Polyurethane/poly(vinyl alcohol) hydrogel coating improves the cytocompatibility of neural electrodes
Mei Li, Hai-han Zhou, Tao Li, Cheng-yan Li, Zhong-yuan Xia,, Yanwen Y. Duan
1 Department of Anesthesiology, Renmin Hospital of Wuhan University, Wuhan, Hubei Province, China
2 Institute of Molecular Science, Shanxi University, Taiyuan, Shanxi Province, China
3 Department of Neurology, Renmin Hospital of Wuhan University, Wuhan, Hubei Province, China
4 College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei Province, China
Polyurethane/poly(vinyl alcohol) hydrogel coating improves the cytocompatibility of neural electrodes
Mei Li1, Hai-han Zhou2, Tao Li3, Cheng-yan Li3, Zhong-yuan Xia1,*, Yanwen Y. Duan4,*
1 Department of Anesthesiology, Renmin Hospital of Wuhan University, Wuhan, Hubei Province, China
2 Institute of Molecular Science, Shanxi University, Taiyuan, Shanxi Province, China
3 Department of Neurology, Renmin Hospital of Wuhan University, Wuhan, Hubei Province, China
4 College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei Province, China
Neural electrodes, the core component of neural prostheses, are usually encapsulated in polydimethylsiloxane (PDMS). However, PDMS can generate a tissue response after implantation. Based on the physicochemical properties and excellent biocompatibility of polyurethane (PU) and poly(vinyl alcohol) (PVA) when used as coating materials, we synthesized PU/PVA hydrogel coatings and coated the surface of PDMS using plasma treatment, and the cytocompatibility to rat pheochromocytoma (PC12) cells was assessed. Protein adsorption tests indicated that the amount of protein adsorption onto the PDMS substrate was reduced by 92% after coating with the hydrogel. Moreover, the PC12 cells on the PU/PVA-coated PDMS showed higher cell density and longer and more numerous neurites than those on the uncoated PDMS. These results indicate that the PU/PVA hydrogel is cytocompatible and a promising coating material for neural electrodes to improve their biocompatibility.
nerve regeneration; cerebral injury; neural electrodes; hydrogel coatings; polyurethane; polydimethylsiloxane; poly(vinyl alcohol); cytocompatibility; protein adsorption; nerve growth factor; rat pheochromocytoma cells; synaptic dif erentiation; neural regeneration
Funding: This study was supported by the National Natural Science Foundation of China, No. 81170768; a grant from the Fundamental Research Project of Shanxi Province of China, No. 2015021079.
Li M, Zhou HH, Li T, Li CY, Xia ZY, Duan YY (2015) Polyurethane/poly(vinyl alcohol) hydrogel coating improves the cytocompatibility of neural electrodes. Neural Regen Res 10(12):2048-2053.
Introduction
Neural electrical stimulation has been used over the last few decades as an alternative treatment to help patients suf ering from neurological disorders such as stroke, paralysis, blindness, deafness, Parkinson’s disease, and epilepsy (Sloviter et al., 1987; Makeig et al., 2002; Rauschecker et al., 2002; Hochberg et al., 2006; Chan CKL et al., 2008; Denheyer et al., 2009). Neural prostheses and modulators are used to recover or replace damaged neurological functions by stimulating target neurons (Talwar et al., 2002; Jackson et al., 2006). The implantable neural electrodes are a crucial component and play a key role in recording and stimulating the nerve tissue (Cogan et al., 2008). However, one major obstacle for the long-term use of neural electrodes is their unstable performance caused by tissue responses after clinical implantation (Zhong et al., 2001; Polikov et al., 2005). These tissue reactions not only lead to a large increase in the impedance between the electrode materials and neural tissue, but also decrease the number of neurons surrounding the electrodes. Over time, the ef ect of the electrical stimulation is weakened and can even disappear (Biran et al., 2005; He et al., 2007). Because certain biomaterials with excellent biocompatibility show decreased tissue responses, there is an urgent need to improve the biocompatibility of implantable electrode materials.
Polydimethylsiloxane (PDMS) has been used extensively as an insulation material for implantable neural electrodes because of its good biocompatibility (Erlich et al., 2003; Stieglitz et al., 2010). However, PDMS induces strong protein adsorption because it is hydrophobic (Anderson et al., 1995), which results in both acute and chronic tissue responses after implantation, leading to a noticeable increase in the electrode-tissue interface impedance caused by tissue encapsulation (Anderson et al., 2008). While several bioactive coating materials have previously been investigated to determine whether they improve neural cell ingrowth and reduce the interactions between the electrode and neural tissue (Zhong et al., 2001; He et al., 2007), most of the research was focused on improving the silicon substrate-tissue interface and few studies reported improvements in the PDMS substrate-tissue interface.
Hydrogels, on the other hand, have been extensively used in the biomedical and pharmaceutical f elds because of their low interfacial tension and large capacity for water uptake in the biological environment (Hamidi et al., 2008). For these reasons, considerable ef ort has been devoted to improving the hydrophobicity of PDMS by coating with hydrophilic hydrogels. Han et al. (2011) demonstrated that compositecoatings mated with hydrogel-electrospun f bers promote the integration of neuron-prostheses and the formation of a stable long-term interface. Winter et al (2007) reported that coating neural electrodes with a neurotrophin-eluting hydrogel improved neurite extension on the electrode surface and decreased the threshold for electrical stimulation.
