Neuroplasticity in post-stroke gait recovery and noninvasive brain stimulation
Yi Xu, Qing-hua Hou, Shawn D. Russell, Bradford C. Bennett, Andrew J. Sellers, Qiang Lin Dong-feng Huang
1 Department of Rehabilitation Medicine, the First Affi liated Hospital of Sun Yat-sen University, Guangzhou, Guangdong Province, China
2 Guangdong Provincial Engineering Technology Research Center for Rehabilitation Medicine and Clinical Translation, Guangzhou, Guangdong Province, China
3 Motion Analysis and Motor Performance Laboratory, Department of Orthopedics and Mechanical Engineering, University of Virginia, Charlottesville, VA, USA
4 Department of Neurology, Guangdong No.2 Provincial People’s Hospital, Guangzhou, Guangdong Province, China
5 H.C Sweere Center for Clinical Biomechanics and Applied Ergonomics, Northwestern Health Science University, Bloomington, MN, USA
6 Department of Radiology, Naval Medical Center Portsmouth, Portsmouth, VA, USA
Neuroplasticity in post-stroke gait recovery and noninvasive brain stimulation
Yi Xu1,2,3, Qing-hua Hou4, Shawn D. Russell3, Bradford C. Bennett5, Andrew J. Sellers6, Qiang Lin1,2, Dong-feng Huang1,2,*
1 Department of Rehabilitation Medicine, the First Affi liated Hospital of Sun Yat-sen University, Guangzhou, Guangdong Province, China
2 Guangdong Provincial Engineering Technology Research Center for Rehabilitation Medicine and Clinical Translation, Guangzhou, Guangdong Province, China
3 Motion Analysis and Motor Performance Laboratory, Department of Orthopedics and Mechanical Engineering, University of Virginia, Charlottesville, VA, USA
4 Department of Neurology, Guangdong No.2 Provincial People’s Hospital, Guangzhou, Guangdong Province, China
5 H.C Sweere Center for Clinical Biomechanics and Applied Ergonomics, Northwestern Health Science University, Bloomington, MN, USA
6 Department of Radiology, Naval Medical Center Portsmouth, Portsmouth, VA, USA
Gait disorders drastically aff ect the quality of life of stroke survivors, making post-stroke rehabilitation an important research focus. Noninvasive brain stimulation has potential in facilitating neuroplasticity and improving post-stroke gait impairment. However, a large inter-individual variability in the response to noninvasive brain stimulation interventions has been increasingly recognized. We fi rst review the neurophysiology of human gait and post-stroke neuroplasticity for gait recovery, and then discuss how noninvasive brain stimulation techniques could be utilized to enhance gait recovery. While post-stroke neuroplasticity for gait recovery is characterized by use-dependent plasticity, it evolves over time, is idiosyncratic, and may develop maladaptive elements. Furthermore, noninvasive brain stimulation has limited reach capability and is facilitative-only in nature. Therefore, we recommend that noninvasive brain stimulation be used adjunctively with rehabilitation training and other concurrent neuroplasticity facilitation techniques. Additionally, when noninvasive brain stimulation is applied for the rehabilitation of gait impairment in stroke survivors, stimulation montages should be customized according to the specifi c types of neuroplasticity found in each individual. This could be done using multiple mapping techniques.
nerve regeneration; stroke; cerebrovascular disorders; transcranial magnetic stimulation; neuroplasticity; transcranial direct current stimulation; electrical stimulation therapy; gait; walking; gait disorders; rehabilitation; neural regeneration
Funding: This work was supported by the National Natural Science Foundation of China, No. 30973165, 81372108, and a grant from Clinical Research 5010 Program Mission Statement of Sun Yat-Sen University, China, No. 2014001.
Xu Y, Hou QH, Russell SD, Bennett BC, Sellers AJ, Lin Q, Huang DF (2015) Neuroplasticity in poststroke gait recovery and noninvasive brain stimulation. Neural Regen Res 10(12):2072-2080.
Introduction
The American Heart Association estimates that approximately 795,000 individuals in the United States have a stroke each year (Go et al., 2014). A lack of mobility is the main obstacle for stroke survivors seeking to regain daily living independence and social integration. Thus, restoring impaired gait is one of the major goals of post-stroke rehabilitation. Recently, traditional rehabilitation techniques have been augmented by the use of a new methodology, noninvasive brain stimulation (NIBS), which facilitates neuroplasticity. To better understand the use of NIBS, this paper reviews literature regarding the neurophysiology of human gait, poststroke neuroplasticity in the motor control system underlying gait, and fi nally, approaches for using NIBS to enhance gait recovery.
Neurophysiology of Human Gait
Involvement of the cerebral cortices
In functional neuroimaging studies of human walking, the premotor cortex (PMC) and the supplementary motor cortex (SMC) are activated prior to step onset (Huppert et al., 2013). However, lesions in these two areas often lead to problems with gait initiation and the negotiation of narrow passages (Jahn et al., 2004), indicating their importance in the initiation and planning of walking. Furthermore, corticospinal inputs signifi cantly facilitate muscular responses in the lower limbs, especially during the swing phase of the step cycle (Pijnappels et al., 1998). These observations suggest that cortical outputs play a critical role in the modulation of lower limb locomotion.
The cerebral cortices are also involved in making adjust-ments during walking. For instance, when vision and proprioceptive inputs degrade during walking (e.g., switching from a lighted fi xed fl oor to a dark sway-referenced fl oor), bilateral temporal-parietal areas are activated (Karim et al., 2013). If, in this condition, experimental participants lose their balance, more cortices, such as the anterior parietal, anterior cingulate, and parietal cortices, are activated (Sipp et al., 2013). These results suggest that the cerebral cortices closely monitor posture and balance during walking. Furthermore, there is evidence that the SMC and PMC are implicated in anticipatory postural adjustments made while walking, which work to maintain equilibrium by counteracting the destabilizing eff ect induced by expected perturbations (Takakusaki et al., 2001; Chang et al., 2010). When dealing with unfamiliar circumstances or overcoming obstacles, multiple cerebral cortices, such as the PMC, SMC, and temporoparietal-posterior parietal cortices, may work closely to integrate diff erent senses and inputs so that adjustments can be made promptly and properly (Takakusaki, 2013).
Nonetheless, the aforementioned observations do not necessarily indicate that supraspinal outputs directly control the locomotion of walking. For example, transcranial magnetic stimulation (TMS) pulses, delivered at various phases of the step cycle, have no eff ect on the timing or duration of phasic tibialis anterior EMG bursts, suggesting that the cerebral cortices do not have a direct infl uence on muscle activities (Capaday et al., 1999). Instead, it is more likely that supraspinal neurons regulate the synergy of walking, rather than participate directly in controlling the locomotion of the lower limbs (Krouchev and Drew, 2013).
Involvement of the subcortical regions and spinal cord
For subcortical control of human gait, the locomotor region, located in the mid-brain, and the reticular formation, located in the ventromedial medulla, generate and maintain the rhythm of walking (Takakusaki, 2013). After receiving proprioceptive feedback that is integrated and distributed by the cerebellum, vestibular somatosensory cortex, and basal ganglion, these two aforementioned brain stem regions signal to motor neurons in the spinal cord to initiate the switch between the swing phase and the stance phase (Martinez et al., 2012). The reticular formation in the brain stem also distributes facilitative or inhibitory information from the cortex, basal ganglion, and cerebellum through descending pathways, which automatically modulates posture and muscle tone during walking (Takakusaki et al., 2004; Takakusaki, 2013). Therefore, in the hierarchical control system underlying human walking, subcortical regions work more as secondary command centers than as relay stations, and are especially responsible for the control of automated locomotion during walking.
