Third-line therapies in patients with Kawasaki disease refractory to first-and second-line intravenous immunoglobulin therapy
Takashi Furuta·Hiroki Yasudo·Seigo Okada ·Yuji Ohnishi·Akiko Kawakami-Miyake·Yasuo Suzuki·Shouichi Ohga·Shunji Hasegawa
Kawasaki disease (KD) is an acute febrile illness characterized by systemic vasculitis affecting the small-and mediumsized arteries in children [1,2].One of the most critical complications of KD is the development of coronary artery lesions (CALs) approximately after 12 days of illness,which may lead to myocardial infarction [3].High-dose intravenous immunoglobulin (IVIG) and oral administration of aspirin have been established as the first-line therapy for KD [4,5].However,10%–20% of patients with KD are resistant to it,necessitating the use of additional therapies.According to the Japanese guideline [4],IVIG,steroids,infliximab (IFX),cyclosporin A (CsA),or plasma exchange are recommended as third-line therapies for KD.A nationwide Japanese survey reported that IVIG,steroids,IFX,other immunosuppressive drugs,and/or plasma exchange were administered as second-line or later therapy in 21.6%,6.3%,2.6%,1.5%,and/or 0.5% patients,respectively [6].If second-line therapy is unsuccessful in treating KD,the optimal choice for the thirdline therapy remains unclear.Therefore,we aimed to review patients with KD refractory to the second-line therapy and study the outcomes according to the different third-line therapies received.
We retrospectively reviewed 221 patients with KD who were treated at 13 hospitals in Yamaguchi Prefecture,Japan between January 2005 and December 2015(Fig. 1).KD was diagnosed according to the 6th revision of the diagnostic guidelines [7].As per our protocol,patients with KD were initially treated with high-dose IVIG (2 g/kg) and oral administration of aspirin.No patient was treated with early administration of prednisolone according to the risk stratifications for KD.When patients showed persistent or recurrent fever within 24 hours after the first-line therapy,another dose of IVIG was administrated.We excluded patients who were treated with IFX as the second-line therapy.When the second-line IVIG therapy was ineffective,we performed the third-line therapy,which was additional IVIG (1-2 g/kg),intravenous methylprednisolone pulse (IVMP,30 mg/kg/day),or IFX (5 mg/kg/dose),according to the attending physician's preference [4,8].We collected patients’ characteristic data and clinical outcomes,including defervescence and the development of CAL in patients with KD refractory to the second-line IVIG therapy.We compared the data and outcomes according to the type of third-line therapy.Defervescence was defined as a body temperature below 37.5 ℃ for 48 hours after the treatment.
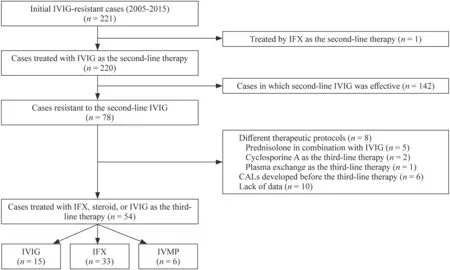
Fig.1 Flowchart of the enrolled patients with refractory Kawasaki disease treated with IVIG,IFX,and IVMP. IFX infliximab,IVIG intravenous immunoglobulin,IVMP intravenous methylprednisolone pulse
Echocardiography was temporally performed to evaluate CALs before and after treatment,at discharge,and four weeks after the onset of KD.CALs were defined as dilatations or aneurysms sized > 3 mm and > 4 mm of the coronary artery in individuals aged < 5 years and > 5 years,respectively,at 4 weeks after the onset of KD.Coronary arterial diameters were described as the absolute diameter andZscore [9].
The clinical characteristics and laboratory data of the three groups were compared using the Kruskal–Wallis test and Fisher's exact test.Further analyses were performed using the Mann–WhitneyUtest and Chi-square test when significant differences were observed among the groups following preliminary analysis.Pvalues < 0.05 were considered significant.Bonferroni corrections were applied to account for multiple testing.The analyses and calculations were performed using JMP Pro version 13.0.0 (SAS Institute Inc.,Tokyo,Japan).
