FeMn、FeMnNa和FeMnK催化劑上合成氣制低碳烯烴的比較
李江兵 馬宏方 張海濤 孫啟文 應衛(wèi)勇,* 房鼎業(yè)
(1華東理工大學大型工業(yè)反應器工程教育部工程中心化學工程聯合國家重點實驗室,上海200237;2石河子大學化學化工學院,新疆兵團化工綠色過程重點實驗室,新疆石河子832003;3煤液化及煤化工國家重點實驗室,上海201203)
1 Introduction
FTS reaction is catalyzed by the group VIII metals,especially iron,cobalt,nickel,and ruthenium.Compared with other metal catalysts,the active phases of iron can be easily formed,especially for producing less methane and more olefins under the same conversion.1Meanwhile,it is a benefit for improving light olefin selectivity in high temperature FT(310-350°C,HTFT)and producing long chain hydrocarbons and waxes in low temperature FT(200-250°C,LTFT).Previous work has showed that the various metal oxides(Mn,K,V,Cu,Ni,Mg)had been extensively added as promoters for improving the light olefin selectivity.1,3The addition of proper amounts of Mn can promote the selectivity of light olefins and decrease CH4selectivity.However,the product distribution of FeMn catalyst is usually far from optimal.Group I alkali metals served as promoters because they can enhance the rate of synthesis and have great impact on the bonding strength of adsorbed molecules(H2and CO),electrons,and the heats of adsorption on iron catalysts.4Potassium has also been applied widely as promoters to modify the activity and selectivity in FTS.Alkali can enhance the bonding strength of Fe and C in the form of donating electrons to Fe,while weakening the Fe―H and C―O bonds.4Therefore,the addition of K can suppress the formation of CH4,improve the olefin and heavy hydrocarbon selectivities,and enhance the carburization degree of Fe.5Additionally,potassium promoter is usually found to be effective in inhibiting the rate of secondary reactions over iron-based catalyst.6But the applications of the other alkalis on iron-based catalyst are seldom reported.Some researches have shown that the FT performances,α-alkene selectivity,and the rate of carburization increased in following order:un-promoted,Li,Na,K,Cs(Rb).7-10Compared with potassium,typical applications of sodium are used to control pH during the coprecipitation and it is difficult to remove the residual sodium completely.Anet al.11has reported that residual sodium on iron-based catalyst had the negative effect on the FTS activity.However,as a promoter,sodium has been proposed to inhibit the formation of CH4and increase the selectivity of light olefins.9,12,13Low concentrations of alkali are favorable to the positive effect on FTS.
Although the importance of the alkali promoter has been realized,researches to describe the effect of alkali promoter over iron-manganese catalyst on the light olefin production are not enough.Moreover,many studies on alkali metals were usually carried out under the different preparation methods and reaction conditions which may not be appropriate for producing light olefins from syngas.
Dry9and Zhang14et al.suggested that the surface basicity depended not only on the amount of alkali present but also on how well it was distributed over the catalyst surface.The present paper dealt with the coprecipitation-calcination-impregnation method to prepare alkali-promoted FeMn catalysts,impregnation of sodium carbonate previously calcined at 500°C.This purpose was to improve the enrichment of promoter on catalyst surface so that the light olefin selectivity was promoted.In order to enhance the stability,the catalysts were reduced with syngas(nH2/nCO=20)and then reacted with syngas(nH2/nCO=3.5)respectively.The Na-promoted and K-promoted catalysts were compared for light olefin syntheses from CO hydrogenation in HTFT process.The effects of sodium or potassium on the catalyst reduction,textural properties,and bulk phase compositions of the precipitated FeMn catalyst after reduction and after FTS reaction were paid particular attention.
2 Experimental
2.1 Catalyst preparation
The catalysts were prepared by a combination of co-precipitation and impregnation method.A solution containing both Fe(NO3)3and Mn(NO3)2(Sinopharm Chemical reagent Co.,Ltd,AR)in an appropriate ratio,as well as an individual solution of NH4OH(Sinopharm Chemical reagent Co.,Ltd.,AR)was prepared and maintained at 80°C in the precipitation reaction.The aqueous salt solution and NH4OH solution were introduced to an agitated vessel at the identical flow rate to hold the pH of a constant of 8.5±0.1.The mixture solution was stirred for 4 h intensively and kept at 80°C during the precipitation process.After the precipitates were aged for 12 h,the suspension was filtered,washed thoroughly with deionized water,dried overnight at 110°C and then calcined at 500°C for 4 h in air.The calcined precipitates were impregnated with K2CO3(Na2CO3or nothing)(Sinopharm Chemical reagent Co.,Ltd,AR)and the samples were dried at 110 °C for 12 h and then treated in air at 500 °C for 4 h.The composition and label of the catalysts used in this study are presented in Table 1.
2.2 Catalyst characterization
The composition of the catalysts was determined by sequential X-ray fluorescence(XRF)spectrometer using a LAB CENTER XRF-1800(Shimadzu,Japan).
Specific Brunauer-Emmett-Teller(BET)surface area and pore size distribution of the as-prepared catalysts were measured by nitrogen physical adsorption at-196°C with a surface area and porosimetry analyzer(Micromeritics ASAP 2020 instrument,America).Prior to the adsorption measurement,the samples were evacuated at 110°C for 4 h.The pore volume and the average pore diameter were calculated by Barrett-Joyner-Halenda(BJH)method from the desorption isotherm.
A spectrometer from surface science instruments(Thermo Fisher ESCALAB 250Xi photoelectron,America)with a monochromatised microfocusedAl X-ray source was employed.Survey spectrum and Fe 2p,Mn 2p,Na 1s,and K 2pspectra were again recorded to decide surface composition of fresh catalysts.Atomic ratios were calculated from atomic sensitivity factors provided by the manufacturer.
H2-TPR and CO/CO2-TPD studies were performed using a mixture gas of 10%H2/Ar or He as carrier gas.H2-TPR of the calcined catalysts was carried out in a conventional atmospheric quartz flow reactor by using a chemical adsorption analyzer(Micromeritics ASAP 2020 instrument,America).About 50 mg of catalyst was treated in 10%H2/90%Ar(V/V)at a flow rate of 50 mL?min-1,and the reduction temperature was heated from room temperature to 900 °C at the rate of 10 °C?min-1.CO-TPD experiments were performed using the same equipment with that of the H2-TPR.About 200 mg samples were loaded in a U-tube quartz reactor.The catalyst was first reduced with H2at 400°C for 4 h.When it was cooled to 50°C,CO adsorption on catalyst was performed for 30 min,and then the CO-TPD was performed at a heating rate of 10 °C?min-1.CO2-TPD was performed following the same equipment.About 200 mg catalysts were loaded in the reactor,and the catalyst samples were heated in He(50 mL?min-1)from room temperature to 500°C for removing the adsorption species in the catalysts.The samples were subsequently cooled to 25°C under the flow of He,and then exposed in CO2for 30 min,followed by purged with He for 30 min to remove the weakly adsorbed species.After this step,the temperature was increased to 500 °C at a rate of 10 °C?min-1.
The composition of the catalysts was determined by sequential powder X-ray diffraction(XRD)analysis on a D/MAX 2550 VB/PC(RIGAKU,Japan)with CuKαradiation(λ=0.154 nm),operated at 40 kV and 100 mA.