In our previous work, we synthesized poly(vinyl alcohol)/ poly(acrylic acid) (PVA/PAA) and polyethylene glycol-containing polyurethane hydrogels as coatings for PDMS-based neural electrodes. The results demonstrated the ability of those hydrogel coatings to improve the electrode-tissue interface in vitro and in vivo (Lu et al., 2009; Rao et al., 2012). Another of our previous studies demonstrated that hydrophilicity is an important material property when modifying neural electrodes (Zhou et al., 2012). PVA is a type of poly hydroxyl polymer that is broadly used as a bioactive material in tissue engineering. It has appropriate physicochemical properties for use as a coating such as hydrophilicity and ease of f lm-formation, as well as good biocompatibility. To develop a better coating material for neural electrodes based on the biocompatible polyurethane (PU) hydrogel coating of PDMS substrates, we introduced PVA and synthesized PU/ PVA hydrogel as a coating for PDMS.
The aim of this study was to synthesize PU/PVA hydrogel and determine its ef ect on the cytocompatibility of PDMS. The adsorption of nonspecif c proteins and cytocompatibility to pheochromocytoma (PC12) cells, including cell attachment and dif erentiation, on PDMS and PU/PVA-coated PDMS were compared. The PU hydrogel coatings were also assessed as a comparison.
Materials and Methods
Preparation of PU/PVA
Before use, polyethylene glycol (CP, molecular weight = 1,000) (Sinopharm, Shanghai, China) was dried at 80°C for 1 day, and the N,N-dimethylformamide (Sinopharm) was dehydrated. As shown in Figure 1, the synthesis of PU/PVA included two steps: f rst, the prepolymers of PU were synthesized. Next, the PU prepolymers were mixed into a PVA (CP, molecular weight = 80,000, Bodi Chemical, Tianjin, China) solution, and then a cross-linking reaction was initiated. The PU prepolymers were synthesized as previously described (Rao et al., 2012). In brief, the N,N-dimethylformamide, polyethylene glycol, and isophorone diisocyanate (Sigma, St. Louis, MO, USA) were mixed in a three-necked f ask, and dibutyltin dilaurate (Sinopharm) was added as a catalyst. Prior to initiating the reaction, the oxygen in the mixture was replaced with argon. Then, the solution was slowly heated to 70°C and placed in an argon atmosphere to react for 12 hours. For the cross-linking reaction, the PVA was dehydrated in a vacuum-drying oven for 8 hours, and then dissolved in dimethyl sulfoxide (AR, Sinopharm) at 100°C for 30 minutes. After the PVA solution in the three-necked f ask was cooled to room temperature, a certain amount of N,N-dimethylformamide was added, and then the f xed solution was cooled to 0°C. Next, the PU prepolymers were slowly added into the mixture with intense stirring and purging with argon. Finally, the reaction conditions were maintained for 2 days. In the above mixture, the volume ratio of dimethyl sulfoxide and N,N-dimethylformamide was 1:1, and the molar ratio of PVA and PU prepolymers was kept at n(–NCO): m(–OH) of 1:12.5. In this study, the PU as the comparison was synthesized using a chain-extending reaction based on the methods from a previous study (Rao et al., 2012).
Fabrication of samples
Platinum silicone elastomer (medical-grade, MDX4-4210, Factor II, Dow Corning Corporation, Midland, MI, USA) and its cross-linking catalyst were degassed under a vacuum after being completely mixed. The mixture was placed in a stainless steel mold and heated to 80°C for 2 hours to create PDMS f lms. After cooling them to room temperature, the PDMS f lms were removed from the mold and cut into small round pieces approximately 0.2 mm thick and 14 mm in diameter. The PDMS f lms were ultrasonically cleaned with deionized water and acetone and dried under a vacuum at 40°C for 12 hours. The f lms were water plasma treated with a CTP-2000k plasma generator (Corona Lab, China) for 2 minutes at 60 W. Next, a 50-μL drop of 1 wt. % polymer solution (PU/PVA or PU) was placed onto the plasma-treated f lms and spread equally over the entire surface to create the PU/PVA- and PU-coated PDMS f lms. The coated PDMS f lms were dried at 80°C for 12 hours under a vacuum. Finally, all of the samples, including the PU/PVA- and PU-coated PDMS f lms and the control PDMS f lms, were washed three times with sterile water and placed in 24-well tissue culture plates (TCPs) after drying at 40°C for 12 hours. All of the prepared samples were sterilized with ethylene oxide before use.
Characterization
Fourier transform-infrared spectroscopy (FT-IR) measurements
PVA, PU/PVA, and PU solutions were coated on potassium bromide plates and dried with an infrared lamp. The FTIR spectra of these materials were measured with an FT-IR spectrometer (Thermo Nicolet Avatar 360) over the range of 4,000–400 cm?1.