Animal studies have demonstrated that the spinal cord also has a subprime control center that regulates walking, which appears to be a network of nerve cells called central pattern generators (CPGs). With modulation from the supraspinal descending pathways, the CPGs organize the activities of motor neurons so that the agonists and antagonists are excited alternately and are reciprocally balanced (Boothe et al., 2013). In this way, the CPGs drive the rhythmic movements of the limbs. Based on the observation that human infants exhibit stepping behavior even before birth, that is, prior to descending corticospinal fi ber myelination (Ivanenko et al., 2013), it seems likely that human beings have CPGs in the spinal cord as well. This extrapolation is also supported by reports of patients who can walk despite complete lateral corticospinal tract injuries (Ahn et al., 2006). Simultaneously, these reports also suggest that spinal cord CPGs can work, to some extent, independently from supraspinal control.
Post-stroke Neuroplasticity in the Motor Control System Underlying Human Gait
The adult brain retains the capability to reorganize itself under conditions of peripheral stimulation, learning, and injury (including stroke), through a process known as neuroplasticity. To date, post-stroke neuroplastic reorganization has been verifi ed at levels ranging from synapses and neurons to brain networks. This has been confi rmed in both animal models and humans (Clarkson et al., 2013; Karabanov et al., 2013), and is increasingly recognized as a critical driving force of post-stroke motor recovery. Some characteristics and mechanisms of post-stroke neuroplasticity in the motor control system underlying human gait are as follows:
Comprehensive neuroplastic reorganization
Using fMRI, researchers have found that post-stroke motor recovery of a paretic lower limb is associated with hyper-activation in multiple brain regions, including those in the contralesional hemisphere. Such regions may include the bilateral M1 cortex, secondary somatosensory cortices, SMC, PMC, cingulate gyrus, cerebellum, and thalamus (Luft et al., 2005; Enzinger et al., 2008). These results are consistent with those obtained using fNIRS (Miyai et al., 2003) or TMS during walking (Yang et al., 2010), and with the results of fMRI connectivity analyses that do not require movement to activate the cortices during examination (Jang et al., 2013). Furthermore, a strong correlation has been documented between the hyperactivation of these brain areas and walking improvement (Enzinger et al., 2009; Yang et al., 2010). Thus, these comprehensive instances of post-stroke hyperactivation are not likely to be merely a refl ection of unmasked interhemispheric or intrahemispheric inhibition.
Increased activity has also been observed in subcortical structures during the recovery of walking after a stroke. For example, researchers examined a group of chronic stroke patients who could walk independently using diff usion tensor imaging. They found increased fractional anisotropy values corresponding to the ipsilesional pedunculopontine nuclei, which is part of the mid-brain locomotor region (Takakusaki et al., 2004), that were positively correlated with the degree of walking recovery (Yeo et al., 2011). Such increased activation has also been reported in the cerebellum and mid-brain (Luft et al., 2008). Furthermore, analyses of muscular EMG activities in the lower limbs of post-stroke patients during hip or knee movement unveiled refl ex-mediated coupling betweenthe rectus femoris and hip adductors (Finley et al., 2008), indicating that neuroplastic reorganization is processed in the spinal cord after stroke. Several important studies have identifi ed comprehensive post-stroke neuroplasticity for gait recovery, as summarized in Table 1.
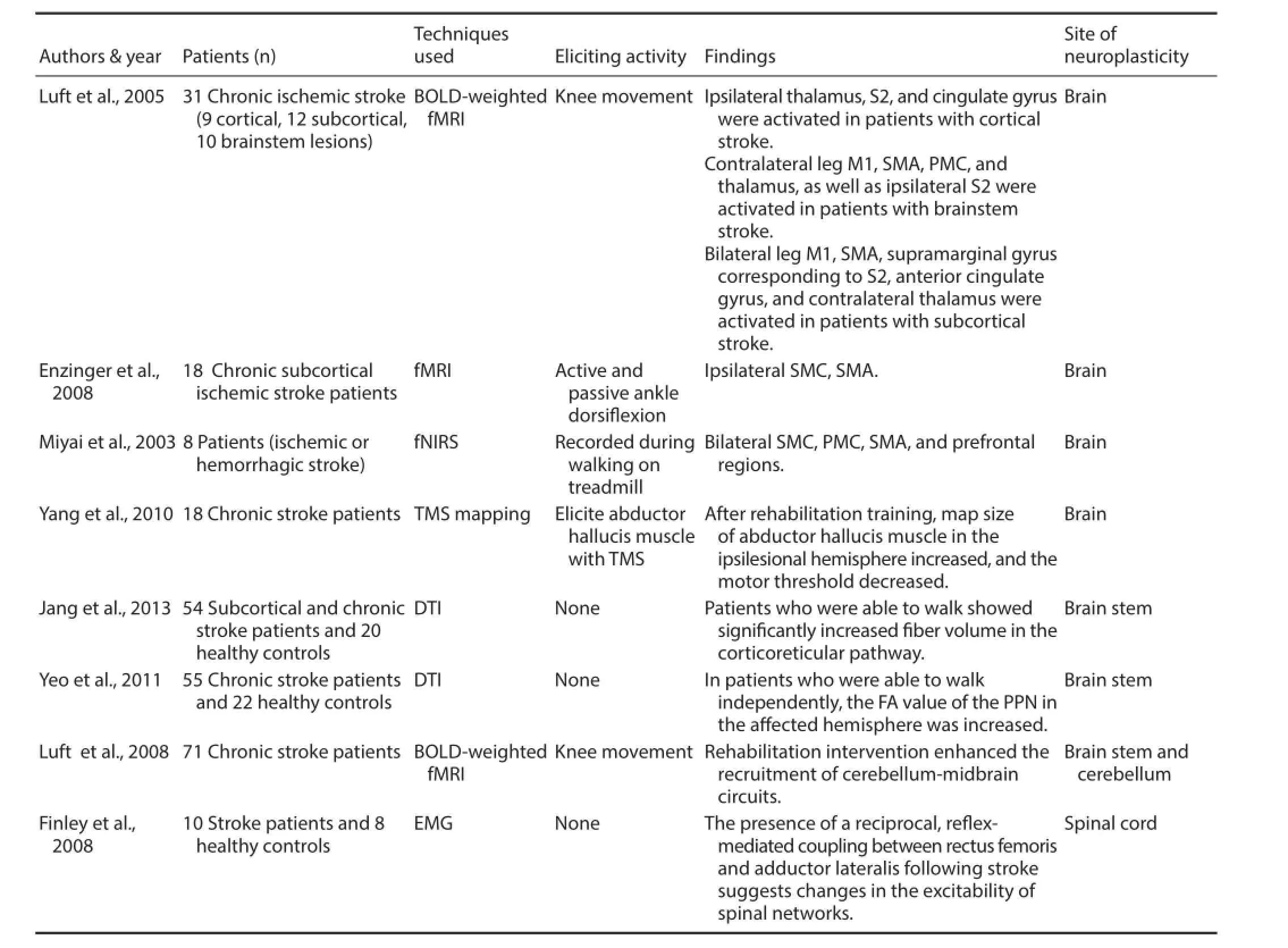
Table 1 Studies identifi ed with post-stroke comprehensive neuroplasticity for gait recovery
Temporally dynamic neuroplastic reorganization
A longitudinal fMRI investigation of 10 stroke patients revealed that paretic lower limb movement-triggered activation of the M1 cortex was initially prominent in the contralesional hemisphere after stroke. However, the original prominent activation pattern in the ipsilesional hemisphere was gradually restored over time. Additionally, the timing of this transition was correlated with the recovery of walking in these patients (Kim et al., 2006). Another investigation further demonstrated that appropriate gait training promotes this activation shift in somatotopic representations (Miyai et al., 2003). These observations provide insight into the temporal dynamics of post-stroke neuroplastic reorganization in the motor control system underlying walking.