The M?ssbauer spectra of catalyst samples were obtained on an MR-351 constant-acceleration M?ssbauer spectrometer(FAST,Germany)at room temperature by using 25 mCi 57Co in a Pd matrix.The spectra were collected over 512 channels in the mirror image format.The spectral components were identified on the basis of their isomeric shift(IS),quadruple splitting(QS),and magnetic hyperfine field(Hhf).
2.3 FTS performance
The FTS performance of the catalysts was conducted in a stainless steel fixed bed reactor(10 mm i.d.,100 mm long).The catalyst precursors(1 g,40-80 mesh particle size)were diluted to 1:5(V/V)with the same size quartz before being loaded into the reactor,The catalysts were reduced with gas(nH2/nCO=20)at 285°C,0.10 MPa,and weight hourly space velocity(WHSV)1000 h-1for 48 h.The FTS catalyst activity tests were maintained at 325°C,1.0 MPa,nH2/nCO=3.5,and 2000 h-1.The detailed description of the reactor and the product analysis system have been given by Qianet al.15
3 Results and discussion
3.1 Textural and surface properties of the fresh catalysts
Specific BET surface area,pore volume,average pore size,and composition of the fresh catalysts with various residual sodium contents are shown in Table 1.It can be seen that the un-promoted FeMncatalysts′specific BET surface area and pore volume increase with the increasing of the Mn content,while the average crystallite size decreases with the increase of the Mn content.Furthermore,at the same Mn content level,the specific BET surface areas and the pore volume of the FeMnNa catalysts are reduced by 39%and 15%,respectively.The average pore diameter is increased by 42%.When the catalyst is promoted by K,the specific BET surface areas decrease by another 50%,the pore volume decreases by another 35%,but the average pore diameter increases by 52%.This result indicates that the decrease of Mn content and the promotion of Na or K result in a low specific BET surface area,small pore volume.This may be attributed to the fact that the increasing content of Mn inhibits the agglomeration of dimension and the introduction of sodium or potassium promoter might lead to the crystallite dimension increases in iron oxide.Similar results have also been found by other investigators.16,17
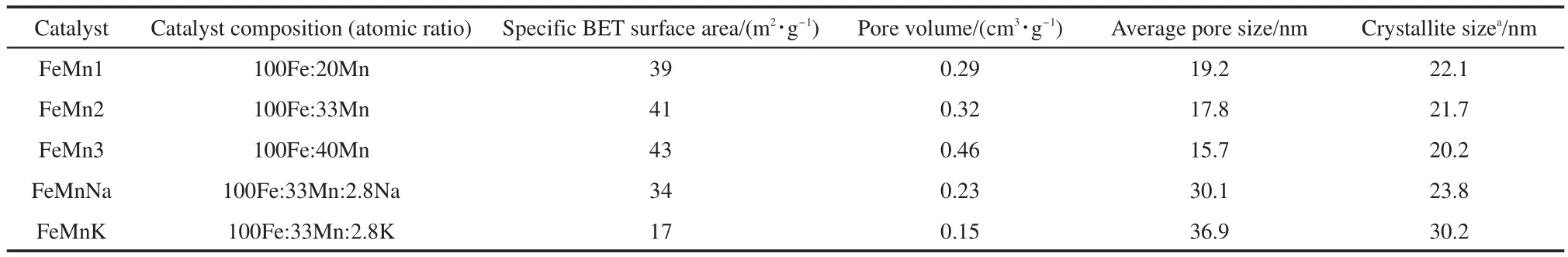
Table 1 Composition and textural properties of the catalysts as prepared
The results of XPS show the surface compositions of the catalyst calculated from the analysis of these spectra.As shown in Table 2,it is found that the Mn/Fe,Na/K,and K/Fe atomic ratios on the surface are much larger than that in the bulk of the samples due to the enrichment of promoters on the surface of the catalysts.In comparison with conventional preparation techniques,10the catalysts prepared by coprecipitation-calcination-impregnation method have higher degree of promoter enrichment on the catalyst surface.Previous studies also reported the enrichment of Mn promoter on the surface of catalysts,which was considered to be one of the reasons for improving olefin selectivity over FeMn catalysts.10,14Thus,it can be speculated that the enrichment of sodium or potassium on the catalyst surface can improve olefin selectivity.
3.2 Reducibility of catalysts
The changes in the reduction behavior of the catalysts were investigated by temperature programmed reduction(TPR)profiles.The TPR profiles of the catalysts are shown in Fig.1.The amounts of H2consumed during different reduction stages are summarized in Table 3.A sharp decline in the hydrogen consumption occurres over the temperature range from 522 to 579°C,depending on the Mn content and the introduction of Na and K promoters,indicating that the reduction process carries out in two distinct stages and all the profiles display three major peaks.The high-temperature region of each TPR curve displays one peak with maximum from 562 to 654°C,respectively.The total hydrogen uptake in these regions(Table 3)is close to 1.0 mol H2per mole Fe(H2consumption of per mole metal reduced in H2-TPR),it can be attributed to the reduction of FeO to Fe,18which is stabilized by the incorporation of Mn2+into FeO.19As shown in Table 3,the hydrogen consumption(from 0.46 to 0.55 mol H2per mole Fe+Mn)during the low temperature reduction steps agrees closely with the theoretical values calculated for the reduction ofα-Mn2O3to MnO andα-Fe2O3to FeO.At the same time,with the increase of Mn level,the total area of the H2-TPR profile peaks decreases slightly,and all the peaks shift to higher temperature.This result indicates that the addition of manganese suppresses the catalyst reduction.Perhaps the main cause is that the Fe-Mn interaction is much stronger with the increased Mn incorporation.But compared with FeMnNa and FeMnK,it can also be observed that the presence of potassium and sodium promoters cause the reduction peaks to shift to higher temperatures.The former does not strongly affect the reduction behavior of the catalyst,but the latter strongly suppresses the reduction of catalyst,implying that there are some interactions between Na and K promoters with manganese oxides and iron oxides,17promoting the aggregation ofα-Fe2O3crystallite,which is consistent with the observed decrease of the specific BET surface area.Beyond this,as shown by the results of XPS,the high enrichments of Mn,Na,and K promoters on the catalyst surface reduce the surface concentration of active Fe and result in a decrease in H2adsorption,which suppresses the reducibility of iron oxide around the Na and K promoters.