Protein adsorption
As previously described (Chen et al., 2004, 2005a, b), fibrinogen (Calbiochem, San Diego, CA, USA) was labeled with125I (ICN Pharmaceuticals, Irvine, CA, USA) using the iodogen method to determine the amount of nonspecif c protein adsorption. For the assessment of f brinogen adsorption, labeled and unlabeled f brinogens were mixed at a ratio of 1:19 and the total f brinogen concentration of the solution was 1 mg/mL. Before the adsorption experiments, the surfaces of the samples were equilibrated in Tris-buffered saline overnight. Samples (PU/PVA-coated PDMS, PU-coated PDMS, and PDMS) were incubated in the125I-labeled protein solution at room temperature for 3 hours, and then rinsed three times with Tris-buf ered saline for 10 minutes each, blotted with f lter paper, and moved to stock tubes. The tubes were placed in a gamma counter (1480 Perkin Elmer) for radioactivity testing. Each sample group was assayed in triplicate. The amount of radioactivity was converted to calculate the amount of adsorbed protein.
Cell culture
Cell attachment
PC12 cells (China Center for Type Culture Collection, Wuhan, China) are widely used as a neural cell model for determining neuronal viability and neurite extension. Here, cell culture was performed for the PU/PVA-coated PDMS, PU-coated PDMS, and PDMS groups, with a TCP group as a control. The dif erent samples were af xed to the bottom of 24-well tissue culture plates and coated with 0.1 mg/mL poly L-lysine (Sigma) at 37°C for 6 hours to promote PC12 cell attachment. The PC12 cells were cultured in RPMI 1640 culture medium containing 5% fetal bovine serum, 2 mM L-glutamine, 10% horse serum, 100 μg/mL streptomycin, and 100 units/mL of penicillin. The cultures were maintained in a 95% humidif ed incubator with 5% CO2at 37°C. The PC12 cells were plated at a density of 2 × 104cells per well in the 24-well plates. DAPI staining was performed as previously described (Webb et al., 2001; Lopez et al., 2006). Digital images of the stained cells were taken on a Ti/S inverted f uorescence microscope equipped with a DS-5MC-U2 digital camera (Nikon, Japan). At least f ve randomly selected f elds of each well were imaged at 100× magnif cation. The cell attachment was quantif ed as the cell number per unit area using Image-Pro Plus? 6.0 software (Media Cybernetics, Silver Spring, MD, USA). The experiments were performed in triplicate.
Cell dif erentiation
After 24 hours of culture, the cells were serum starved for 6 hours. The culture medium was replaced with low-serum medium containing 50 ng/mL nerve growth factor (Sigma). Half of the culture medium was replaced every 2 days. After culture in the presence of nerve growth factor for 7 days, digital images of the PC12 cells were taken with an inverted phase-contrast microscope (Nikon). At least f ve randomly selected f elds were imaged from each well at 200× magnif cation. To assess the cell dif erentiation, 50 cells from each well were randomly selected and the average number and length of their neurites were calculated. The cellular dif erentiation was quantif ed as the number and length of neurites per cell using IPP 6.0 software (Media Cybernetics).
The cells were stained with calcein to visualize the neurite outgrowths from the PC12 cells on the dif erent samples, as was done in a previous study (Hashimoto et al., 2001). Briefly, the culture medium was removed and the samples were washed with 0.1 M PBS. The cells were then cultured in RPMI 1640 medium with 5 mM calcein AM (Sigma) at 37°C for 30 minutes in a CO2atmosphere. The samples were washed with 0.1 M PBS after calcein loading. Fluorescence images were taken with a Ti/S inverted f uorescence microscope (Nikon) using a 520-nm emission f lter and 490-nm excitation f lter. The cell culture experiments were conducted in triplicate. Each sample was assayed for cell attachment and dif erentiation in 16 independent wells of the 24-well plates (n = 16).
Statistical analysis
The statistical analysis was performed using SPSS 20.0 software (IBM Corp, Armonk, NY, USA). The measurement data were normally distributed and are expressed as the mean ± SEM. Differences between the PU-coated PDMS, PU/PVA-coated PDMS, PDMS, and TCP f lms were analyzed by one-way analysis of variance followed by Student-Newman-Keuls post hoc analysis. P-values less than 0.05 were considered statistically signif cant.
Results
FT-IR spectra
The FT-IR spectra of PVA, PU/PVA, and PU are shown in Figure 2. The main characteristic spectrum bands for the pure PVA f lms were: the O-H stretching vibration at 3,300 cm?1, the C-H stretching vibration at 2,941 and 2,910 cm?1, the C-H deformation vibration at 1,421 and 1,328 cm?1, the C-O stretching vibration at 1,241 cm?1, the C-O stretching vibration at 1,090 cm?1, and the O-H bend at 917 cm?1. The main characteristic FT-IR spectrum bands for the pure PU f lms were: the C-O-C stretching vibration at 1,106 cm?1, C-O and C-N stretching vibrations at 1,252 cm?1, C-H bending vibration of O-CH2at 1,452 cm?1, N-H stretching vibration of carbamate at 1,541 cm?1, C=O stretching vibration of carbamate at 1,712 cm?1, C-H stretching vibration of O-CH2at 2,872 cm?1, and N-H stretching vibration at 3,330 cm?1.
