Reflections and perspectives on adjuvant treatment in the setting of resected hepatocellular carcinoma
Matteo Donadon, Marcello Di Martino, Paolo Baroffio, Michela Anna Polidoro
1Department of Health Sciences, University of Piemonte Orientale, Novara 28100, Italy.
2Department of Surgery, University Maggiore Hospital della Carità, Novara 28100, Italy.
3Hepatobiliary Immunopathology Laboratory, IRCCS Humanitas Research Hospital, Milan 20089, Italy.
Abstract Hepatectomy is a curative procedure in selected patients affected by hepatocellular carcinoma (HCC).However,recurrence rates are as high as 70% five years after resection, and having a valid postoperative systemic regimen would represent a significant improvement in the care of HCC patients.While burgeoning evidence is emerging around the use of immunotherapy in the setting of resected HCC, little is yet known to allow the widespread use of immunotherapy after surgery.Here, we pointed out some reflections and perspectives on adjuvant strategies for patients with resected HCC and discussed potential benefits and drawbacks.
Keywords: Hepatocellular carcinoma, surgery for hepatocellular carcinoma, HCC, adjuvant chemotherapy,immunotherapy
MAIN TEXT
To date, there is a lack of standard protocols for adjuvant systemic therapy for hepatocellular carcinoma(HCC) following curative intent treatments, such as surgical resection or ablation.While the rationale is clear - to prevent or delay HCC recurrence (up to 70% at 5 years) by eradicating occult metastases -previous clinical trials on systemic therapy have failed to demonstrate improvements in recurrence-free survival (RFS) compared with placebo[1-3].
A valid postoperative systemic regimen would represent a significant improvement in the care of a disease where surgery plays a limited role, at least according to current Western guidelines[4].Despite acceptable surgical results for intermediate and advanced non-metastatic HCC[5,6], hepatectomy in these cases is still considered not indicated[4].Notably, effective adjuvant therapy could encourage the hepatologists’ and medical oncologists’ community to expand the proportion of patients who could benefit from surgery,knowing that the surgical treatment might be supported by effective postoperative systemic treatment.
Following the promising results of recent randomized clinical trials (RCTs) assessing the survival benefits of combining immune and targeted therapies compared to sorafenib [Table 1][7-12], researchers have begun evaluating the potential benefits of contemporary systemic therapies in resected HCC.Building on the promising results of the IMbrave150 study, which reported significant survival improvement with atezolizumab plus bevacizumab versus sorafenib in advanced HCC, Qinet al.recently reported the interim results of the IMbrave050 study[13].In this study, the same systemic regimen was administered in an adjuvant setting after resection or ablation of high-risk HCC patients.Accordingly, RFS was improved in those who received atezolizumab plus bevacizumab (median RFS NE; 95%CI: 22.1-NE) compared with surveillance (RFS NE; 95%CI: 21.4-NE), giving a reduction of 12% (95%CI: 5.6-19.5) at 12 months.
Therefore, infectious enthusiasm has spread worldwide about the potential to consider that regimen in patients undergoing resection for HCC, which is also in line with current guidelines where immune oncology-based regimens are noted as the standard of care in advanced HCC[4].However, while immunebased systemic therapy represents a valuable therapeutic asset, caution is advised in the setting of resected HCC.The high recurrence rate of HCC is not only related to tumoral factors, such as tumor number, size,presence of micro- or macro-vascular invasion, but also to underlying liver disease, such as chronic hepatitis, which is still the primary cause of most HCC cases.Consequently, systemic therapy may not prevent HCC recurrence after surgery in patients where chronic hepatitis is the main carcinogenic stimulus.The balance between prognostic tumoral factors and liver factors cannot be precisely defined in each HCC patient, representing a limitation in the decision-making process.This challenge might be addressed in multidisciplinary meetings involving liver surgeons, hepatologists, medical oncologists, and pathologists.However, in the absence of standard protocols, a truly personalized approach is desirable.
Following these arguments, patients at higher risk of HCC recurrence after surgery or ablation could be identified according to the surgeon’s and pathologist’s findings, aiming to add data to weigh the different prognostic factors, both tumoral and liver-related, and make the decision-making process more effective.A typical example might be the case of R0- versus R1- or even versus R2-surgery.Assuming that the surgical margin might be of prognostic significance, further studies should be done to investigate the role of adjuvant systemic treatment in these three different categories.As a matter of fact, we do not have today that information.