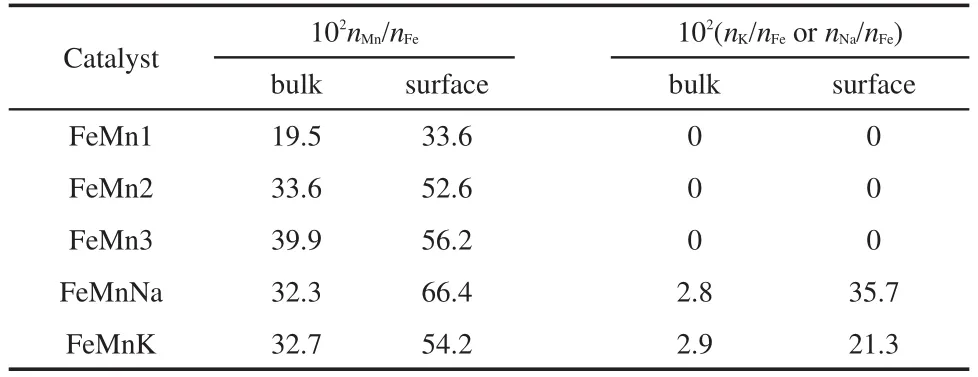
Table 2 Composition and textural properties of the catalysts as prepared
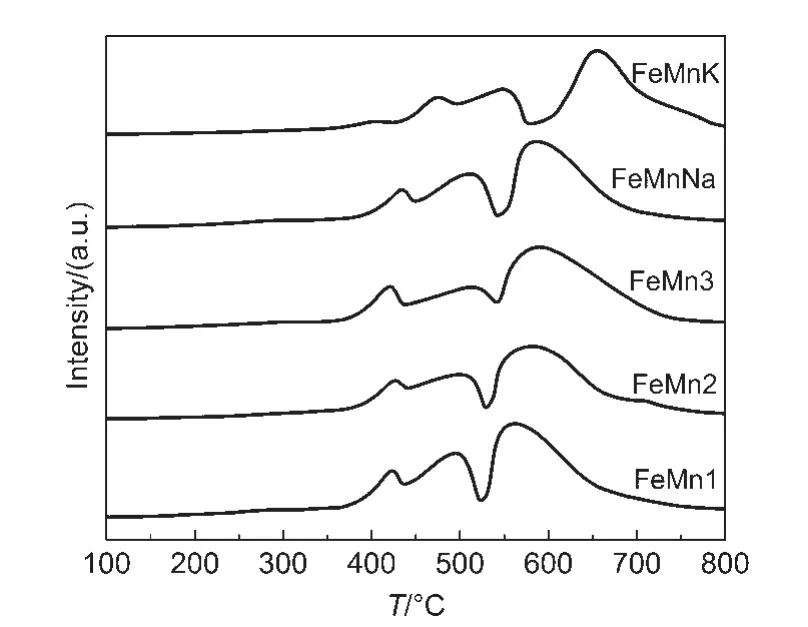
Fig.1 H2-TPR profiles of FeMnK,FeMnNa,and un-promoted FeMn catalysts
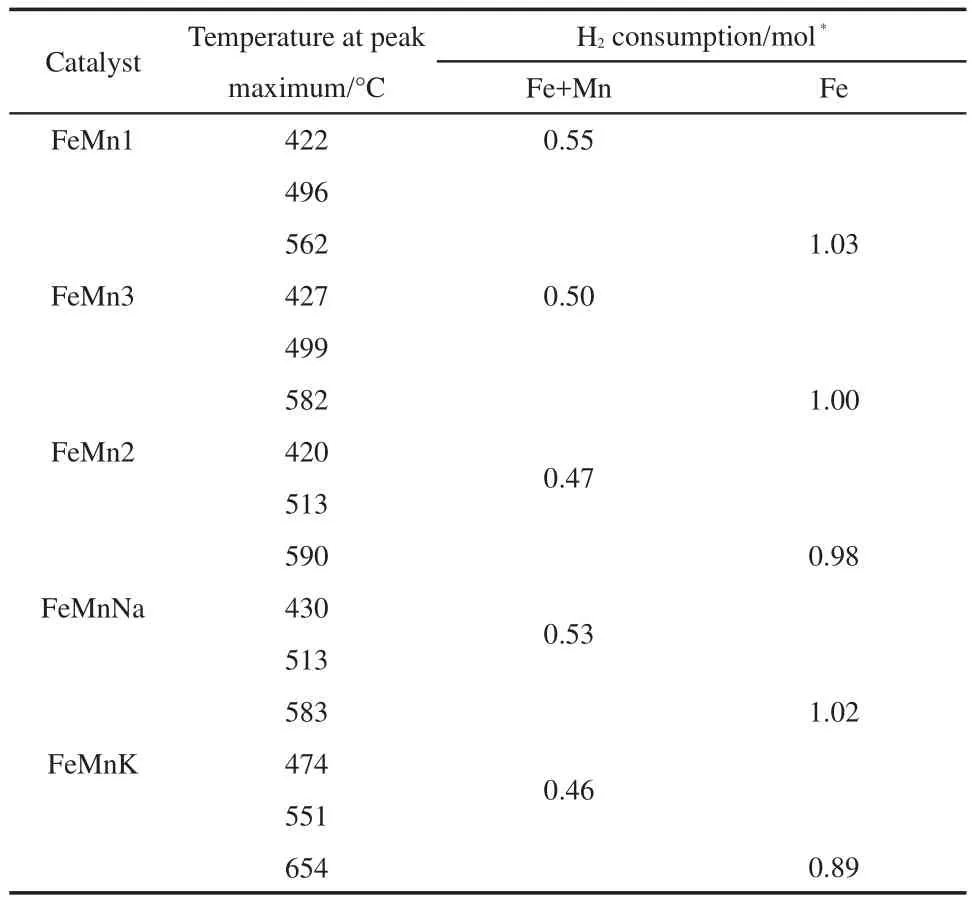
Table 3 Quantitative results of H2-TPR
3.3 Surface basicity
The basicity properties of catalysts were characterized by CO2-TPD and the results are shown in Fig.2.As shown in Fig.2,for the un-promoted FeMn catalysts,only a single weak overlap of desorption peak is observed in the CO2-TPD profile,which can be divided into the two desorptions of CO2.At the same time,with the increase of Mn content,the CO2-TPD profile has no significant change.But the FeMnNa and FeMnK catalysts show two obvious desorption peaks in the temperature range of 160-300°C,corresponding to the desorption of CO2that has some bearing on surface basic sites.Given the low specific surface area of catalysts,no physically adsorbed CO2on the catalyst surface is observed in the present work.The first peak at low temperature may be attributed to the weakly adsorbed CO2on the catalyst surface,and the second peak at high temperature may be attributed to desorption of the strongly chemisorbed CO2.20To compare the CO2-TPD profiles of FeMnNa and FeMnK catalysts,low-temperature desorption peak of K-promoted catalyst is weaker,whereas high-temperature desorption peak is stronger.Na-promoted catalyst however is quite opposite.In addition,the total area of the CO2-TPD profile of K-promoted catalyst is larger than that of Napromoted catalyst.It implies that the catalyst basicity decreases in the order of FeMnK>FeMnNa>un-promoted FeMn catalysts.This result can be ascribed to the fact that the enrichment of potassium and sodium oxides on the catalyst surface enhances the catalyst surface basicity,since alkali promoter exhibits surface basicity,and the surface basicity of alkali-promoted catalysts is stronger than that of FeMn oxide.
