated nerve fibers was found in the nerve allograft group. Blood capillaries between the perineurium proliferated and scarring nerve fi bers were obvious. In the ganglioside + nerve allograft group, there were a large number of myelinated nerve fi bers in the rabbit sciatic nerve. Only a few nerve fibers were scarred and blood capillaries between the perineurium were clearly visible (Figure 1).
Ganglioside improved the function of sciatic nerve defects repaired with cryopreserved peripheral nerve allograft
At 2 and 4 weeks after modeling, sciatic nerve conduction velocity and conduction latency were similar to potential amplitude in the two groups (P> 0.05). At 8 and 12 weeks after modeling, sciatic nerve potential amplitude and conduction velocity in the ganglioside + nerve allograft group were higher than that in the nerve allograft group (P< 0.05). The ganglioside + nerve allograft group had a lower conduction latency than the nerve allograft group at 12 weeks only (P< 0.05; Figure 2).
Ganglioside increased gastrocnemius wet weight in rabbit sciatic nerve repaired with cryopreserved peripheral nerve allograft
At 2-12 weeks after modeling, gastrocnemius wet weight ratio in the ganglioside + nerve allograft group was higher than that in the nerve allograft group (P< 0.05; Figure 3).
Ganglioside improved the morphology of nerve fi bers in sciatic nerve repaired with cryopreserved peripheral nerve allograft
At 2 weeks after modeling, the quantity of myelinated fi bers, myelin thickness and fi ber diameter in the regenerated sciatic nerve showed no difference between the ganglioside +nerve allograft group and nerve allograft group (P> 0.05). At 4, 8, and 12 weeks after modeling, the ganglioside + nerve allograft group had greater myelinated fi bers, myelin thickness and fi ber diameter than the nerve allograft group (P<0.05; Figure 4).
Discussion
Growing evidence from previous studies has shown that pre-implantation of Schwann cells contributed to repairing long nerve defects and improving nerve regeneration (Zhang, 2011; Yu et al., 2012). The results of this study demonstrated that ganglioside injection attenuated myelin disintegration in myelinated nerve fi bers and promoted the proliferation of Schwann cells. An increase in blood capillaries between the perineurium was also clearly visible. Nerve injury is highly associated with the deterioration of neurological function, especially nerve conduction velocity, conduction latency and potential amplitude (Ji and Qi, 2012). The present study showed that, ganglioside-treated rabbits had a higher potential amplitude and conduction velocity than the non-treated rabbits at 8 and 12 weeks after modeling. Previous studies revealed that nerve damage causes muscle atrophy because the nerve provides nutrition for the muscle and can secrete hormones and substances that promote and maintain muscle activity (Han, 2011). In this study, gastrocnemius wet weight ratio in the ganglioside-treated rabbits was higher than that in the model rabbits at 2, 4, 8, and 12 weeks after modeling. This evidence indicates that ganglioside solution enhances the nutrition effect on rabbit nerves, and is conducive to maintaining and promoting the survival and function of muscles. After nerve injury occurs, the nerve has self-repair potential in humans and animals (Cao et al., 2013; Chen and Guo, 2014). Regenerated nerve fi bers will continuously grow to minimize the inconvenience caused by nerve injury (Liu et al., 2012; Yu, 2013). Ganglioside solution increased the quantity of myelinated fibers, myelin sheath thickness and fi ber diameter signi fi cantly in the regenerated sciatic nerve of rabbits after transplantation of peripheral nerve allograft.
In summary, ganglioside promotes the regeneration of peripheral nerve allograft after transplantation. Ganglioside can also accelerate nerve conduction velocity, increase the potential amplitude, shorten conduction latency, promote nerve fiber remodeling, and repair peripheral nerve defects. The same drug may exert signi fi cantly different effects at different time points after peripheral nerve injury (Hou et al., 2009). Therefore, further studies are needed to explore the exact durations of ganglioside injection for promoting the restoration of peripheral nerve allograft, so as to provide theoretical evidence for the choices made in the clinical setting.
Author contributions:Wang YD and Liu YG designed the study and were responsible for the article. Wang YD, Liu YG and Liu Q implemented the experiment. Liu YG evaluated the study. Liu YG and Liu Q collected experimental data. Wang YD drafted the manuscript. Liu YG revised the manuscript. All authors approved the final version of the manuscript.
Con fl icts of interest:None declared.
Cao MR, Hua HQ, Qin SK (2013) Preventive effect of ganglioside on peripheral neurotoxicity induced by vincristine. Shandong Yiyao 53:57-59.
Chen J, Lin LX, Ma B, Pang J (2012) Meta-analysis of ganglioside treatment for hypoxic-ischemic encephalopathy in newborn infants. Shiyong Erke Linchuang Zazhi 27:132-135.
Chen TL, Guo QL (2014) Observation of the ganglioside combined with nerve growth factor in the treatment of children brain damage. Zhongguo Fuyou Baojian 29:728-730.
den Dunnen WF, Meek MF (2001) Sensory nerve function and auto-mutilation after reconstruction of various gap lengths with nerve guides and autologous nerve grafts. Biomaterials 22:1171-1176.