The -OH absorption peak at 3,330 cm?1was clearly decreased in the PU/PVA spectrum compared with that in the PVA spectrum, while the absorption peaks of C-O-C at 1,106 cm?1and N-H from carbamates at 1,541 cm?1were stronger, indicating that the -OH of the PVA reacted with the -N=C=O of the PU. The FT-IR characterization of the sample spectra indicated that the PU/PVA hydrogel was successfully created.
Protein adsorption
Fibrinogen has been widely used as a model for the nonspecif c adsorption of proteins on implants (Chen et al., 2004; Chen et al., 2005; Rao et al., 2012). The amounts of f brinogen adsorbed onto the surface of the PDMS, PU-coated PDMS, and PU/PVA-coated PDMS f lms are shown in Figure 3.
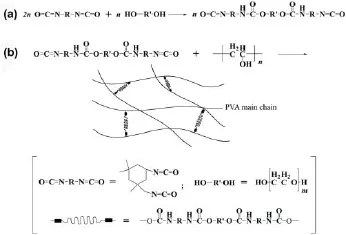
Figure 1 Schematic diagram of the polyurethane/poly(vinyl alcohol) (PU/PVA) synthesis.
The amount of nonspecif c protein adsorption on the uncoated PDMS surface was 442.67 ng/cm2, indicating that a signif cant amount of f brinogen had assembled and formed a protein layer. In contrast, the f brinogen adsorption was only 36.67 ng/cm2on the PU/PVA-coated surfaces (a reduction of ~92 %), and 32 ng/cm2on the PU-coated surfaces.
PC12 cell attachment
As shown in Figure 4, the cells on the PDMS films were more aggregated and showed a lower density per area than those on the PU-PU/PVA-coated PDMS films. The PC12 cells on the PU- and PU/PVA-coated PDMS displayed a similar degree of dispersion and cell density. As expected, the smallest number of PC12 cells attached to the TCP. The quantitative comparisons of cell density on the different samples are shown in Figure 4E, which mirror the qualitative results described above.
The cell densities on the PU- and PU/PVA-coated films were significantly higher than that on the PDMS film (P< 0.01), but were not dif erent to each other. These results demonstrated that the PU/PVA f lms had a better af nity for the PC12 cells than the PDMS alone, and that the PU/PVA and PU had similar affinities for the PC12 cells. Thus, the hydrophilic surfaces improved PC12 cell viability and neurite outgrowth. The introduction of PVA, a type of hydrophilic poly hydroxyl polymer, into the PU polymers resulted in a more hydrophilic surface that had more af nity to the PC12 cells than the hydrophobic PDMS surface.
PC12 cell dif erentiation
Neurite outgrowth from PC12 cells can be induced in the presence of nerve growth factor, and cytocompatible biomaterials promote neurite outgrowth in PC12 cells (Rao et al., 2012). The dif erentiation of PC12 cells on the dif erent materials is shown in Figure 5A–D. The cells on the PDMS f lms aggregated together and extended fewer and shorter neurites than those on the other f lms. In contrast, the PC12 cells exhibited better neurite extension and formed an interconnected neurite network when cultured on the surface of the PU, PU/PVA, and TCP materials on day 7. The quantitative results indicated that the neurite number per cell (Figure 5E) and neurite length per cell (Figure 5F) for cells on the PU/PVA were signif cantly higher and longer than those on the PDMS (P < 0.01). The PC12 cells on the PU/PVA and PU showed more and longer neurites than those on the TCP.
Discussion
Neural prostheses such as cochlear implants, deep brain stimulation devices, and eye prostheses have played an important role in the treatment of brain injury and neurological disability. However, tissue encapsulation of the electrode-tissue interface caused by tissue responses after implantation increases the distance between the electrode and neurons, leading to large increases in the impedance between the electrode materials and the neural tissue, which weakens the ef ect of the electrical stimulation over time. Therefore, there is an urgent need to decrease the tissue responses by improving the biocompatibility of the implantable electrode materials. Coating the electrode surface with a biocompatible hydrogel is one feasible and simple technique to achieve this goal. The aim of the present study was to synthesize a PU/PVA hydrogel and investigate its ef ect on the cytocompatibility of PDMS.
First, the PU/PVA hydrogel was synthesized and the FT-IR spectrum was measured to verify the formation of the PU/ PVA hydrogel. Previous studies indicated that the common, nonspecif c adsorption of a protein layer was related to the tissue response and harmful to the performance of implants (Chen et al., 2004). The amount of nonspecific protein adsorption was 442 ng/cm2on the PDMS surface, but the f brinogen adsorption was decreased to 36.7 ng/cm2on the PU/PVA-coated surfaces and 32 ng/cm2on the PU-coated surfaces. PU had previously exhibited good biocompatibility and protein resistance (Rao et al., 2012). The PU/PVA hydrogel coatings showed similar levels of nonspecif c protein adsorption to the PU hydrogel coatings. These results indicate that the PU/PVA hydrogel possesses good nonspecif c protein resistance, which should improve the stability of the electrode/tissue interface.