Concerns arise on the use of immune oncology-based regimens in the adjuvant setting.Data on resistance development in patients treated with atezolizumab plus bevacizumab are emerging (up to 22% of patients in the IMbrave150 study experienced disease progression due to resistance), and while the mechanisms behind this process remain to be investigated[14], the same phenomenon could occur in the adjuvant setting.Clearly,that proportion of patients would not be eligible to receive the same regimen in case of recurrence amenable to first-line therapy.Moreover, in the adjuvant setting of resected HCC, the risk of toxicity must be considered.In the IMbrave050 trial, up to 98% of patients experienced adverse events (AEs), with 41%encountering severe AEs (G3-4), leading to the withdrawal of treatment for 37% of these patients[13].Notably, these resected patients should be considered cured by surgery, albeit at risk of recurrence.Whethersuch toxicity is acceptable remains to be determined.
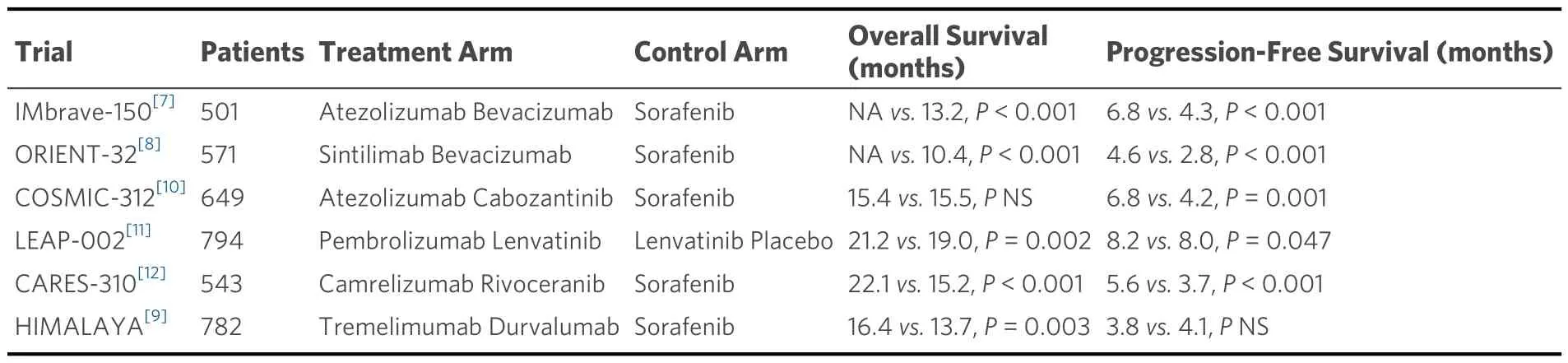
Table 1.Randomized clinical trials assessing the survival benefits of combining immune and targeted therapies compared to sorafenib
Certainly, in the adjuvant setting of resected patients, there is a significant advantage in having the surgical specimen available for translational research.Utilizing cutting-edge omics technologies, comprehensive translational research should be conducted to unveil the inferences and detect molecular or tissue predictors that may help select which patients may truly benefit from immunotherapy[15,16].Considering the inflammation that is present in patients with chronic hepatitis, there is an increased production of proinflammatory and immunosuppressive cytokines, which may contribute to the development of a pro-tumor microenvironment, unique compared to other solid tumors[17,18].As a result of that inflammatory state, HCC has a rich immune infiltrate, with tumor-infiltrating lymphocytes being one of the most abundant populations within the tumor microenvironment[19,20][Figure 1].Altered immune cells such as macrophages, CD8+ cells, CD4+ cells, and CD3+ cells have been reported to various extents, which might be responsible for the breadth of clinical presentations and responsiveness to treatments[21].Tumorassociated Treg may favor tumor immune evasion by remarkedly reducing the effector cells through secretion of both IL-10 and TGFβ and by cell-cell interaction[22].Besides, the inhibitory PD-L1 molecule was found to be distinctly expressed in HCC patients from KCs, tumor cells, and LSECs, correlating with PD-1 upregulation on CD8+ cytotoxic cells.These mechanisms lead to the altered activity of CD8+ cells, resulting in an exhausted phenotype [Figure 2].Notably, the balance between Treg and cytotoxic T cell infiltration has been found to correlate with prognosis.In other words, resected HCC patients should be stratified according to available immunoscores to predict outcome and response to treatment[23-25].Importantly, based on the study from Siaet al., from a large cohort of 956 patients, only 25% expressed markers of an immuneinflammatory response and were classified as “immune active”, while the larger remaining proportion was classified as “immune excluded” or “immune exhausted”[25].Consistently, the proportion of patients who responded to immunotherapy in the clinical trial did not exceed 20%-30%.Nothing different should be expected in the adjuvant setting.