The temporal dynamics of neuroplastic reorganization appear to be driven mainly by a dynamic imbalance in neuronal excitability that lies between the aff ected brain areas and those regions with which they have functional connections. After a stroke, the interhemispheric inhibition between the aff ected lesion and contralesional hemisphere is destroyed, and so sequential hyperactivation of the latter region may, in turn, suppress the excitability of the perilesional neurons (Manganotti et al., 2008; Clarkson et al., 2010). However, the excitability of perilesional neurons generally recovers, and may even exceed normal levels approximately 8 weeks after a stroke, according to observations from a mouse stroke model (Brown et al., 2009). Furthermore, the inhibitory strength from the contralateral hemisphere decreases over time (Kim et al., 2014; Takechi et al., 2014). These dynamic diff erences in excitability facilitate the unmasking of silent synapses, the formation of new synapses, and help adjust the threshold of selectivity in neuronal processing, thus allowing neuronsthat have been recruited for walking recovery to build new anatomic connections (Winship and Murphy, 2008).
The critical role of excitability diff erences in driving the evolution of cortical reorganization was demonstrated by a pharmacological prevention experiment, in which researchers found that abolishing hyperexcitability via diazepam within the fi rst 3 weeks after a brain injury delayed functional recovery, while applying the same treatment more than 3 weeks after the brain injury did not have the same eff ect (Schallert et al., 1986). Moreover, the observation that treatment-associated cortical reorganization preferentially occurs where intracortical inhibitory properties are low further supports the role of varied excitability as a driving force in neuroplastic reorganization after stroke (Liepert et al., 2006).
Heterogeneity among individual reorganization
The size and location of the lesion and the extent of secondary motor cortex involvement is thought to add to the diversifi cation of post-stroke neuroplastic reorganization. However, as suggested by Weiller and colleagues in their study of patients with capsular limited stroke, there are likely many additional factors governing neuroplastic reorganization that have yet to be discovered. They found that, despite relative homogeneity in lesion size, location, and clinical symptoms, patterns of cortical activation, as a measure of neuroplastic reorganization, were idiosyncratic (Weiller et al., 1993). This observation is not surprising considering how the brain remedies the loss of the cerebrospinal tract (CST) via stroke. After the projections from the M1 cortex to the spinal cord are destroyed by a stroke, survivors tend to recruit the impaired descending fi bers arising from the SMC and PMC to “take over” the role of the lost CST in recovering walking ability. This may be possible because both the SMC and PMC have projections to the bilateral M1 cortices and the spinal cord, and the outputs of their projections to the spinal cord have a facilitating eff ect on muscle activity (Boudrias et al., 2006; Dancause, 2006). This “take over”could be achieved by 1) enhancing the intrasulcus (Starkey et al., 2012), intrahemispheric (Carmichael et al., 2001), or interhemispheric connectivities (Wang et al., 2012a) between the two aforementioned areas in the bilateral hemispheres, as well as enhancing their connectivities with the aff ected M1 cortex, 2) by building up a bypass via the corticoreticular pathway (Jang et al., 2013), or by 3) recruiting additional relay connections at the spinal cord level (Courtine et al., 2008). The extent of the “take over” and the contribution of these diff erent processes likely varies markedly from person to person. In a longitudinal fMRI functional connectivity network analysis of 10 patients with stroke, who were analyzed across fi ve consecutive time points in a single year, the authors discovered that the motor execution network in the patients gradually shifted towards a random mode during the recovery process (Wang et al., 2010). Therefore, poststroke reorganization of walking control might be individualized by the varying degrees of participation of the entire residual motor system during the recovery process.
Not every incidence of post-stroke hyperactivation aids functional recovery
Because there are large diff erences in lesion characteristics among existing studies, it is difficult to compare different types of post-stroke hyperactivation among the literature. Additionally, the diff ering clinical states of the patients among studies infl uences whether contralesional reorganization can be classifi ed as “benefi cial” or “non-benefi cial.” For example, contralesional reorganization-related compensatory movements from the trunk and proximal limb may be benefi cial for severely impaired patients, but may not be benefi cial for complete recovery in mild or moderately impaired patients. Madhavan et al. (2010) studied this eff ect in a carefully selected cohort of stroke survivors and demonstrated that, regardless of lesion location or size, individuals with strong ipsilesional motor projections to the paretic lower limb showed inversely greater degradation of tracking accuracy in the non-paretic limb. Additionally, a higher rate of mirror movements has been documented in patients with greater degrees of ipsilesional cortical or cerebellum recruitment after stroke (Luft et al., 2005). These fi ndings indicate that not all poststroke cortical activation contributes to functional recovery, and suggest that some activation might even be maladaptive, for example, leading to mirror movements, spasm, dystonia, or interjoint coupling movements (Luft et al., 2005; Finley et al., 2008; Levin et al., 2009; Huynh et al., 2013).
Intra- and interhemispheric competitive interaction has been reported to be the main mechanism of maladaptive plasticity (Takeuchi and Izumi, 2012). First, neurophysiological studies have revealed a long-lasting interhemispheric imbalance after stroke, with the unaff ected hemisphere inhibiting the aff ected hemisphere, while widespread disinhibition exists in the aff ected hemisphere (Carmichael et al., 2001; Sun et al., 2012). Furthermore, motor function of the paretic limb is improved by inhibiting the contralesional hemisphere or nearby ipsilesional motor areas (Floel et al., 2008; Takeuchi and Izumi, 2012), while excessive training of the non-paretic limb can impair or delay functional recovery of the paretic limb (Kerr et al., 2013). Second, researchers have demonstrated that task-specifi c rehabilitative training, even when conducted for a short period of 5 consecutive days, can induce remapping of cortical activation from the contralesional hemisphere to the ipsilesional hemisphere (Boyd et al., 2010). Additionally, 6 weeks of unilateral high-intensity dorsifl exor resistance training has been found to produce bilateral neuromuscular plasticity in stroke survivors (Dragert and Zehr, 2013). This evidence suggests that the two hemispheres compete in terms of rewiring to the aff ected limbs, which might hinder, rather than facilitate, further recovery.
Not surprisingly, during the complex process of poststroke repair, some of the numerous outgrowths of new connections may be maladaptive (Wang et al., 2010).