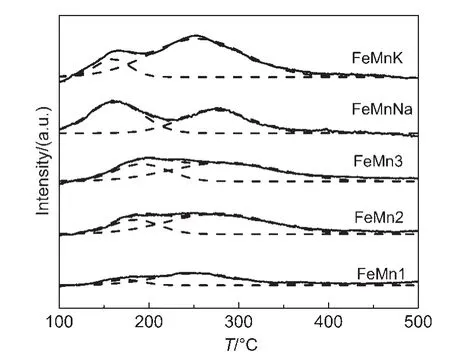
Fig.2 CO2-TPD profiles of FeMnK,FeMnNa,and un-promoted FeMn catalysts
3.4 CO adsorption on catalysts
As shown in Fig.3,un-promoted FeMn catalysts have two groups of desorption peaks;one at the lower temperatures(about 140°C)corresponding to the weak CO adsorption,while the other at higher temperatures(about 320°C)is ascribed to the strong CO adsorption.Meanwhile,with the increase of Mn concentration,the peak intensities of the weak CO adsorption gradually increase and the strong CO adsorption peaks have few changes.FeMnNa and FeMnK catalysts also have two groups of desorption peaks:one at the lower temperature(at about 140°C)corresponding to the weak CO adsorption,while the others at higher temperatures(at about 500 and 750°C)are attributed to the strong CO adsorption.It clearly showed that the addition of Mn promoter improves the CO adsorption slightly,while the addition of K or Na promoter promotes the CO adsorption strongly.It is well known that K and Na promoters have donating electron ability while CO tends to accept electrons.21As a result,the promotion of CO adsorption on K-promoted and Na-promoted FeMn catalysts can be due to that K and Na donated electrons to iron.In addition,compared with K promoted catalyst,it is interesting to find that the strong desorption peak shifts to lower temperature for Na-promoted catalyst,indicating that the dissociative of adsorbed CO is more likely to happen for FeMnNa at the FTS reaction temperature.CO dissociative adsorption can enhance the FTS activity.9Reviewing the CO2adsorption in previous section of this paper,a clear relationship between alkali and the ability of CO adsorption is found;the introduction of an appropriate amount of Na and K into the catalyst enhances the surface basic sites and further results in strong CO adsorption,which is favored to the increment of the selectivity towards olefins.
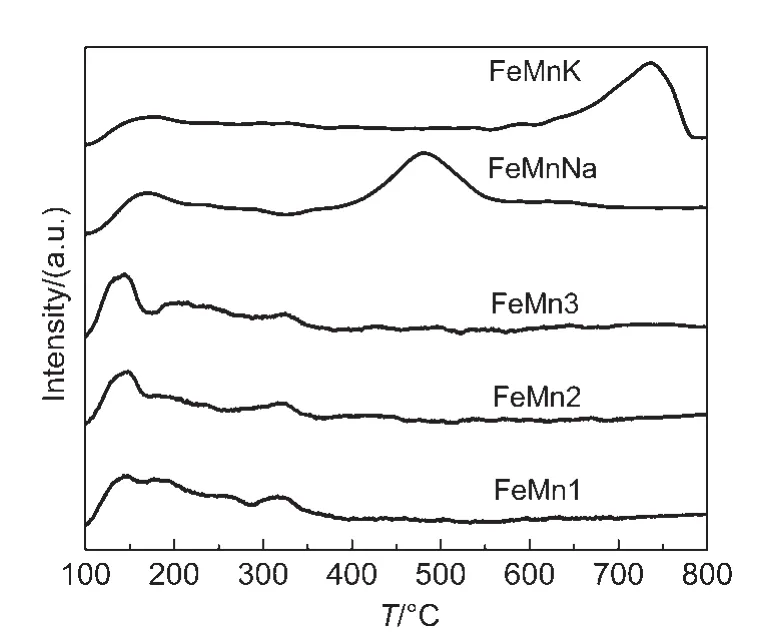
Fig.3 CO-TPD profiles for FeMnK,FeMnNa,and un-promoted FeMn catalysts
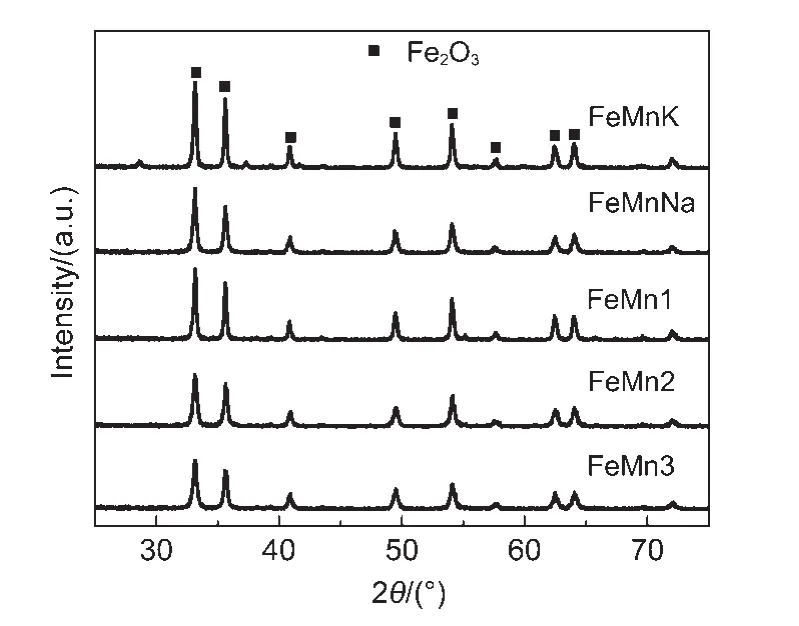
Fig.4 XRD patterns of FeMnK,FeMnNa,and un-promoted FeMn catalysts as-prepared
3.5 Crystallite structure of the catalysts
The crystalline structure of the fresh catalysts was measured by XRD.Bulk iron phases in catalysts after reduction and after FTS reaction were detected by XRD or MES.The X-ray diffraction spectra have been plotted over 2θvalues ranging from 20°to 75°.Considering the poor dispersion in the un-supported catalysts caused by low specific surface area,the MES measured at room temperature can give the detailed phase composition.
3.5.1 Catalysts as prepared
The powder X-ray diffraction patterns for un-promoted and alkali-promoted catalysts are shown in Fig.4.The diffraction data show that rhombohedral oxides ofα-Fe2O3with a corundum-type structure at values of 33.2°,35.7°,40.9°,49.5°,54.1°,57.7°,62.5°and 64.1°are mainly detectable phase identified in all of catalysts as-prepared.18Simultaneously,the Mn promoter improves the dispersion ofα-Fe2O3,and the intensity of the diffraction peaks ofα-Fe2O3is gradually strengthened with the decrease of Mn content of un-promoted FeMn catalysts.However,the XRD peak intensities ofα-Fe2O3in catalysts impregnated with sodium or potassium are stronger than that of un-promoted FeMn catalysts,indicating that impregnation of sodium or potassium into the precipitated Fe/Mn catalyst promotes the aggregation ofα-Fe2O3.It is consistent with the obtained decline in the catalyst textural properties.This is consistent with the results of BET and H2-TPR mentioned above.The disappearance of diffraction peaks for the manganese phase is probably due to the dissolving of Mn atoms in the hematite phase.17,22
3.5.2 Catalysts after reduction
The MES parameters of the catalysts after reduction with syngas(nH2/nCO=20)at 285°C,0.1 MPa,and 1000 h-1for 48 h are summarized in Fig.5 and Table 4.It would help us to compare the effects of Na and K promoters on catalyst reduction.As shown in Table 4,the sextets with Hhf of 488-490 and 443-456 kOe can be attributed to Fe3O4(A)and Fe3O4(B),respectively.The former represents the Fe3+at the tetrahedral site(A site),and the latter represents the Fe3+and Fe2+at octahedral site(B site)in Fe3O4.The IS values of the central doublet can be attributed to the superparamagnetic(spm)Fe3+ion in small crystallites.The MES results indicate that the main phases of FeMn2 and FeMnNa catalysts are Fe3O4and superparamagnetic Fe2+.With potassium addition,a small amount of spm Fe3+and FeCxare observed.It indicates that 1.6%Fe3+is not transformed to Fe3O4and spm Fe2+after 48 h of reduction.The percentage of reduction,which was expressed by 100 minus the amount of Fe3+remaining after the reduction,23is the lowest for the K-promoted catalyst,followed by Na-promoted catalyst and un-promoted catalysts.This is consistent with the reduction results of H2-TPR and CO2-TPD.The formation of iron carbides is only observed for K-promoted catalyst,indicating that sodium has a relatively small promotional influence on the overall carbon deposition rate and potassium has a more significant effect on the carbon formation.4As a stronger basicity promoter and electron donor than sodium,potassium has a greater effect on the rate of CO adsorption dissociation and decomposition on catalyst surface,and thus improves the carburization of the catalyst.Moreover,the lack of solubility of carbon by usingnH2/nCO=20 reductant is probably another important factor.Compared with the reduction in hydrogen atmosphere,the formation of elemental iron is not observed,implying that elemental iron transforms partly into Fe3O4and partly into FeCxunder the reduction conditions.These results indicate that the catalyst reduced with syngas(nH2/nCO=20)suppresses the formation of surface carbonaceous species and thus inhibits the carbon deposition on the surface.