Han WD (2011) Effects of ganglioside on neurologic impairment in cerebral infarction patients. Shandong Yiyao 51:67-68.
Hou ZJ, Yang S, Meng XL, Xia HC, Fan CD, Yan JL (2009) Comparative study on repairing effects at different time points after peripheral nerve injury. Zhongguo Zuzhi Gongcheng Yanjiu yu Linchuang Kangfu 13:9084-9087.
Huang YZ, Chen QH, Ren MM (2012) Injection of polylactic acid-polyglycolic acid-recombinant human erythropoietin microspheres into chitin nerve regeneration chamber can promote sciatic nerve regeneration. Zhongguo Zuzhi Gongcheng Yanjiu 16:2115-2119.
Ji SY, Qi PJ (2012) The ef fi cacy of gangliosides on the situation after decompression surgery of severe traumatic brain injury induced intracranial hypertension. Zhongguo Shenghua Yaowu Zazhi 33:181-184.
Krause NP, Dworski S, Feinberg K, Jones K, Johnston AP, Paul S, Paris M, Peles E, Bagli D, Forrest CR, Kaplan DR, Miller FD (2014) Direct genesis of functional rodent and human schwann cells from skin mesenchymal precursors. Stem Cell Reports 3:85-100.
Li QM, Huang HH, Zhang GS, Lin GX, Mou X, Chen QH (2012) Efficacy and safety of ganglioside treatment on Alzheimer’s disease. Shiyong Yixue Zazhi 28:804-806.
Liu JX, Chen JG, Chen HY, Lin Q, Ye CH, Chen J, Cheng MT (2012) Changes and signi fi cance of ganglioside on IL-6, NSE expression in the serum and brain ultrasound of premature infants with leukodystrophy. Guangdong Yixue 33:2410-2412.
Luo P, Peng QL, Xiang JP, Qi J (2013) Synthetic nerve conduit versus autogenous nerve transplantation for repair of peripheral nerve defects. Zhongguo Zuzhi Gongcheng Yanjiu 17:3010-3017.
Ning N, Chen BH (2009) Biological activity of ganglioside. Shengli Kexue Jinzhan 40:24-30.
Tang Y, Liu XH, Liu D, Yu HX (2013) Difficulties in tissue engineering construction technology for repair of peripheral nerve injuries. Zhongguo Zuzhi Gongcheng Yanjiu 17:1295-1304.
Wang SX, Peng LF, Qu SX, Jiang MX, Jiang JC, Ji YM (2012) Effect of ganglioside on Nogo-A expression in the serum of patients with severe traumaticbrain injury. Shandong Yiyao 52:72-74.
Xiang J, Huang JF, Li DZ, Li SP, Yan YH, Yan QJ (2012) Histological observation of dog peripheral nerve regeneration induced by a novel biomimetic nerve conduit. Zhongguo Zuzhi Gongcheng Yanjiu 16:3801-3805.
Yang XH, Han JB, Zhang CB, Tian DH, Han JH (2011) Allogeneic nerve complex for repair of peripheral nerve defects in rabbits. Zhongguo Zuzhi Gongcheng Yanjiu yu Linchuang Kangfu 15:6315-6318.
Yu L, Wan AQ, Wang MX (2012) Effect observation on the ganglioside combined with edaravone in treatment of acute cerebral infarction and its affection on in fl ammatory cytokines. Xiandai Yufang Yixue 39:3182-3183, 3185.
Yu ZJ (2013) Analysis on the clinical efficacy of ganglioside in treatment of neonatal hypoxic-ischemic encephalopathy. Zhongguo Fuyou Baojian 28:5478-5480.
Yuksel TN, Halici Z, Demir R, Cakir M, Calikoglu C, Ozdemir G, Unal D (2014) Investigation of the effect of telmisartan on experimentally induced peripheral nerve injury in rats. Int J Neurosci Doi:103109/002074542014948115.
Zhang Y (2011) Clinical observation of ganglioside combined hyperbaric oxygen therapy on neonatal hypoxic-ischemic encephalopathy:125 cases. Shandong Yiyao 51:87-88.
Copyedited by Apricò K, Norman C, Yu J, Yang Y, Li CH, Song LP, Zhao M
Yuguang Liu, Ph.D., Brain Science Research Institute, Shandong University, Jinan City Cultural Road No. 107, Jinan 250012, Shandong Province, China, wangyaodong741@126.com.
10.4103/1673-5374.143429
http://www.nrronline.org/
Accepted: 2014-08-20
- 中國神經(jīng)再生研究(英文版)的其它文章
- Meta analysis of olfactory ensheathing cell transplantation promoting functional recovery of motor nerves in rats with complete spinal cord transection
- Ultrasonographic reference values for assessing normal radial nerve ultrasonography in the normal population
- Penile erectile dysfunction after brachial plexus root avulsion injury in rats
- Synergistic actions of olomoucine and bone morphogenetic protein-4 in axonal repair after acute spinal cord contusion
- Three-dimensional conformal intensity-modulated radiation therapy of left femur foci does not damage the sciatic nerve
- Combining acellular nerve allografts with brainderived neurotrophic factor transfected bone marrow mesenchymal stem cells restores sciatic nerve injury better than either intervention alone