Noninvasive Brain Stimulation
TMS and transcranial direct current stimulation (tDCS) are the two most common types of noninvasive brain stimulation. Neither of these are new to the medical practice, asTMS has been used clinically since 1985, and the use of tDCS can be traced back to the 1800s. In both NIBS techniques, an electric current is applied to cortical neurons (directly in tDCS or indirectly in TMS), which modulates the excitability of cortical circuits and augments neural plasticity (Romero Lauro et al., 2014). The focal modulating eff ect of NIBS can be either excitatory or inhibitory, depending on the stimulation protocols used. For example, with “conventional”repetitive TMS (rTMS) protocols (TMS pulses are delivered at a constant rate), the low-frequency stimulation (1Hz or less) is inhibitory and the high-frequency stimulation (5 Hz or more) is excitatory. In theta burst stimulation (TBS, TMS pulses are delivered in short rTMS bursts at frequency rates in the theta range, and with pauses between each stimulation burst), continuous TBS (pause periods = 2 seconds, cTBS) is inhibitory and intermittent TBS (pause periods = 10 seconds, iTBS) is excitatory. Likewise, both paired associative stimulation (PAS) with an interval duration of 10 milliseconds (PAS10) and cathodal tDCS (c-tDCS) are inhibitory, while PAS25 and anodal tDCS (A-tDCS) are excitatory. In addition to the instant focal excitability modulating eff ect upon stimulation, tDCS and TMS also have remote eff ects (via projecting fi bers to distant structures) and after-eff ects on the brain network that facilitate neural plasticity (Lang et al., 2004; Chib et al., 2013; Notturno et al., 2014).
The use of NIBS has been infrequent until recently, when the effi cacy of these techniques in facilitating neural plasticity was determined. To date, NIBS has shown promising effi -cacy in improving the motor function of paretic upper limbs (Takeuchi et al., 2009) and in treating aphasia (Khedr et al., 2014). Additionally, several primary studies have reported that NIBS techniques are effi cacious in the rehabilitation of post-stroke gait impairments (Wang et al., 2012b; Kakuda et al., 2013). However, a large inter-individual variability in response to NIBS interventions has been increasingly documented (Lefaucheur et al., 2014; Lopez-Alonso et al., 2014). Thus, we believe it necessary to reevaluate our understanding of gait impairment recovery after stroke.
Based on the above review of the neurophysiology of human gait and post-stroke reorganization in the motor control system underlying human gait, we herein propose several guidelines for the optimization of future NIBS applications:
NIBS should be used in combination with meaningful rehabilitation training
Neuroplastic reorganization is, above all, a use-dependent plasticity. It is therefore not surprising that functional recovery achieved by NIBS is usually greater when applied in combination with active task performance (Zimerman et al., 2012).
Central to this point is a discussion regarding what constitutes meaningful rehabilitation training: First, rehabilitation training should be meaningful with respect to skill learning. For instance, non-skill and passive training, that is, repeated voluntary and assisted dorsi- and plantarfl exion movements, did not increase cortical excitability, while motor skill training had a positive effect (Perez et al., 2004). Second, the combined training should be task-specifi c. In animals with complete spinal cord transections, those that were trained to stand did not walk well on a treadmill, while those that were trained to walk did not stand well (Wolpaw and Tennissen, 2001). Thus, by extrapolation, we surmise that specifi c task training facilitates individual performance of that task. Third, to reduce the risk of maladaptive reorganization, pathological movement should be avoided in jointly applied rehabilitation training. We therefore recommend the use of body weight support or robotics support in gait training, as these two therapeutic devices allow both automatic and manual correction of pathological movement patterns during gait training, and thus facilitate near normal patient gait. These recommendations are supported by recent successful experiences with the combined application of NIBS and rehabilitation training strategies (Wang et al., 2012b; Danzl et al., 2013).
NIBS should be used in combination with other concurrent neuroplasticity facilitation techniques
Each neuroplasticity facilitation measure has limitations and might only target limited parts of cortical circuits. For instance, when healthy adults learn motor adaptations, anodal tDCS stimulation can increase anterograde interference, but not savings (Leow et al., 2014). In a study that compared three diff erent experimental models of the organization of the human motor cortex, TMS only increased the amplitudes of motor evoked potentials (MEP), and had no effect on short-interval intracortical inhibition (Rosenkranz and Rothwell, 2006).
Therefore, to activate neuroplastic reorganization at multiple levels, it is advisable to administer NIBS in combination with other concurrent neuroplasticity facilitation techniques. For this purpose, body weight support treadmill training, functional electrical stimulation of the lower limbs, and augmented bio-feedback treatments can facilitate activation of the locomotor circuits of the spinal cord (Stein et al., 2013), thus strengthening the remote eff ects of NIBS treatment. Balance training can enhance cerebellar–brainstem interactions, and therefore can be jointly used to facilitate the rebuilding of connectivity at the brain stem level (Reisman et al., 2007). Additionally, as constraint-induced movement therapy can reduce interhemispheric inhibition and prevent learned non-use of the paretic limbs, it appears to enhance the therapeutic eff ects of NIBS (Williams et al., 2010). Furthermore, pharmacological treatments may also work with NIBS to yield greater rehabilitation.
Timing of NIBS treatment
The natural recovery of the residual brain proceeds at a defi ned pace after an injury. For instance, perilesional tissues exhibit a depression in metabolism and a decrease in neurite density within several days after a stroke (Ito et al., 2006; Jablonka et al., 2010) and new prosperous connectivity is not observed until 2 weeks after stroke onset (Nudo, 2006). Thus, NIBS therapy is likely to be most effective when it is performed in consideration of the natural pace of endogenous neuroplastic reorganization.
In animal studies, the diff erence in hyperexcitability that drives neuroplastic reorganization subsequently dissipatesover several weeks following a stroke (Schallert et al., 1986), indicating that there is a critical time window for rehabilitative interventions. Accordingly, rehabilitative effi cacy declined over time in an ischemic stroke animal model within a 30-day observation period (Takeuchi et al., 2004), signifying the importance of early NIBS treatment. Nonetheless, limited observations in animal models also suggest that tDCS treatment may be less eff ective if initiated too soon after an injury. These models favor initiation of tDCS therapy 1 week after a stroke rather than 1 day after a stroke (Kim et al., 2010; Jiang et al., 2012). Consistent with this, in a recent clinical trial, researchers applied anodal tDCS stimulation to the aff ected motor cortex of 25 patients 2 days after a stroke (20 minutes once a day for 5 consecutive days), and did not fi nd any signifi cant functional improvements between the treatment group and controls (Nudo et al., 2006). Therefore, the optimal timing for the initiation of NIBS treatment in the acute phase after a stroke is still unclear. Thus, although results regarding the application of NIBS to subacute and chronic stroke patients are mostly favorable, no studies have precisely identifi ed the optimal timing for postacute phase NIBS treatment.
Based on the above, it is clear that attention should be paid to the temporal relationships between NIBS interventions, the application of other neuroplasticity facilitation rehabilitative techniques, and the timing of NIBS delivery during a gait cycle. For instance, improvements in behavioral performance were observed only when tDCS was delivered prior to, but not during a task (Pirulli et al., 2014). Additionally, when the PAS protocol of TMS is applied in diff erent phases of gait cycle, it can increase the muscular response if it is delivered in the late swing phase, or suppress it if it is delivered in the mid swing phase (Prior and Stinear, 2006). Thus, the timing of NIBS delivery during a gait cycle should be considered with respect to the design of NIBS protocol.
Customized considerations for NIBS treatment
Because post-stroke neuroplastic reorganization evolves over time, is idiosyncratic, and may develop maladaptive characteristics, NIBS application requires a customized stimulation paradigm designed for each individual patient.