A total of 221 patients were diagnosed with KD refractory to the first-line IVIG therapy.We excluded one patient who was treated with IFX as the second-line IVIG.Among 220 patients treated with the second-line IVIG therapy,IVIG was effective in 142 (64.3%) patients.Therefore,we reviewed 78 patients with KD refractory to the second-line IVIG therapy.Of these,ten patients were excluded due to incomplete data set,six patients due to the development of CALs prior to undergoing a third-line therapy,and eight patients due to other third-line treatments (combination of IVIG and prednisolone in five patients,CsA in two patients,and plasma exchange in one patient).Thus,we studied 54 patients (24.4% of the initial cohort),including 15,33,and 6 patients treated with IVIG,IFX,and IVMP,respectively(Fig. 1).Patients’ characteristic data are shown in Table 1.There were no significant differences in the patients' baseline data,including age,sex,febrile days until the start of treatment,the day of the illness when the third-line therapy started,Egami score [10],and laboratory findings.
The ratios of non-responders for IVIG,IFX,and IVMP were 46.7%,21.2%,and 50.0%,respectively,which did not differ between the groups (Table 2).There were no differences in the duration of fever between the groups.However,hospitalization days in the IVMP group were significantly longer than those in other groups (Table 3).
Median absolute diameters of right coronary,left main trunk,left anterior descending,and left circumflex arteries were 1.9,2.4,1.9,and 1.7 mm,respectively,while medianZscores of right coronary,left main trunk,left anterior descending,and left circumflex arteries were 0.68,1.30,1.10,and 0.85,respectively (Table 2).There was a significant association between the maximum Z score of 2.5 or more and CALs diagnosed using the absolute diameter criteria (Supplementary Fig.1).A total of five patients had CALs.Out of six patients who were treated with IVMP,4(66.7%) patients developed CALs (Table 2).The ratio of patients with CAL in the IVMP groups was significantly higher than those in other groups (IVMP vs.IFX: 66.7% vs.3.0%,P=0.003;IVMP vs.IVIG: 66.7% vs.0%,P=0.008;Table 3).
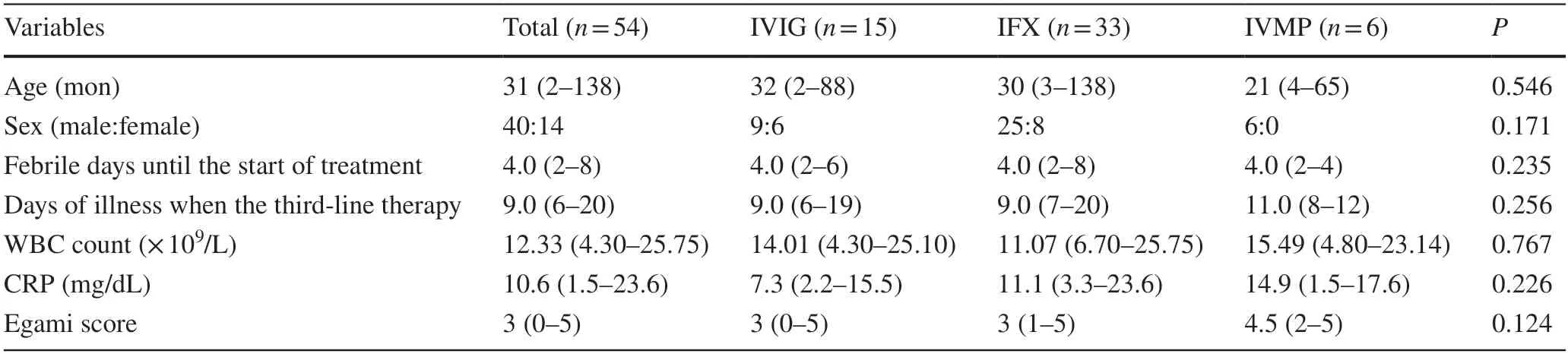
Table 1 Clinical profiles of refractory Kawasaki disease patients treated with IVIG,IFX,or IVMP
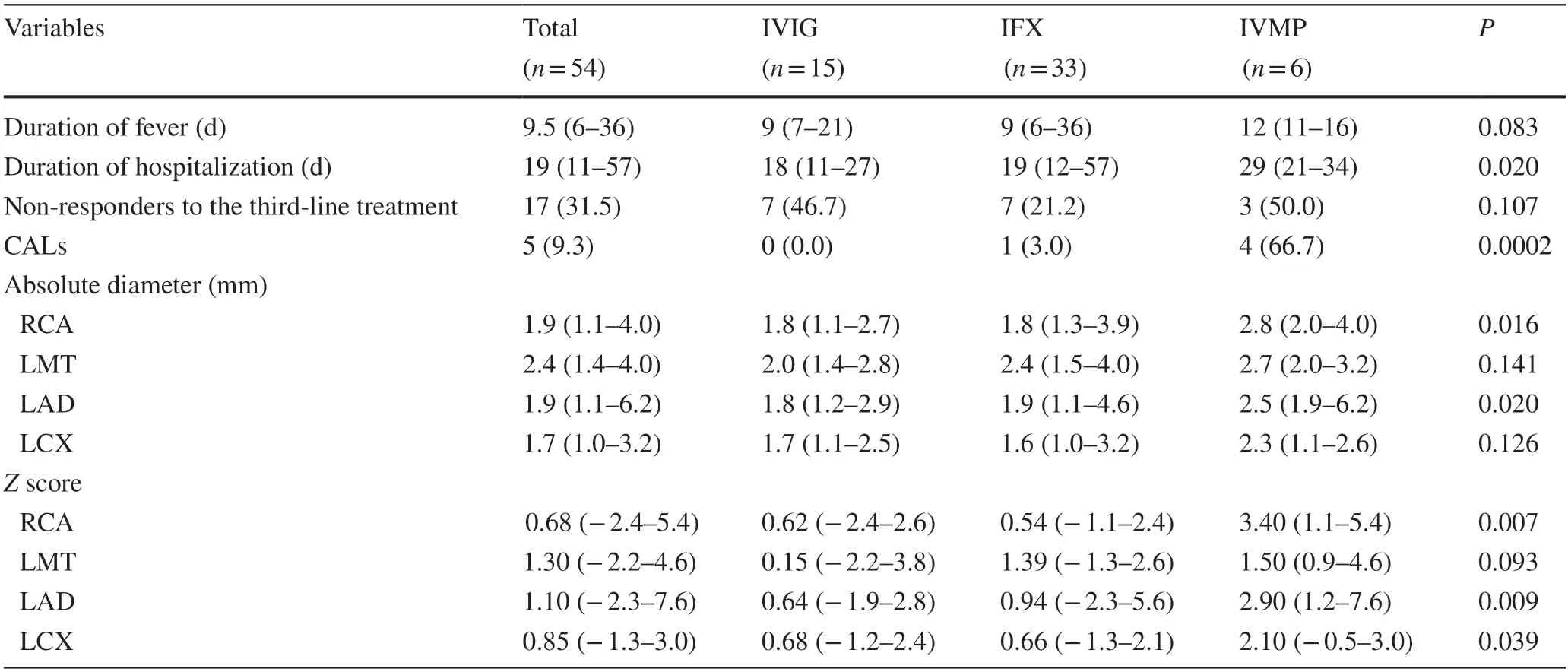
Table 2 Results of refractory Kawasaki disease patients treated with IVIG,IFX,or IVMP
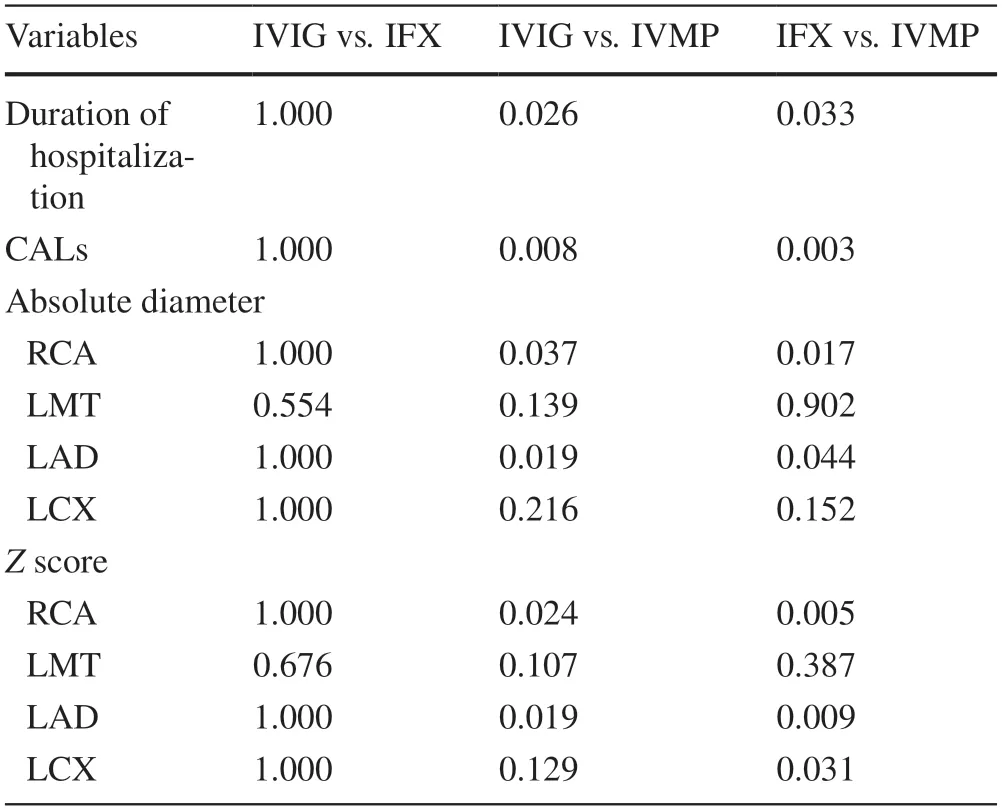
Table 3 Multiple testing evaluating the duration of hospitalization and developing CALs in the three groups
In this study,IVIG and IFX were found to be equally efficacious as third-line therapies in patients with refractory KD.The IVMP group showed a longer duration of hospitalization and higher incidence of CALs compared with the IFX and IVIG groups.The efficacy of treatment with IFX in refractory KD has recently been reported [11,12].Some randomized trials have compared the efficacy of IFX with that of IVIG as second-line therapy.Evidence shows that IFX helps achieve quicker defervescence and consequently,results in a shorter duration of hospitalization;however,IFX does not contribute to preventing