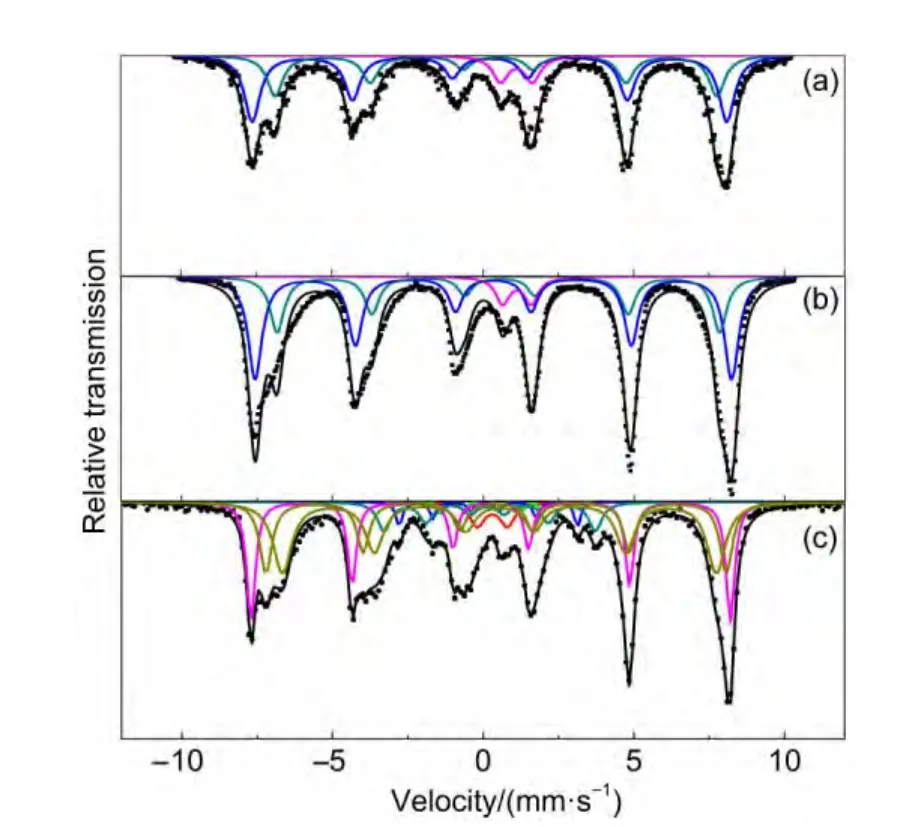
Fig.5 M?ssbauer spectra of FeMnK,FeMnNa,and un-promoted FeMn catalysts after reduction
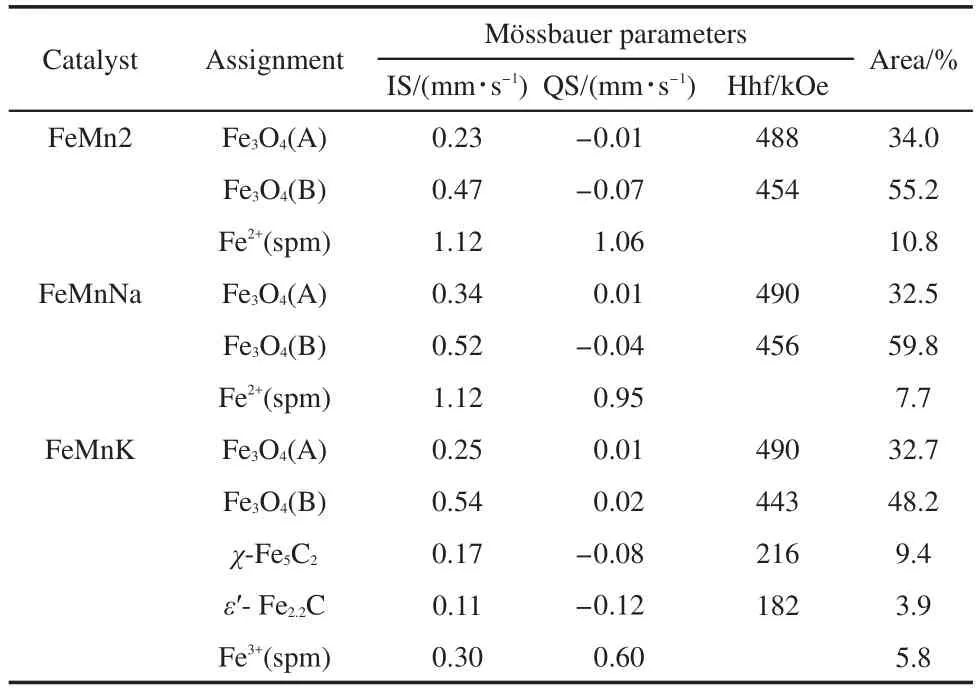
Table 4 MES parameters of FeMnK,FeMnNa,and FeMn catalysts after reduction
3.5.3 Catalysts after reaction
X-ray powder diffraction patterns of the samples after reaction in syngas(nH2/nCO=3.5)at 325°C,1.0 MPa,and 2000 h-1are depicted in Fig.6.The XRD patterns of all catalysts show the main characteristic diffraction peaks of magnetite and iron carbide.According to the JCPDS,the broad and weak peaks at 2θvalues of 31.0°,and 44.5°can be assigned to the FeCx,in whichε′-Fe2.2C andχ-Fe5C2are main iron carbides.2,10,18Besides,peaks at 2θvalues of 43.0°is are the overlap of iron carbide(s)with magnetite.24On the contrary,strong peaks are clearly observed at 30°,35°,43°,53°,57°,and 63°as well in the diffraction profiles assigned to Fe3O4.