First, decisions regarding which hemisphere to stimulate are not trivial. Although ipsilesional facilitation stimulation has been found to be benefi cial, recent research is more in support of inhibitory stimulation of the contralesional hemisphere. A study comparing the motor recovery of 36 patients who were randomly divided into 3 groups to receive ipsilesional facilitating stimulation, contralesional inhibitory stimulation, or sham stimulation, showed that contralesional inhibitory stimulation was more eff ective than ipsilesional facilitating stimulation (Khedr et al., 2009). Also, greater degrees of functional recovery and activated connectivities have been reported for protocols involving contralesional inhibitory stimulation compared with those without (Takeuchi et al., 2009; Sehm et al., 2013). However, as the role of the contralesional hemisphere in stroke recovery varies for each individual patient and at diff erent stages of recovery (Schallert et al., 1986; Ago et al., 2003; Lotze et al., 2006), only careful cortical mapping can delineate which patients will most benefi t from ipsilesional, contralesional or bihemispheric stimulation.
Second, the location and extent of stimulation for each hemisphere needs to be identifi ed. Although experimental investigations have indicated that distributed stimulation works better than focalized stimulation (Boychuk et al., 2011), non-selective extensive stimulation may not be acceptable for clinical application. Cortical stimulation might reduce or even reverse the motor outputs from the representations for a nearby body part. For example, stimulation of the face or hand representations can cancel and even reverse the increase of motor output from the arm representation (Ziemann et al., 2002). Additionally, stimulating different portions of non-motor cortices might result in completely diff erent eff ects on the excitability of bilateral M1 regions. For example, stimulation of the anterior portion of the inferior-parietal lobule resulted in inhibition of the ipsilateral M1 in both hemispheres, while stimulation of the central and posterior portion of this region facilitated the excitability of ipsilateral M1 in the left but not the right hemisphere (Karabanov et al., 2013). However, diff erent TMS protocols may target diff erent preferential cortical circuits, e.g., low frequency rTMS selectively excites late I-wave producing circuits, while cTBS preferentially inhibits early I-wave producing circuits (Di Lazzaro et al., 2005, 2008). Even reproducible“excitability” or “inhibitory” eff ects can be achieved based on tissue physiology. Thus, the functional outcome of a specifi c NIBS protocol is still highly related to the pre-stimulation state of the tissue (Daskalakis et al., 2006; Giacobbe et al., 2013; Pirulli et al., 2014). Accordingly, clinical applications of NIBS have stringent requirements regarding the determination of stimulation targets and methods. While at present, stimulation sites are most commonly determined by fMRI and/or TMS mapping, these two methods have limitations. Because of gantry size and image degradation, fMRI studies are only able to incorporate very limited limb movements during activation mapping, such as using fi nger movement to mimic the movement of the whole upper limb, and using ankle fl exion to mimic walking. TMS also has limitations with respect to mapping changes in the somatosensory cortices, as it basically relies on testing muscle activities. Ideally, multiple mapping techniques should be integrated for identifi cation of the sites and extent of NIBS interventions. Subsequently, we recommend the use of computer modeling to test the accuracy of the stimulation montage, or alternatively, the use of magnetoencephalography to monitor neuromagnetic brain activity during tDCS stimulation (Datta et al., 2011).
Third, the optimal stimulation paradigm for each patient should be determined. Recent studies have indicated that rTMS can improve the gait performance of stroke survivors after either being applied alone or in combination with task-oriented training (Wang et al., 2012b; Kakuda et al., 2013). PAS applied to chronic stroke patients has also resulted in positive eff ects regarding the recovery of lower limb motor function (Rogers et al., 2011). In a head-to-head comparative study of the local and remote eff ects of diff erent TMS paradigms, 6 most commonly used TMS paradigms were applied to the M1 cortex of 10 healthy subjects in a randomized crossover manner. iTBS was found to be mosteff ective in increasing cortical excitability among the three excitatory paradigms (iTBS, PAS25, and 5 Hz rTMS), while PAS10 was the most successful among the three inhibitory paradigms in inhibiting cortical and intra-cortical excitability (cTBS, PAS10, 1 Hz rTMS) (Di Lazzaro et al., 2011). As this comparison was performed among young healthy subjects, this fi nding likely cannot be extrapolated to the applications of NIBS on non-motor cortical regions or for the treatment of stroke patients. Thus, further investigation is required to identify optimal stimulation paradigms for the rehabilitation of walking disorders after stroke.
Three recent small sample studies tested the eff ect of tDCS (specifi cally A-tDCS over the ipsilesional hemisphere) stimulation on lower limb function or postural stability (Danzl et al., 2013; Sohn et al., 2013; Cha et al., 2014) with positive results. However, inter-individual diff erences in the response to NIBS have been reported for applications over the lower limb motor cortex (Madhavan and Stinear, 2010). Additionally, different stroke subtypes might be differentially susceptible to the benefi cial eff ects of a specifi c stimulation paradigm (Ameli et al., 2009).
Additional research is needed to optimize the intensity, frequency, and even the direction of NIBS. Changes in these parameters can have drastic impacts on therapeutic eff ect. For instance, doubling the duration of stimulation can reverse the outcome from inhibition to excitation and vice versa (Gamboa et al., 2010).
Conclusions
Given the high inter-individual variability in responses to NIBS, we recommend a customized stimulation montage for each individual, and a real-time monitoring system that allows for adjustments during stimulation. In this regard, the following issues need to be addressed in future research: (1) Identify genetic or imaging biomarkers that can be used to predict responses to NIBS interventions. (2) Defi ne target sites more precisely. This includes a need for improved surrogate models for approximating gait during imaging, or alternatively, incorporating brain computer interface techniques into future target site-mapping procedures. This may improve interpretations of the role of detected hyper-activations in the recovery of post-stroke gait impairment. (3) Explore methods for high precision stimulation of specifi c targets and develop new protocols that have less variable eff ects on cortical excitability. (4) Develop a system that monitors the eff ect of stimulation and provides real-time feedback during the stimulation, thus allowing for any needed adjustments.
Acknowledgments: We thank our colleagues at the Department of Rehabilitation Medicine of the First Affi liated Hospital of Sun Yat-Sen University, Guangdong Provincial Research Center for Rehabilitation Medicine and Translational Engineering Technology, China, and the Motion Analysis and Motor Performance Laboratory of University of Virginia, USA, for their assistance and support.
Author statements: The views expressed in this presentation are those of the author Bradford C. Bennett and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, or the United States government. I am a military service member. This work was prepared as part of my offi cial duties. Title 17, USC, §105 provides that‘Copyright protection under this title is not available for any work of the U.S. Government. ‘Title 17, USC, §101 defi nes a U.S. Government work as a work prepared by a military service member or employee of the U.S. government as part of that person’s offi cial duties.
Author contributions: YX and QHH researched and reviewed literature and prepared the manuscript. DFH defined the intellectual content of the paper and edited the manuscript. SDR, BCB, AJS, and QL proofread and made edits and comments to the manuscript. All authors approved the fi nal version of this paper. Confl icts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Ago T, Kitazono T, Ooboshi H, Takada J, Yoshiura T, Mihara F, Ibayashi S, Iida M (2003) Deterioration of pre-existing hemiparesis brought about by subsequent ipsilateral lacunar infarction. J Neurol Neurosurg Psychiatry 74:1152-1153.
Ahn YH, Ahn SH, Kim H, Hong JH, Jang SH (2006) Can stroke patients walk after complete lateral corticospinal tract injury of the aff ected hemisphere? Neuroreport 17:987-990.