CALs compared with additional IVIG [13–15].In terms of preventing CALs and acute phase response,our results showed that the efficacy of IFX as a third-line therapy was comparable to that of IVIG therapy.Administration of IFX is especially applicable to patients with IVIG resistance who have iatrogenic hypergammaglobulinemia as a result of repeated IVIG.Furthermore,the previous study reported that IFX may contribute to the early regression of CALs in patients with refractory KD compared with other treatments [16].Therefore,there seem to be potential benefits in treating patients with refractory KD with IFX as the third-line therapy.
The role of steroids in the treatment of refractory KD remains controversial.A retrospective study reported the efficacy of steroids in reducing the risk of CAL development in patients with refractory KD [17].However,some prospective randomized trials reported that IVMP as second-line therapy was not beneficial in preventing the development of CALs compared to IVIG [18,19].In our study,the IVMP group showed a higher incidence of CALs than other groups.The delayed steroid therapy might have influenced the higher incidence of CALs,although there were no significant differences in the timing of third-line therapy for any regimen.In a previous study,steroid therapy during the prolonged phase resulted in a risk of CAL development in a patient non-responsive to initial IVIG[8].Furthermore,the IVMP group showed longer hospitalization compared with other groups;this was mainly due to the necessity of tapering down the steroid dosage before discharge.