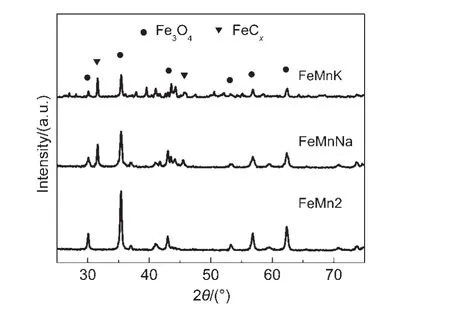
Fig.6 XRD patterns of FeMnK,FeMnNa,and un-promoted FeMn catalysts after reaction
As shown in Fig.6,by comparing the XRD patterns of unpromoted and promoted catalysts,the patterns of catalysts after promotion have a higher amount of iron carbides than un-promoted catalysts.Meanwhile,it is found that there is slight decrease in the intensity of Fe3O4for promoted catalysts.These phenomena can be interpreted as that Na and K promoters enhance the surface basicity of FeMn catalysts.The basicity of the catalyst is essential to the chemisorption of CO,and increases the probability of contact between CO and the iron surface on which the dissociation of the adsorbed CO into C is more likely to carry out,thus the formation of more FeCxspecies is formed.Aprevious study has shown that the formation of FeCxspecies was controlled by oxygen diffusivity from the bulk phase to the surface of catalyst.16In this process,a great amount of large carbide crystallites were generated on the FeMnNa and FeMnK catalyst surface.The external carbides hindered the internal oxygen diffusion from the bulk.The diffusion of carbon to form carbide and the hydrogenation of carbon to form hydrocarbons competed with each other on the catalyst surface.Moreover,the main catalytic active phase in FTS isε′-Fe2.2C,which can be further converted into theχ-Fe2.5C and subsequently converts into theθ-Fe3C.25Owing to the poor crystallographic and limited amount of FeCx,iron carbides were still indistinguishable from the magnetite background,which could subsequently be measured by MES.
The MES parameters of the samples after reaction are presented in Fig.7 and Table 5.As noted above,the sextets with Hhf of about 488-491 and 452-467 kOe can be attributed to Fe3O4(A and B sites),respectively.The fitted Hhf value of 217-219 and 90-103 kOe are consistent with those ofχ-Fe5C2;The IS of 0.20-0.22 mm?s-1and Hhf of 181-186 kOe imply the presence ofε-Fe2.2C.For FeMn2 catalyst,we can see that there is little change of the bulk phase compositions before and after a reaction.However,there is a significant increase in the proportion of FeCxof used FeMnNa and FeMnK catalysts.The MES parameters demonstrates that potassium or sodium loading can improve the carburization of iron-manganese catalysts in HTFT and the degree of carbonation follows in the order of surface basicity.This results in a high CO conversion and olefin selectivity.Furthermore,Table 5 shows that the used FeMnK and FeMnNa catalysts were mainly composed of Fe3O4and FeCx.These results accord with FTS activity and prove that FeCxare the main active phases for FTS reaction.In contrast,Fe3+species were only observed in FeMn catalyst after reaction.It implies that FeMnNa and FeMnK catalysts are favorable for the carburization of iron oxides.
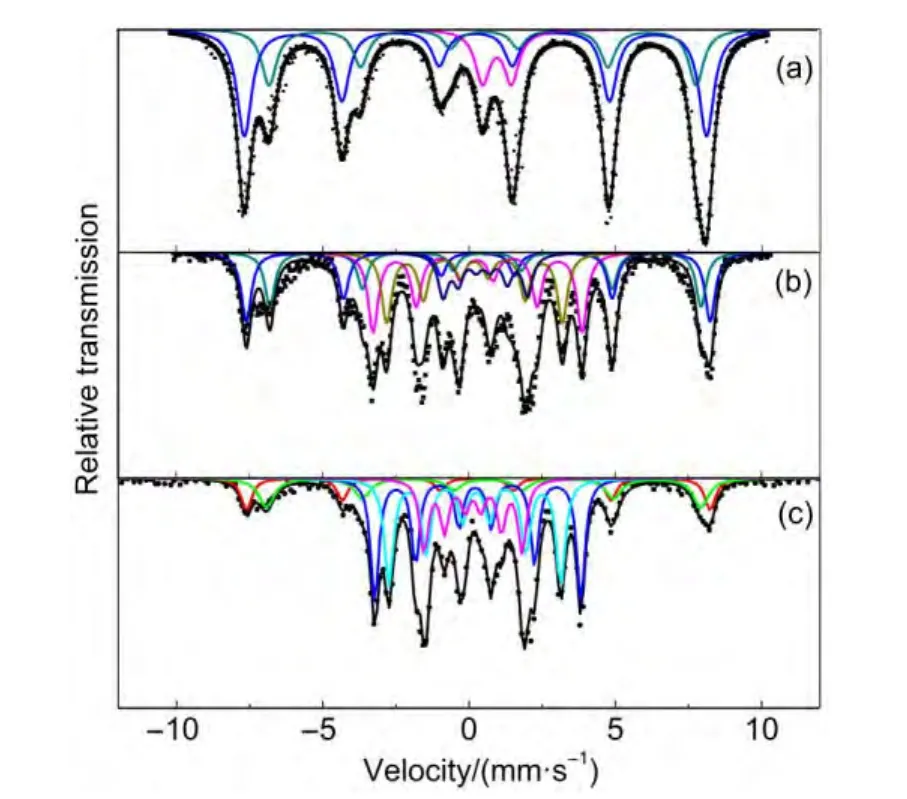
Fig.7 M?ssbauer spectra of FeMnK,FeMnNa,and un-promoted FeMn catalysts after reaction
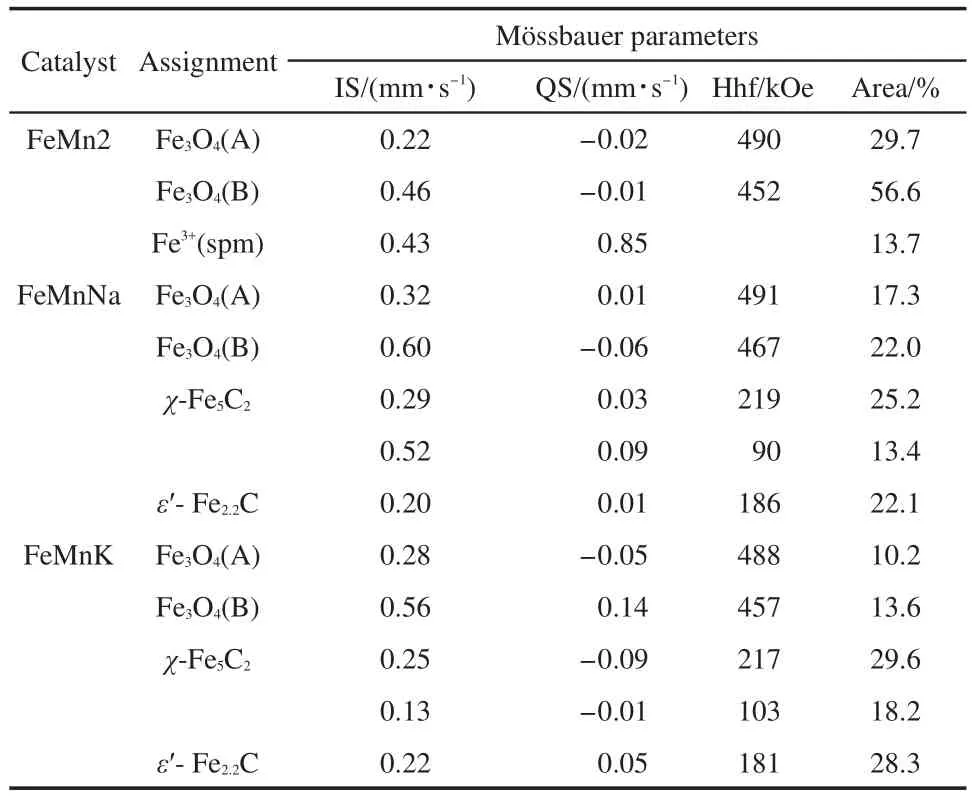
Table 5 MES parameters of FeMnK,FeMnNa,and FeMn catalysts after reaction
3.6 Catalytic performances of the catalysts
The CO hydrogenation performances of the catalysts were measured with the reaction conditions of 325°C,1.0 MPa,2000 h-1,andnH2/nCO=3.5.The activities and product selectivity were tested over a period of 36 h steady-state runs.