Ameli M, Grefkes C, Kemper F, Riegg FP, Rehme AK, Karbe H, Fink GR, Nowak DA (2009) Diff erential eff ects of high-frequency repetitive transcranial magnetic stimulation over ipsilesional primary motor cortex in cortical and subcortical middle cerebral artery stroke. Ann Neurol 66:298-309.
Boothe DL, Cohen AH, Troyer TW (2013) Phase locking asymmetries at fl exor-extensor transitions during fi ctive locomotion. PLoS One 8:e64421.
Boudrias MH, Belhaj-Saif A, Park MC, Cheney PD (2006) Contrasting properties of motor output from the supplementary motor area and primary motor cortex in rhesus macaques. Cereb Cortex 16:632-638.
Boychuk JA, Adkins DL, Kleim JA (2011) Distributed versus focal cortical stimulation to enhance motor function and motor map plasticity in a rodent model of ischemia. Neurorehabil Neural Repair 25:88-97.
Boyd LA, Vidoni ED, Wessel BD (2010) Motor learning after stroke: is skill acquisition a prerequisite for contralesional neuroplastic change? Neurosci Lett 482:21-25.
Brown CE, Aminoltejari K, Erb H, Winship IR, Murphy TH (2009) In vivo voltage-sensitive dye imaging in adult mice reveals that somatosensory maps lost to stroke are replaced over weeks by new structural and functional circuits with prolonged modes of activation within both the peri-infarct zone and distant sites. J Neurosci 29:1719-1734.
Capaday C, Lavoie BA, Barbeau H, Schneider C, Bonnard M (1999) Studies on the corticospinal control of human walking. I. Responses to focal transcranial magnetic stimulation of the motor cortex. J Neurophysiol 81:129-139.
Carmichael ST, Wei L, Rovainen CM, Woolsey TA (2001) New patterns of intracortical projections after focal cortical stroke. Neurobiol Dis 8:910-922.
Cha HK, Ji SG, Kim MK, Chang JS (2014) Eff ect of transcranial direct current stimulation of function in patients with stroke. J Physical Ther Sci 26:363-365.
Chang WH, Tang PF, Wang YH, Lin KH, Chiu MJ, Chen SH (2010) Role of the premotor cortex in leg selection and anticipatory postural adjustments associated with a rapid stepping task in patients with stroke. Gait Posture 32:487-493.
Chib VS, Yun K, Takahashi H, Shimojo S (2013) Noninvasive remote activation of the ventral midbrain by transcranial direct current stimulation of prefrontal cortex. Transl Psychiatry 3:e268.
Clarkson AN, Huang BS, Macisaac SE, Mody I, Carmichael ST (2010) Reducing excessive GABA-mediated tonic inhibition promotes functional recovery after stroke. Nature 468:305-309.
Clarkson AN, Lopez-Valdes HE, Overman JJ, Charles AC, Brennan KC, Thomas Carmichael S (2013) Multimodal examination of structural and functional remapping in the mouse photothrombotic stroke model. J Cereb Blood Flow Metab 33:716-723.
Courtine G, Song B, Roy RR, Zhong H, Herrmann JE, Ao Y, Qi J, Edgerton VR, Sofroniew MV (2008) Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nat Med 14:69-74.
Dancause N (2006) Vicarious function of remote cortex following stroke: recent evidence from human and animal studies. Neuroscientist 12:489-499.
Danzl MM, Chelette KC, Lee K, Lykins D, Sawaki L (2013) Brain stimulation paired with novel locomotor training with robotic gait orthosis in chronic stroke: a feasibility study. NeuroRehabilitation 33:67-76.
Daskalakis ZJ, Moller B, Christensen BK, Fitzgerald PB, Gunraj C, Chen R (2006) The eff ects of repetitive transcranial magnetic stimulation on cortical inhibition in healthy human subjects. Exp Brain Res 174:403-412.
Datta A, Baker JM, Bikson M, Fridriksson J (2011) Individualized model predicts brain current fl ow during transcranial direct-current stimulation treatment in responsive stroke patient. Brain Stimul 4:169-174.
Di Lazzaro V, Pilato F, Dileone M, Profice P, Oliviero A, Mazzone P, Insola A, Ranieri F, Tonali PA, Rothwell JC (2008) Low-frequency repetitive transcranial magnetic stimulation suppresses specifi c excitatory circuits in the human motor cortex. J Physiol 586:4481-4487.
Di Lazzaro V, Pilato F, Saturno E, Oliviero A, Dileone M, Mazzone P, Insola A, Tonali PA, Ranieri F, Huang YZ, Rothwell JC (2005) Theta-burst repetitive transcranial magnetic stimulation suppresses specific excitatory circuits in the human motor cortex. J Physiol 565:945-950.
Di Lazzaro V, Dileone M, Pilato F, Capone F, Musumeci G, Ranieri F, Ricci V, Bria P, Di Iorio R, de Waure C, Pasqualetti P, Profi ce P (2011) Modulation of motor cortex neuronal networks by rTMS: comparison of local and remote eff ects of six diff erent protocols of stimulation. J Neurophysiol 105:2150-2156.
Dragert K, Zehr EP (2013) High-intensity unilateral dorsiflexor resistance training results in bilateral neuromuscular plasticity after stroke. Exp Brain Res 225:93-104.
Enzinger C, Johansen-Berg H, Dawes H, Bogdanovic M, Collett J, Guy C, Ropele S, Kischka U, Wade D, Fazekas F, Matthews PM (2008) Functional MRI correlates of lower limb function in stroke victims with gait impairment. Stroke 39:1507-1513.
Enzinger C, Dawes H, Johansen-Berg H, Wade D, Bogdanovic M, Collett J, Guy C, Kischka U, Ropele S, Fazekas F, Matthews PM (2009) Brain activity changes associated with treadmill training after stroke. Stroke 40:2460-2467.
Finley JM, Perreault EJ, Dhaher YY (2008) Stretch refl ex coupling between the hip and knee: implications for impaired gait following stroke. Exp Brain Res 188:529-540.
Floel A, Hummel F, Duque J, Knecht S, Cohen LG (2008) Infl uence of somatosensory input on interhemispheric interactions in patients with chronic stroke. Neurorehabil Neural Repair 22:477-485.
Gamboa OL, Antal A, Moliadze V, Paulus W (2010) Simply longer is not better: reversal of theta burst after-eff ect with prolonged stimulation. Exp Brain Res 204:181-187.
Giacobbe V, Krebs HI, Volpe BT, Pascual-Leone A, Rykman A, Zeiarati G, Fregni F, Dipietro L, Thickbroom GW, Edwards DJ (2013) Transcranial direct current stimulation (tDCS) and robotic practice in chronic stroke: the dimension of timing. NeuroRehabilitation 33:49-56.
Go AS, Mozaff arian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huff man MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, et al. (2014) Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation 129:e28-292.
Huppert T, Schmidt B, Beluk N, Furman J, Sparto P (2013) Measurement of brain activation during an upright stepping reaction task using functional near-infrared spectroscopy. Hum Brain Mapp 34:2817-2828.
Huynh W, Krishnan AV, Lin CS, Vucic S, Katrak P, Hornberger M, Kiernan MC (2013) Botulinum toxin modulates cortical maladaptation in post-stroke spasticity. Muscle Nerve 48:93-99.