PC12 cells are a commonly used model for assessing cell viability and neurite outgrowth, which were done here on the PU/PVA hydrogel coatings. Based on the protein adsorption and PC12 cell attachment and dif erentiation results, the PU/ PVA coating improved the cytocompatibility of PDMS to PC12 cells, which can be attributed to the hydrophilicity of the coating yielding higher cell viability and more neurite extension. Furthermore, the cytocompatibility of the PU/PVA is similar to that of PU, which is a biocompatible material appropriate for long-term use in implantable neural prostheses (Rao et al., 2012). These results suggest that the PU/PVA hydrogel coating improves the cell attachment and neurite outgrowth of PC12 cells and is cytocompatible. However, hydrogel coatings have certain disadvantages, including being insulators and swelling as a result of water absorption. Improving the ionic conductivity and decreasing the swelling of PU/PVA hydrogels should be investigated further in future studies.
In summary, the PU/PVA hydrogel coatings prepared here could improve the cytocompatibility of the PDMS used in neural electrodes. The PU/PVA-coated PDMS f lms decreased the amount of protein adsorption by 92%, while the PC12 cell assays revealed that the PU/PVA coatings enhanced the attachment and neurite extension of PC12 cells. All of the measured characteristics of the PU/PVA hydrogels satisfy the requirements necessary for hydrogel coatings on implantable neural electrodes. The tissue responses after implantation could be assessed to further verify the improvement in PDMS biocompatibility using the PU/PVA hydrogel coatings.
Acknowledgments: The authors would like to thank Yi Lu from Shenzhen Institute of Advanced Technology, China and Ding-fang Wang (College of Chemistry and Molecular Science, Wuhan University, China) for their assistance in the synthesis of polyurethane and its characterization.
Author contributions: ML performed experiments and wrote the paper. YYD designed the study and provided technical support. ZYX provided critical revision and technical support.
HHZ analyzed data and performed experiments. TL performed experiments and provided materials. CYL revised the paper and analyzed the data. All authors approved the f nal version of the paper.
Conf icts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Anderson JM, Rodriguez A, Chang DT (2008) Foreign body reaction to biomaterials. Semin Immunol 20:86-100.
Anderson JM, Ziats NP, Azeez A, Brunstedt MR, Stack S, Bonfield TL (1995) Protein adsorption and macrophage activation on polydimethylsiloxane and silicone rubber. J Biomater Sci Polym Ed 7:159-169.
Biran R, Martin DC, Tresco PA (2005) Neuronal cell loss accompanies the brain tissue response to chronically implanted silicon microelectrode arrays. Exp Neurol 195:115-126.
Chan CKL (2008) A preliminary study of functional electrical stimulation in upper limb rehabilitation after stroke: an evidence-based review. Hong Kong J Occup Th 18:52-58.
Chen H, Brook MA, Sheardown H (2004) Silicone elastomers for reduced protein adsorption. Biomaterials 25:2273-2282.
Chen H, Chen Y, Sheardown H, Brook MA (2005a) Immobilization of heparin on a silicone surface through a heterobifunctional PEG spacer. Biomaterials 26:7418-424.
Chen H, Zhang Z, Chen Y, Brook MA, Sheardown H (2005b) Protein repellant silicone surfaces by covalent immobilization of poly (ethylene oxide). Biomaterials 26:2391-2399.
Choi J, Bodugoz-Senturk H, Kung HJ, Malhi AS, Muratoglu OK (2007) Ef ects of solvent dehydration on creep resistance of poly (vinyl alcohol) hydrogel. Biomaterials 28:772-780.
Cogan SF (2008) Neural stimulation and recording electrodes. Annu Rev Biomed Eng 10:275-309.
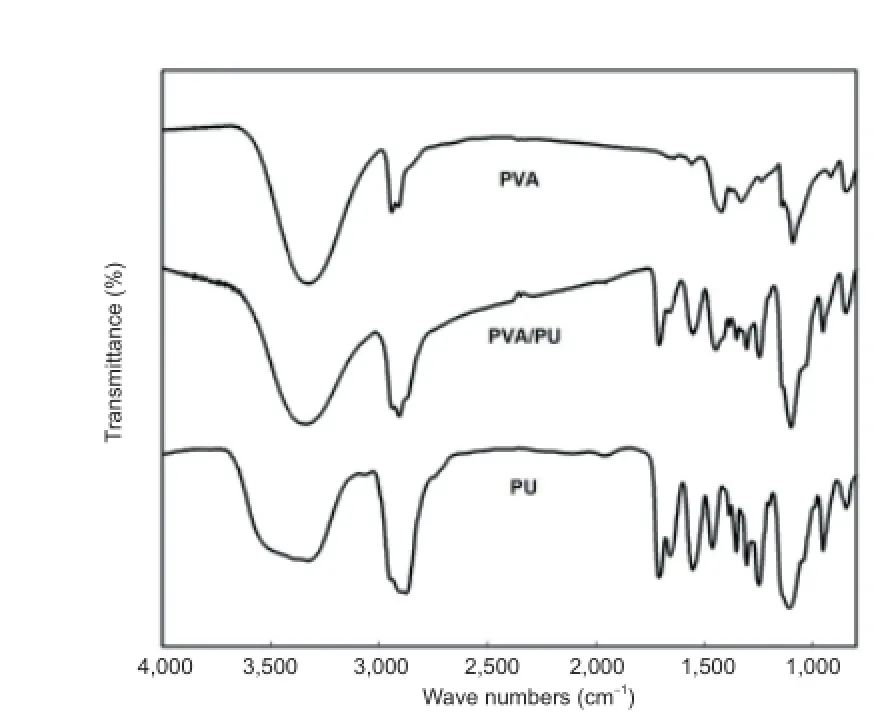
Figure 2 Fourier transform-infrared spectra of the poly(vinyl alcohol) (PVA), polyurethane/poly(vinyl alcohol) (PU/PVA), and polyurethane (PU) f lms.