Intriguingly, adjuvant immunotherapy can become neoadjuvant in the perspective of further treatments.That is the case of transplantation that could be done in selected cases treated with surgery, then with adjuvant immunotherapy, and finally with liver transplantation.As said before, clinical and translational research should be planned to study this treatment sequence in depth.
In conclusion, much remains to be understood about the mechanisms of immunotherapy.The comprehension of the orchestration among immune and liver cancer cells on an individual basis might be the key to a truly personalized approach, which will likely allow for understanding the clinical, pathological,and immunological intricacies of HCC patients.
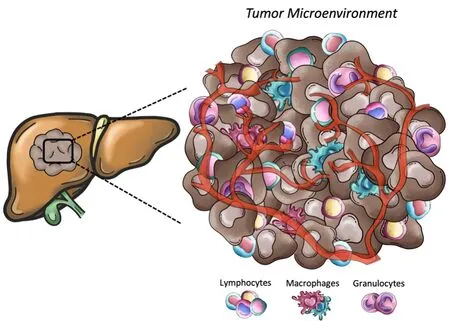
Figure 1.Representative scheme of the main cellular component of tumor microenvironment.Tumors present a wide array of cells from both innate and adaptative immunity (i.e., macrophages, natural killer cells, neutrophils, and lymphocytes) able to foster cancer growth and malignancy.
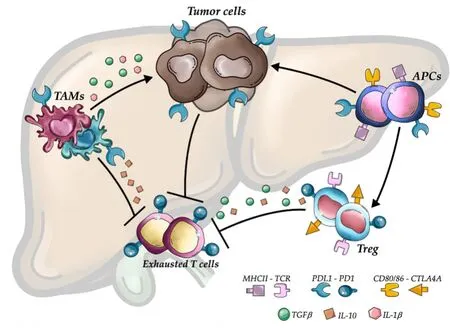
Figure 2.Mechanisms involved in HCC immune evasion.Upregulation of inhibitory programmed death-ligand 1 molecule from tumor cells, macrophages, and antigen-presenting cells, together with the release of interleukin-10 and transforming growth factor beta, lead to an exhausted phenotype of CD8+ cells and prevent tumor cells from immune damage.APCs: Antigen-presenting cells; TAMs:Tumor-associated macrophages; PDL1: Programmed death ligand 1; CTL4A: Cytotoxic T lymphocyte antigen 4; PD1: Programmed cell death protein 1; TGFβ: Transforming growth factor beta; IL-10: Interleukin-10; IL-1β: Interleukin-1 beta.
DECLARATIONS
Authors’ contributions
Made substation contribution to conception, design, acquisition, analysis, and interpretation, drafting of initial manuscript, and critical review of final manuscript: Donadon M, Di Martino M, Baroffio P, Polidoro MA.
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
Di Martino M is an Editorial Board Member of the journal Hepatoma Research.The other authors declare that there are no conflicts of interest.
Ethical approval and consent to participate.
Not applicable.
Consent for publication
Not applicable.
Copyright
? The Author(s) 2024.
- Hepatoma Research的其它文章
- Introduction of Chinese expert consensus on neoadjuvant therapy for primary liver cancer (2023 edition)
- Introduction to 2023 Chinese expert consensus on the whole-course management of hepatocellular carcinoma
- Dysmetabolic comorbidities and non-alcoholic fatty liver disease: a stairway to metabolic dysfunctionassociated steatotic liver disease
- Progression of liver disease and associated risk of hepatocellular carcinoma
- Role of temporary portosystemic surgical shunt during liver resection to prevent a post-resection small for size-like syndrome
- The role of percutaneous hepatic perfusion (PHP) in the treatment of cholangiocarcinoma