Ito U, Kuroiwa T, Nagasao J, Kawakami E, Oyanagi K (2006) Temporal profi les of axon terminals, synapses and spines in the ischemic penumbra of the cerebral cortex: ultrastructure of neuronal remodeling. Stroke 37:2134-2139.
Ivanenko YP, Dominici N, Cappellini G, Di Paolo A, Giannini C, Poppele RE, Lacquaniti F (2013) Changes in the spinal segmental motor output for stepping during development from infant to adult. J Neurosci 33:3025-3036a.
Jablonka JA, Burnat K, Witte OW, Kossut M (2010) Remapping of the somatosensory cortex after a photothrombotic stroke: dynamics of the compensatory reorganization. Neuroscience 165:90-100.
Jahn K, Deutschlander A, Stephan T, Strupp M, Wiesmann M, Brandt T (2004) Brain activation patterns during imagined stance and locomotion in functional magnetic resonance imaging. NeuroImage 22:1722-1731.
Jang SH, Chang CH, Lee J, Kim CS, Seo JP, Yeo SS (2013) Functional role of the corticoreticular pathway in chronic stroke patients. Stroke 44:1099-1104.
Jiang T, Xu RX, Zhang AW, Di W, Xiao ZJ, Miao JY, Luo N, Fang YN (2012) Eff ects of transcranial direct current stimulation on hemichannel pannexin-1 and neural plasticity in rat model of cerebral infarction. Neuroscience 226:421-426.
Kakuda W, Abo M, Nakayama Y, Kiyama A, Yoshida H (2013) High-frequency rTMS using a double cone coil for gait disturbance. Acta Neurol Scand 128:100-106.
Karabanov AN, Chao CC, Paine R, Hallett M (2013) Mapping diff erent intra-hemispheric parietal-motor networks using twin Coil TMS. Brain Stimul 6:384-389.
Karim H, Fuhrman SI, Sparto P, Furman J, Huppert T (2013) Functional brain imaging of multi-sensory vestibular processing during computerized dynamic posturography using near-infrared spectroscopy. NeuroImage 74:318-325.
Kerr AL, Wolke ML, Bell JA, Jones TA (2013) Post-stroke protection from maladaptive eff ects of learning with the non-paretic forelimb by bimanual home cage experience in C57BL/6 mice. Behav Brain Res 252:180-187.
Khedr EM, Abdel-Fadeil MR, Farghali A, Qaid M (2009) Role of 1 and 3 Hz repetitive transcranial magnetic stimulation on motor function recovery after acute ischaemic stroke. Eur J Neurol 16:1323-1330.
Khedr EM, Abo El-Fetoh N, Ali AM, El-Hammady DH, Khalifa H, Atta H, Karim AA (2014) Dual-hemisphere repetitive transcranial magnetic stimulation for rehabilitation of poststroke aphasia: a randomized, double-blind clinical trial. Neurorehabil Neural Repair 28:740-750.
Kim SJ, Kim BK, Ko YJ, Bang MS, Kim MH, Han TR (2010) Functional and histologic changes after repeated transcranial direct current stimulation in rat stroke model. J Korean Med Sci 25:1499-1505.
Kim YH, You SH, Kwon YH, Hallett M, Kim JH, Jang SH (2006) Longitudinal fMRI study for locomotor recovery in patients with stroke. Neurology 67:330-333.
Kim YK, Yang EJ, Cho K, Lim JY, Paik NJ (2014) Functional Recovery After Ischemic Stroke Is Associated With Reduced GABAergic Inhibition in the Cerebral Cortex: A GABA PET Study. Neurorehabil Neural Repair 28:576-583.
Krouchev N, Drew T (2013) Motor cortical regulation of sparse synergies provides a framework for the fl exible control of precision walking. Front Comput Neurosci 7:83.
Lang N, Nitsche MA, Paulus W, Rothwell JC, Lemon RN (2004) Eff ects of transcranial direct current stimulation over the human motor cortex on corticospinal and transcallosal excitability. Exp Brain Res 156:439-443.
Lefaucheur JP, André-Obadia N, Antal A, Ayache SS, Baeken C, Benninger DH, Cantello RM, Cincotta M, de Carvalho M, De Ridder D, Devanne H, Di Lazzaro V, Filipovi? SR, Hummel FC, J??skel?inen SK, Kimiskidis VK, Koch G, Langguth B, Nyff eler T, Oliviero A, et al. (2014) Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin Neurophysiol 125:2150-2206.
Leow LA, Hammond G, de Rugy A (2014) Anodal motor cortex stimulation paired with movement repetition increases anterograde interference but not savings. Eur J Neurosci 40:3243-3252.
Levin MF, Kleim JA, Wolf SL (2009) What do motor “recovery” and“compensation” mean in patients following stroke? Neurorehabil Neural Repair 23:313-319.
Liepert J, Haevernick K, Weiller C, Barzel A (2006) The surround inhibition determines therapy-induced cortical reorganization. Neuro-Image 32:1216-1220.
Lopez-Alonso V, Cheeran B, Rio-Rodriguez D, Fernandez-Del-Olmo M (2014) Inter-individual variability in response to non-invasive brain stimulation paradigms. Brain Stimul 7:372-380.
Lotze M, Markert J, Sauseng P, Hoppe J, Plewnia C, Gerloff C (2006) The role of multiple contralesional motor areas for complex hand movements after internal capsular lesion. J Neurosci 26:6096-6102.
Luft AR, Forrester L, Macko RF, McCombe-Waller S, Whitall J, Villagra F, Hanley DF (2005) Brain activation of lower extremity movement in chronically impaired stroke survivors. NeuroImage 26:184-194.
Luft AR, Macko RF, Forrester LW, Villagra F, Ivey F, Sorkin JD, Whitall J, McCombe-Waller S, Katzel L, Goldberg AP, Hanley DF (2008) Treadmill exercise activates subcortical neural networks and improves walking after stroke: a randomized controlled trial. Stroke 39:3341-3350.
Madhavan S, Stinear JW (2010) Focal and bi-directional modulation of lower limb motor cortex using anodal transcranial direct current stimulation. Brain Stimul 3:42.
Madhavan S, Rogers LM, Stinear JW (2010) A paradox: after stroke, the non-lesioned lower limb motor cortex may be maladaptive. Eur J Neurosci 32:1032-1039.
Manganotti P, Acler M, Zanette GP, Smania N, Fiaschi A (2008) Motor cortical disinhibition during early and late recovery after stroke. Neurorehabil Neural Repair 22:396-403.
Martinez M, Delivet-Mongrain H, Leblond H, Rossignol S (2012) Incomplete spinal cord injury promotes durable functional changes within the spinal locomotor circuitry. J Neurophysiol 108:124-134.
Miyai I, Yagura H, Hatakenaka M, Oda I, Konishi I, Kubota K (2003) Longitudinal optical imaging study for locomotor recovery after stroke. Stroke 34:2866-2870.
Notturno F, Marzetti L, Pizzella V, Uncini A, Zappasodi F (2014) Local and remote eff ects of transcranial direct current stimulation on the electrical activity of the motor cortical network. Hum Brain Mapp 35:2220-2232.
Nudo RJ (2006) Plasticity. NeuroRx 3:420-427.
Perez MA, Lungholt BK, Nyborg K, Nielsen JB (2004) Motor skill training induces changes in the excitability of the leg cortical area in healthy humans. Exp Brain Res 159:197-205.