There are some limitations to this study.First,the retrospective design and small number of participants might have led to a selection bias.Second,the choices of drugs used for third-line therapy and the timing of discharge from the hospital were at the discretion of the attending physician.Third,we could not evaluate the efficacy of CsA and plasma exchange as third-line therapies.In conclusion,IVIG and IFX were equally effective as third-line therapy for refractory KD in terms of the nonresponse ratio and duration of fever and hospitalization.Compared with IVMP,administration of IVIG and IFX as third-line therapy may be associated with a lower risk for developing CALs.Further prospective,well-designed randomized controlled trials are required to evaluate the efficacy of third-line therapies in refractory KD.
Supplementary InformationThe online version contains supplementary material available at https:// doi.org/ 10.1007/ s12519-022-00602-9.
AcknowledgementsWe would like to thank Dr.Kiyoshi Ichihara(Faculty of Health Sciences,Yamaguchi University Graduate School of Medicine,Ube,Japan) for assisting with the statistical analysis.We would also like to thank Drs.Yasumasa Tsuda,Noriko Ohbuchi(Division of Pediatrics,Yamaguchi Red Cross Hospital,Yamaguchi,Japan),Norimichi Tashiro (Division of Pediatrics,Yamaguchi Rosai Hospital,Sanyo-Onoda,Japan),Shoji Kawano (Division of Pediatrics,Shimonoseki City Hospital,Shimonoseki,Japan),Masanari Hasegawa (Division of Pediatrics,Yamaguchi Grand Medical Center,Hofu,Japan),Takashi Iwai (Division of Pediatrics,Yamaguchi-ken Saiseikai Shimonoseki General Hospital,Shimonoseki,Japan),Mai Kawamura,Keisuke Takada (Division of Pediatrics,National Hospital Organization Iwakuni Clinical Center,Iwakuni,Japan),and Yukifumi Sakamoto,Masashi Uchida (Division of Pediatrics,JCHO Tokuyama Central Hospital,Shunan,Japan) for collecting clinical data.
Author contributionsFT contributed to conceptualization,data curation,formal analysis,investigation,and writing (original draft preparation).YH and OS contributed equally to this paper.They equally contributed to data curation,formal analysis,and writing (original draft preparation).OY,MA,and SY contributed to investigation,who performed the clinical management with helpful discussion for the completion of the study.OS and HS supervised and validated the data and revised the manuscript (review and editing).All the authors approved the final version of the manuscript.
FundingThis work was supported in part by Japan Society for the Promotion of Science (JSPS) KAKENHI [Nos.16K19647 (SY),19K08323 (SH),and 21K15906 (OS)] from the Ministry of Education,Culture,Sports,Science and Technology.
Data availabilityThe data that support the findings of this study are not openly available due to clinical data and are available from the corresponding author upon reasonable request.
Declarations
Ethical approvalThis work does not involve any human/animal experimentation.All procedures contributing to this work comply with the Helsinki Declaration of 1975,as revised in 2008,and has been approved by the Institutional Review Board at Yamaguchi University Hospital (H27-173).Informed consent was obtained from the parents of all patients.
Conflict of interestNo financial or non-financial benefits have been received or will be received from any party related directly or indirectly to the subject of this article.The authors have no conflict of interest to declare.
 World Journal of Pediatrics2022年11期
World Journal of Pediatrics2022年11期
- World Journal of Pediatrics的其它文章
- Instructions for Authors
- Incident respiratory disease among youths using combustible tobacco,electronic nicotine products,or both: a longitudinal analysis
- A novel HNF4A mutation identified in a child with maturity onset diabetes of the young
- Neutropenia: diagnosis and management
- Integration of multiscale entropy and BASED scale of electroencephalography after adrenocorticotropic hormone therapy predict relapse of infantile spasms
- Effect of early atopic sensitization in children aged 0–2 years on the development of asthma symptoms at 9–11 years of age