FTS activities of the catalysts,indicated as CO conversion,are shown in Table 6.It can be seen that the CO conversion increases firstly and then decreases at different Mn contents,and the FeMn2 catalyst has the highest CO conversion(94.5%).Beyond this Mn concentration,a significant decrease in CO conversion is observed with the increase in Mn content.It is generally accepted that the amounts of active Fe sites on catalyst surface and the CO desorption behavior of them play a crucial role in FTS activity.The optimized amount of Mn promoter for the highest catalyst activity may be caused by the synergetic effects that Mn promoter improves the dispersion of active component iron but the enrichment of Mn promoter reduces the iron content on the catalyst surface.
The effects of Na and K promoters on the CO conversion are shown in Table 6 and Fig.8.The un-promoted catalysts give the CO conversion in the range from 86.6%to 94.5%,while the Napromoted and K-promoted catalysts present a higher CO conversion of 96.2%and 95.0%respectively and no obvious deactivation for all catalysts during the 36 h FTS reaction is observed.It is apparent that the CO conversion improves with the introduction of Na and K promoters.Iron carbides are generally ac-cepted to be the most likely active phase for the FTS reaction,and iron carbide content may be used to measure the quantity of FTS active sites to some extent.14,26According to the MES results and other references,10,24there should be a positive relationship between the carburization extent and FTS activity for catalysts.Table 6 shows that the CO conversion of FeMnK and FeMnNa increases(from 95.0%to 96.2%)with decreasing iron carbides content(from 76.1%to 60.7%),but on the catalyst containing higher FeCx,the activity of the reaction is not higher.This result implies that the catalyst containing high levels of iron carbides has high FTS activity.This is probably because surface basicity of catalyst can modify the adsorption pattern of H2and CO by affecting electronic state of surface iron atoms.21However basicity is too strong,CO dissociation exceeds carbon hydrogenation,which leads to an excessive carbon deposition.It eventually decreases the CO conversion and light olefin selectivity.The influence on FTS activity obtained in the present research is similar to some previous studies.10,27
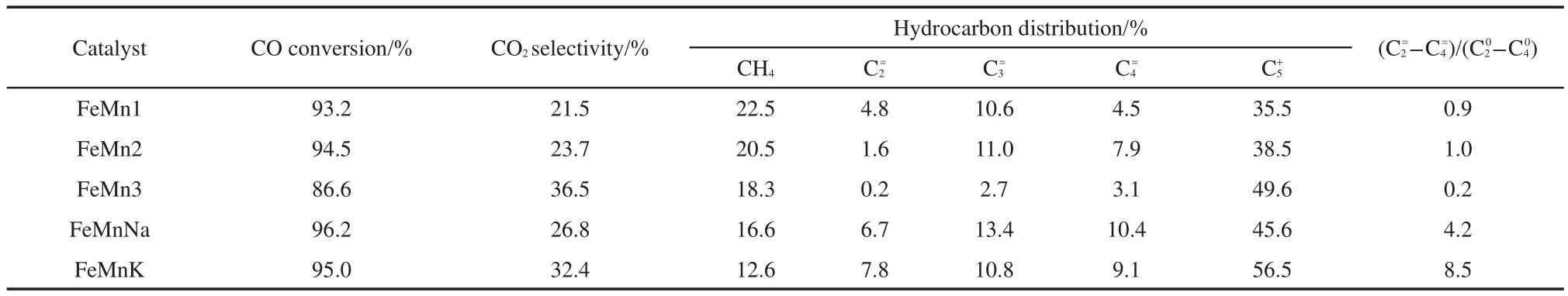
Table 6 Activity and light olefins distribution of FeMnK,FeMnNa,and un-promoted FeMn catalysts
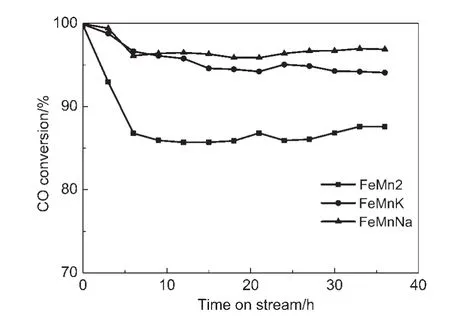
Fig.8 Carbon monoxide conversion over the catalysts
The hydrocarbon product distribution and light olefin selectivity are also shown in Table 6.On FeMnNa and FeMnK catalysts,higher selectivity of light olefins is always accompanied with morehydrocarbons and less methane,while the inverse is obtained on un-promoted catalysts.One reason is that addition of Na and K promoters can restrain the hydrogen adatom required for saturation of few olefins and intermediates.It can be seen that FeMnNa has the highest light olefin selectivity of about 30.5%.FeMnK lists about 27.7%light olefin selectivity.The un-promoted FeMn catalysts show the lower light olefin selectivity in the range of 6.0%to 20.5%(molar fraction).The phenomenon can be explained that dissociative CO chemisorption and H2adsorption on the active sites of catalysts are in competition.Stronger basic of potassium promoter results in the CO dissociative and associative adsorption significantly increasing,leading to the deposition of carbon on the K-promoted catalyst,and the H2chemisorption is markedly suppressed.Thus selectivity to-over Na-promoted catalysts is higher than that over the K-promoted catalyst.Moreover,the formation of FeCxis also responsible for improving selectivity towards-andhydrocarbons.More fraction ofhydrocarbons is formed due to the strong basicity and more FeCxof FeMnK catalyst.
CH4selectivity over the three catalysts decreased in the order of FeMnK>FeMnNa>un-promoted FeMn catalysts,as well as that tohydrocarbons and the(-)/(-)molar ratio because the surface basicity of catalysts followed the same order.These results can be associated with strongly basic alkaline promoter.In addition,the FeMnNa and FeMnK catalysts were prepared by a coprecipitation-calcination-impregnation method,and it favors the enrichment of Na and K promoters on catalyst surface which may change the local electron density of iron,and then the dissociative adsorption of CO is improved,thereby the second hydrogenation of primary olefins may be depressed and the light olefin selectivity and chain growth reaction are enhanced on the catalyst incorporated with Na and K promoters.
Zhanget al.28reported that the selectivity of olefin was affected by the relative rate of primary reaction and secondary reaction,and proved that iron promoted with alkali could improve the selectivity ofα-olefin.For FeMnNa and FeMnK catalysts,olefins are easier produced than alkanes since the activation energy of alkanes are higher than those of olefins.Light olefins may have a very low tendency for secondary reactions except for ethylene by addition of alkali.6In this study,more ethenes than the other alkenes are reabsorbed onto the active site to initiate a new chain growth reaction,resulting in the selectivity ofof 6.7%(molar fraction)less than that of(13.4%)and(10.4%)for FeMnNa.Similar to the FeMnK catalyst,the selectivities ofandare about 7.8%,10.8%,9.1%,respectively.As a result,the Na promoted catalysts exhibits better ability in the direct synthesis of light alkenes.