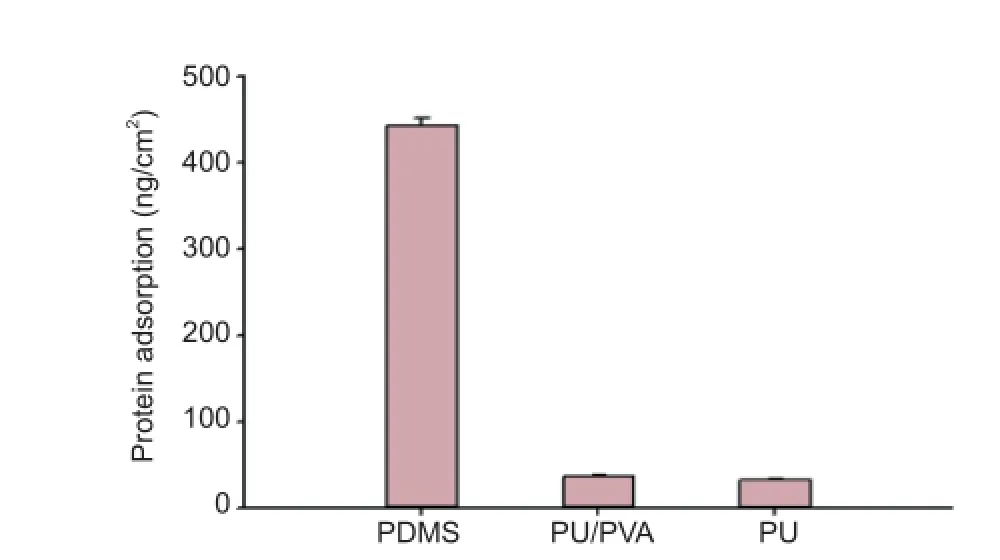
Figure 3 Fibrinogen adsorption on polydimethylsiloxane (PDMS), polyurethane/poly (vinyl alcohol) (PU/PVA)-coated PDMS, and polyurethane (PU)-coated PDMS f lms.
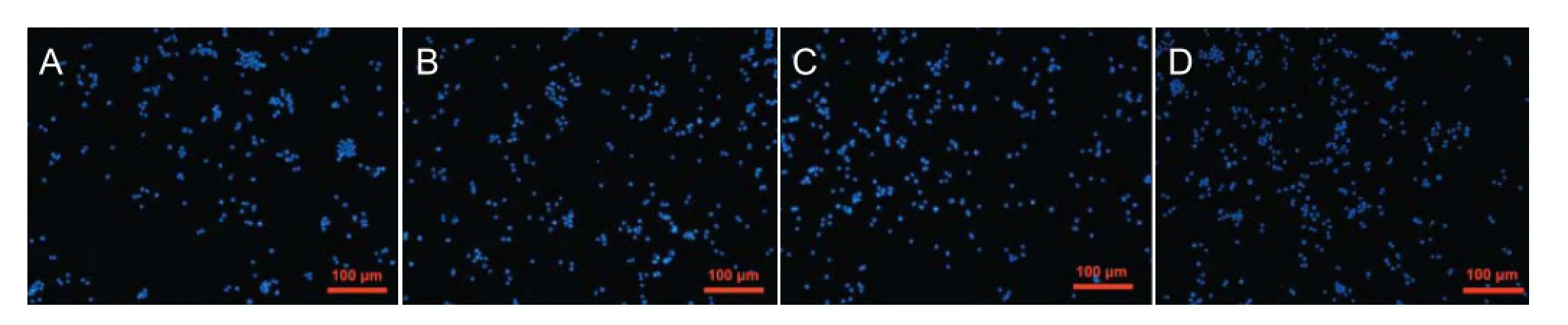
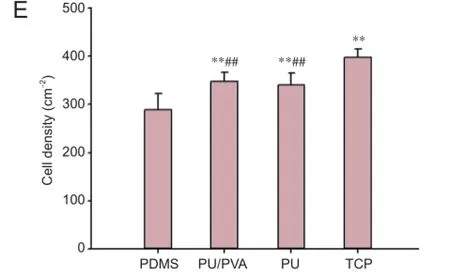
Figure 4 Representative 4′6-diamidino-2-phenylindole (DAPI)-stained f uorescent images of the PC12 cells cultured on dif erent materials.