Pijnappels M, Van Wezel BM, Colombo G, Dietz V, Duysens J (1998) Cortical facilitation of cutaneous refl exes in leg muscles during human gait. Brain Res 787:149-153.
Pirulli C, Fertonani A, Miniussi C (2014) Is neural hyperpolarization by cathodal stimulation always detrimental at the behavioral level? Front Behav Neurosci 8:226.
Prior MM, Stinear JW (2006) Phasic spike-timing-dependent plasticity of human motor cortex during walking. Brain Res 1110:150-158.
Reisman DS, Wityk R, Silver K, Bastian AJ (2007) Locomotor adaptation on a split-belt treadmill can improve walking symmetry poststroke. Brain 130:1861-1872.
Rogers LM, Brown DA, Stinear JW (2011) The eff ects of paired associative stimulation on knee extensor motor excitability of individuals post-stroke: a pilot study. Clin Neurophysiol 122:1211-1218.
Romero Lauro LJ, Rosanova M, Mattavelli G, Convento S, Pisoni A, Opitz A, Bolognini N, Vallar G (2014) TDCS increases cortical excitability: Direct evidence from TMS-EEG. Cortex 58:99-111.
Rosenkranz K, Rothwell JC (2006) Diff erences between the eff ects of three plasticity inducing protocols on the organization of the human motor cortex. Eur J Neurosci 23:822-829.
Schallert T, Hernandez TD, Barth TM (1986) Recovery of function after brain damage: severe and chronic disruption by diazepam. Brain Res 379:104-111.
Sehm B, Kipping J, Schafer A, Villringer A, Ragert P (2013) A comparison between uni- and bilateral tDCS eff ects on functional connectivity of the human motor cortex. Front Hum Neurosci 7:183.
Sipp AR, Gwin JT, Makeig S, Ferris DP (2013) Loss of balance during balance beam walking elicits a multifocal theta band electrocortical response. J Neurophysiol 110:2050-2060.
Sohn MK, Jee SJ, Kim YW (2013) Eff ect of transcranial direct current stimulation on postural stability and lower extremity strength in hemiplegic stroke patients. Ann Rehabil Med 37:759-765.
Starkey ML, Bleul C, Zorner B, Lindau NT, Mueggler T, Rudin M, Schwab ME (2012) Back seat driving: hindlimb corticospinal neurons assume forelimb control following ischaemic stroke. Brain 135:3265-3281.
Stein RB, Everaert DG, Roy FD, Chong S, Soleimani M (2013) Facilitation of corticospinal connections in able-bodied people and people with central nervous system disorders using eight interventions. J Clin Neurophysiol 30:66-78.
Sun F, Xie L, Mao X, Hill J, Greenberg DA, Jin K (2012) Eff ect of a contralateral lesion on neurological recovery from stroke in rats. Restor Neurol Neurosci 30:491-495.
Takakusaki K (2013) Neurophysiology of gait: from the spinal cord to the frontal lobe. Mov Disord 28:1483-1491.
Takakusaki K, Kohyama J, Matsuyama K, Mori S (2001) Medullary reticulospinal tract mediating the generalized motor inhibition in cats: parallel inhibitory mechanisms acting on motoneurons and on interneuronal transmission in refl ex pathways. Neuroscience 103:511-527.
Takakusaki K, Habaguchi T, Saitoh K, Kohyama J (2004) Changes in the excitability of hindlimb motoneurons during muscular atonia induced by stimulating the pedunculopontine tegmental nucleus in cats. Neuroscience 124:467-480.
Takechi U, Matsunaga K, Nakanishi R, Yamanaga H, Murayama N, Mafune K, Tsuji S (2014) Longitudinal changes of motor cortical excitability and transcallosal inhibition after subcortical stroke. Clin Neurophysiol 125:2055-2069.
Takeuchi N, Izumi S (2012) Maladaptive plasticity for motor recovery after stroke: mechanisms and approaches. Neural Plast 2012:359728. Takeuchi N, Tada T, Toshima M, Matsuo Y, Ikoma K (2009) Repetitive transcranial magnetic stimulation over bilateral hemispheres enhances motor function and training eff ect of paretic hand in patients after stroke. J Rehabil Med 41:1049-1054.
Wang L, Yu C, Chen H, Qin W, He Y, Fan F, Zhang Y, Wang M, Li K, Zang Y, Woodward TS, Zhu C (2010) Dynamic functional reorganization of the motor execution network after stroke. Brain 133:1224-1238.
Wang LE, Tittgemeyer M, Imperati D, Diekhoff S, Ameli M, Fink GR, Grefkes C (2012a) Degeneration of corpus callosum and recovery of motor function after stroke: a multimodal magnetic resonance imaging study. Hum Brain Mapp 33:2941-2956.
Wang RY, Tseng HY, Liao KK, Wang CJ, Lai KL, Yang YR (2012b) rTMS combined with task-oriented training to improve symmetry of interhemispheric corticomotor excitability and gait performance after stroke: a randomized trial. Neurorehabil Neural Repair 26:222-230.
Weiller C, Ramsay SC, Wise RJ, Friston KJ, Frackowiak RS (1993) Individual patterns of functional reorganization in the human cerebral cortex after capsular infarction. Ann Neurol 33:181-189.
Williams JA, Pascual-Leone A, Fregni F (2010) Interhemispheric modulation induced by cortical stimulation and motor training. Phys Ther 90:398-410.
Winship IR, Murphy TH (2008) In vivo calcium imaging reveals functional rewiring of single somatosensory neurons after stroke. J Neurosci 28:6592-6606.
Wolpaw JR, Tennissen AM (2001) Activity-dependent spinal cord plasticity in health and disease. Annl review Neurosci 24:807-843.
Yang YR, Chen IH, Liao KK, Huang CC, Wang RY (2010) Cortical reorganization induced by body weight-supported treadmill training in patients with hemiparesis of diff erent stroke durations. Arch Physl Med Rehabil 91:513-518.
Yeo SS, Ahn SH, Choi BY, Chang CH, Lee J, Jang SH (2011) Contribution of the pedunculopontine nucleus on walking in stroke patients. Eur Neurol 65:332-337.
Ziemann U, Wittenberg GF, Cohen LG (2002) Stimulation-induced within-representation and across-representation plasticity in human motor cortex. J Neurosci 22:5563- 5571.
Zimerman M, Heise KF, Hoppe J, Cohen LG, Gerloff C, Hummel FC (2012) Modulation of training by single-session transcranial direct current stimulation to the intact motor cortex enhances motor skill acquisition of the paretic hand. Stroke 43:2185-2191.
Copyedited by Koke S, Raye W, Li CH, Song LP, Zhao M
*Correspondence to: Dong-feng Huang, M.D., huangdf_sysu@163.com.
orcid: 0000-0002-1932-9581 (Yi Xu)
10.4103/1673-5374.172329 http://www.nrronline.org/
Accepted: 2015-03-28
- 中國神經(jīng)再生研究(英文版)的其它文章
- Structural and functional connectivity in traumatic brain injury
- Neglected corticospinal tract injury for 10 months in a stroke patient
- Polyurethane/poly(vinyl alcohol) hydrogel coating improves the cytocompatibility of neural electrodes
- Transplantation of human telomerase reverse transcriptase gene-transfected Schwann cells for repairing spinal cord injury
- Electroacupuncture promotes the recovery of motor neuron function in the anterior horn of the injured spinal cord
- A novel method for evaluating brain function and microstructural changes in Parkinson’s disease