4 Conclusions
The increase of manganese shrinks the catalyst crystallite size,and enhances the catalyst specific BET surface area and pore volume.The introduction of manganese improves the enrichment of manganese on the catalyst surface.An increase in manganese inhibits the catalyst reduction and promotes the weak CO adsorption.Small amount of Mn promoter can improve the CO conversion,but the CO conversion decreases with the further increase of Mn content.In addition,the FeMnNa and FeMnK catalysts were prepared with coprecipitation-calcination-impregnation method,which improves promoter enrichment on the catalyst surface and stabilizes the oxide phase for avoiding preferably chemically interact with Na and K promoters.The FTS performance was carried out in HTFT for improving the selectivity of-olefins.The addition of sodium or potassium facilitates the enhancement of surface basicity and promotes the CO adsorption and formation of iron carbides.Incorporation of sodium or potassium promoter can inhibit the formation of methane and improve thehydrocarbons.The selectivity of light olefins selectivity followes in the order of FeMnNa>FeMnK>unpromoted FeMn catalysts in HTFT.
(1) Janardanarao,M.Ind.Eng.Chem.Res.1990,29,1735.doi:10.1021/ie00105a001
(2)Ma,W.P.;Ding,Y.J.;Luo,H.Y.;Lin,P.Z.;Lin,L.W.Chinese Journal of Catalysis2001,22,279. [馬文平,丁云杰,羅洪原,林培滋,林勵吾.催化學報,2001,22,279.]
(3) Mirzaei,A.A.;Habibpour,R.;Kashi,E.Appl.Catal.A-Gen.2005,296,222.doi:10.1016/j.apcata.2005.08.033
(4) Dry,M.E.;Shingles,T.;Botha,C.S.V.H.J.Catal.1970,17,341.doi:10.1016/0021-9517(70)90109-0
(5) Lohitharn,N.;Goodwin,J.G.,Jr.J.Catal.2008,260,7.doi:10.1016/j.jcat.2008.08.011
(6) Botes,F.G.;Govender,N.S.Energ.Fuel.2007,21,3095.doi:10.1021/ef7003464
(7)Ngantsoue-Hoc,W.;Zhang,Y.Q.;O’Brien,R.J.;Luo,M.;Davis,B.H.Appl.Catal.A-Gen.2002,236,77.doi:10.1016/S0926-860X(02)00278-8
(8)Wang,C.;Zhao,H.B.;Wang,H.;Liu,L.T.;Xiao,C.X.;Ma,D.Catal.Today2012,183,143.doi:10.1016/j.cattod.2011.10.020
(9) Dry,M.E.;Oosthuizen,G.J.J.Catal.1968,11,18.doi:10.1016/0021-9517(68)90004-3
(10)Yang,Y.;Xiang,H.W.;Xu,Y.Y.;Li,Y.W.Appl.Catal.A-Gen.2004,266,181.doi:10.1016/j.apcata.2004.02.018
(11)An,X.;Wu,B.S.;Hou,W.J.;Wan,H.J.;Tao,Z.C.;Li,T.Z.;Zhang,Z.X.;Xiang,H.W.;Li,Y.W.;Xu,B.;Yi,F.J.Mol.Catal.A:Chem.2007,263,266.doi:10.1016/j.molcata.2006.09.003
(12)Abbot,J.;Clark,N.J.;Baker,B.G.Appl.Catal.A-Gen.1986,26,141.doi:10.1016/S0166-9834(00)82547-6
(13) Galvis,H.M.T.;Bitter,J.H.;Khare,C.B.;Ruitenbeek,M.;Dugulan,A.I.;Jong,K.P.D.Science2012,335,835.doi:10.1126/science.1215614
(14) Zhang,J.L.;Ma,L.H.;Fan,S.B.;Zhao,T.S.;Sun,Y.H.Fuel2013,109,116.doi:10.1016/j.fuel.2012.12.081
(15)Qian,W.X.;Zhang,H.T.;Ying,W.Y.;Fang,D.Y.J.Nat.Gas Chem.2011,20,389.doi:10.1016/S1003-9953(10)60203-4
(16) Herranz,T.;Rojas,S.;Pérez-Alonso,F.J.;Ojeda,M.;Terreros,P.;Fierro,J.L.G.Appl.Catal.A-Gen.2006,311,66.doi:10.1016/j.apcata.2006.06.007
(17)Tao,Z.C.;Yang,Y.;Wan,H.J.;Li,T.Z.;An,X.;Xiang,H.W.;Li,Y.W.Catal.Lett.2007,114,161.doi:10.1007/s10562-007-9060-6
(18)Cao,C.J.;Liu,X.G.;Ju,X.R.;Chen,X.R.Acta Phys.-Chim.Sin.2013,29,2475.[曹崇江,劉曉庚,鞠興榮,陳曉榮.物理化學學報,2013,29,2475.]doi:10.3866/PKU.WHXB201310101
(19) Leith,I.R.;Howden,M.G.Appl.Catal.1988,37,75.doi:10.1016/S0166-9834(00)80752-6
(20)An,X.;Wu,B.S.;Wan,H.J.;Li,T.Z.;Tao,Z.C.;Xiang,H.W.;Li,Y.W.Catal.Commun.2007,8,1957.doi:10.1016/j.catcom.2007.03.016
(21)Wang,H.L.;Yang,Y.;Xu,J.;Wang,H.;Ding,M.Y.;Li,Y.W.J.Mol.Catal.A:Chem.2010,326,29.doi:10.1016/j.molcata.2010.04.009
(22) Motjope,T.R.;Dlamini,H.T.;Hearne,G.R.;Coville,N.J.Catal.Today2002,71,335.doi:10.1016/S0920-5861(01)00460-6
(23)Yang,Y.;Xiang,H.W.;Tian,L.;Wang,H.;Zhang,C.H.;Tao,Z.C.;Xu,Y.Y.;Zhong,B.;Li,Y.W.Appl.Catal.A-Gen.2005,284,105.doi:10.1016/j.apcata.2005.01.025
(24) Jung,H.;Thomson,W.J.J.Catal.1992,134,654.doi:10.1016/0021-9517(92)90350-Q
(25)Tao,Z.C.;Yang,Y.;Zhang,C.H.;Li,T.Z.;Wang,J.H.;Wan,H.J.;Xiang,H.W.;Li,Y.W.Catal.Commun.2006,7,1061.
(26) Dry,R.E.;Shingles,T.;Bboshoff,L.J.;Oosthuizen,G.J.J.Catal.1969,15,190.doi:10.1016/0021-9517(69)90023-2
(27) Miller,D.G.;Moskovits,M.The Journal of Physical Chemistry1988,92,6081.doi:10.1021/j100332a047
(28) Zhang,X.J.;Liu,Y.;Liu,G.Q.;Tao,K.;Jin,Q.;Meng,F.Z.;Wang,D.;Tsubaki,N.Fuel2012,92,122.doi:10.1016/j.fuel.2011.07.041