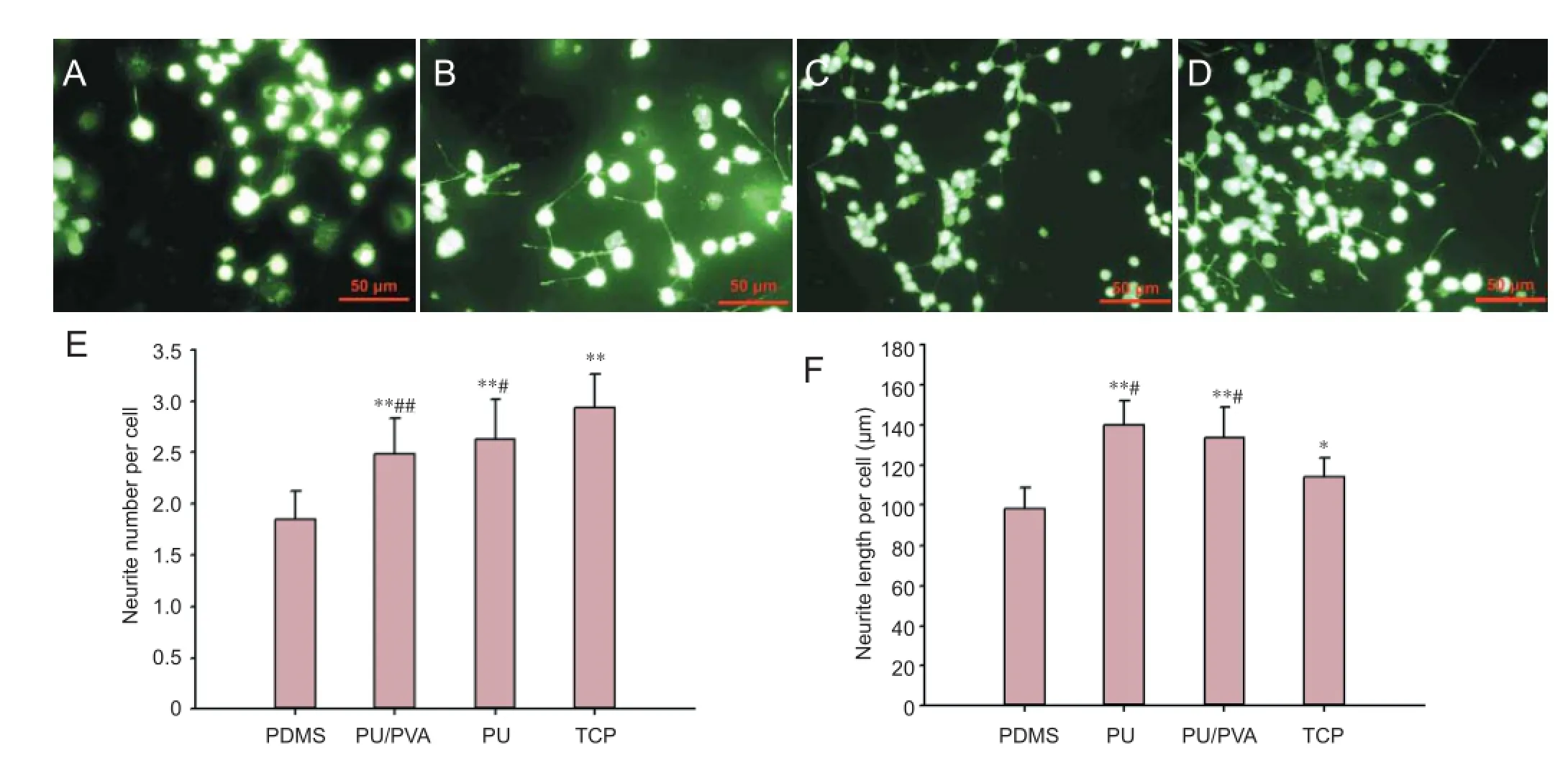
Figure 5 Representative calcein AM-stained f uorescent images of the PC12 cells cultured on dif erent materials.
Denheyer M, Kiss ZH, Haf enden AM (2009) Behavioral ef ects of subthalamic deep brain stimulation in Parkinson’s disease. Neuropsychologia 47:3203-3209.
Erlich MA, Parhiscar A (2003) Nasal dorsal augmentation with silicone implants. Facial Plast Surg 19:325-330.
Hamidi M, Azadi A, Rafiei P (2008) Hydrogel nanoparticles in drug delivery. Adv Drug Deliv Rev 60:1638-1649.
Han N, Rao SS, Johnson J, Parikh KS, Bradley PA, Lannutti JJ, Winter JO (2011) Hydrogel-electrospun f ber mat composite coatings for neural prostheses. Front Neuroeng 4:2.
Hashimoto Y, Niikura T, Tajima H, Yasukawa T, Sudo H, Ito Y (2001) A rescue factor abolishing neuronal cell death by a wide spectrum of familial Alzheimer’s disease genes and A beta. Proc Natl Acad Sci U S A 98:6336-6341.
He W, McConnell GC, Schneider TM, Bellamkonda RV (2007) A novel anti-inf ammatory surface for neural electrodes. Adv Mater 19:3529-3533.
Hochberg LR, Serruya MD, Friehs GM, Mukand JA, Saleh M, Caplan AH, Branner A, Chen D, Penn RD, Donoghue JP (2006) Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature 442:164-171.
Jackson A, Mavoori J, Fetz EE (2006) Long-term motor cortex plasticity induced by an electronic neural implant. Nature 444:56-60.
Lopez CA, Fleischman AJ, Roy S, Desai TA (2006) Evaluation of silicon nanoporous membranes and ECM-based microenvironments on neurosecretory cells. Biomaterials 27:3075-3083.
Lu Y, Wang D, Li T, Zhao X, Cao Y, Yang H, Duan YY (2009) Poly (vinyl alcohol)/poly (acrylic acid) hydrogel coatings for improving electrode-neural tissue interface. Biomaterials 30:4143-4151.
Makeig S, Westerf eld M, Jung TP, Enghof S, Townsend J, Courchesne E, Sejnowski TJ (2002) Dynamic brain sources of visual evoked responses. Science 295: 690-694.
Mawad D, Martens PJ, Odell RA, Poole-Warren LA (2007) The ef ect of redox polymerisation on degradation and cell responses to poly (vinyl alcohol) hydrogels. Biomaterials 28:947-955.
Polikov VS, Tresco PA, Reichert WM (2005) Response of brain tissue to chronically implanted neural electrodes. J Neurosci Methods 148:1-18.
Rao L, Zhou H, Li T, Li C, Duan YY (2012) Polyethylene glycol-containing polyurethane hydrogel coatings for improving the biocompatibility of neural electrodes. Acta Biomater 8:2233-2242.
Rauschecker JP, Shannon RV (2002) Sending sound to the brain. Science 295:1025-1029.
Sloviter RS (1987) Decreased hippocampal inhibition and a selective loss of interneurons in experimental epilepsy. Science 235:73-76.
Stieglitz T, Huang W, Chen SC, Morley JW, Lovell NH, Suaning G (2010) A transparent electrode array for simultaneous cortical potential recording and intrinsic signal optical imaging. Conf Proc IEEE Eng Med Biol Soc 2010:1796-1799.
Talwar SK, Xu SH, Hawley ES, Weiss SA, Moxon KA, Chapin JK (2002) Behavioural neuroscience: Rat navigation guided by remote control-Free animals can be ‘virtually’ trained by microstimulating key areas of their brains. Nature 417:37-38.
Teramura Y, Kaneda Y, Iwata H (2007) Islet-encapsulation in ultra-thin layer-by-layer membranes of poly(vinyl alcohol) anchored to poly(ethylene glycol)-lipids in the cell membrane. Biomaterials 28:4818-4825.
Webb K, Budko E, Neuberger TJ, Chen S, Schachner M, Tresco PA (2001) Substrate-bound human recombinant L1 selectively promotes neuronal attachment and outgrowth in the presence of astrocytes and f broblasts. Biomaterials 22:1017-1028.
Winter J O, Cogan S F, Rizzo J F (2007) Neurotrophin-eluting hydrogel coatings for neural stimulating electrodes, J Biomed Mater Res B Appl Biomater 81:551-563.
Zhong YH, Yu XJ, Gilbert R, Bellamkonda RV (2001) Stabilizing electrode-host interfaces: a tissue engineering approach. J Rehabil Res Dev 38:627-632.
Zhou HH, Li T, Duan YY (2012) Reduce impedance of intracortical iridium oxide microelectrodes by hydrogel coatings. Sensor Actuat B-Chem 161:198-202.
Copyedited by McCarty W, PacK M, Wang J, Qiu Y, Li CH, Song LP, Zhao M
*Correspondence to: Zhong-yuan Xia, M.D. or Yanwen Y. Duan, Ph.D., xiazhongyuan2005@aliyun.com or yduan@whu.edu.cn.
orcid: 0000-0002-5807-9554 (Zhong-yuan Xia) 0000-0001-6937-9977 (Yanwen Y. Duan)
10.4103/1673-5374.172325 http://www.nrronline.org/
Accepted: 2015-10-21
 中國(guó)神經(jīng)再生研究(英文版)2015年12期
中國(guó)神經(jīng)再生研究(英文版)2015年12期
- 中國(guó)神經(jīng)再生研究(英文版)的其它文章
- Neuroplasticity in post-stroke gait recovery and noninvasive brain stimulation
- Structural and functional connectivity in traumatic brain injury
- Neglected corticospinal tract injury for 10 months in a stroke patient
- Transplantation of human telomerase reverse transcriptase gene-transfected Schwann cells for repairing spinal cord injury
- Electroacupuncture promotes the recovery of motor neuron function in the anterior horn of the injured spinal cord
- A novel method for evaluating brain function and microstructural changes in Parkinson’s disease
