Tooth loss inhibits neurogenesis in the dentate gyrus of adult mice
Shaochen Su, Tao Qi, Baoli Su, Huibin Gu, Jianlin Wang, Lan Yang
1 School of Life Sciences, Lanzhou University; Second Hospital, Lanzhou University, Lanzhou, Gansu Province, China
2 First Hospital, Lanzhou University, Lanzhou, Gansu Province, China
3 Changzhou Institute of Mechatronic and Technology, Changzhou, Jiangsu Province, China
Tooth loss inhibits neurogenesis in the dentate gyrus of adult mice
Shaochen Su1, Tao Qi2, Baoli Su3, Huibin Gu3, Jianlin Wang1, Lan Yang1
1 School of Life Sciences, Lanzhou University; Second Hospital, Lanzhou University, Lanzhou, Gansu Province, China
2 First Hospital, Lanzhou University, Lanzhou, Gansu Province, China
3 Changzhou Institute of Mechatronic and Technology, Changzhou, Jiangsu Province, China
Tooth loss has been shown to affect learning and memory in mice and increases the risk of Alzheimer’s disease. The dentate gyrus is strongly associated with cognitive function. This study hypothesized that tooth loss affects neurons in the dentate gyrus. Adult male mice were randomly assigned to either the tooth loss group or normal control group. In the tooth loss group, the left maxillary and mandibular molars were extracted. Normal control mice did not receive any intervention. Immuno fl uorescence staining revealed that the density and absorbance of doublecortin- and neuronal nuclear antigen-positive cells were lower in the tooth loss group than in the normal control group. These data suggest that tooth loss may inhibit neurogenesis in the dentate gyrus of adult mice.
nerve regeneration; neurogenesis; neurons; tooth loss; hippocampus; dentate gyrus; doublecortin; neuronal nuclear antigen; neural regeneration
Funding:This study was supported by the Science and Technology Key Project of Ministry of Education of China, No. 106152; the Scientific Research Project of Second Hospital of Lanzhou University of China, No. C1708.
Su SC, Qi T, Su BL, Gu HB, Wang JL, Yang L. Tooth loss inhibits neurogenesis in the dentate gyrus of adult mice. Neural Regen Res. 2014;9(17):1606-1609.
Introduction
A relationship between tooth loss and memory decline has become increasingly accepted. Epidemiological investigations have demonstrated that tooth loss increases the risk of senile dementia (Nakata, 1998). Animal studies have con fi rmed that a restrictive relationship exists between the teeth and memory. In mice, a large loss in the number of teeth reduces their learning and memory (Kato et al., 1997; Bergdahl et al., 2007). The hippocampus is a key region for higher neural activities such as emotion, behavior, learning, and memory. In particular, neurons in the dentate gyrus of animals and humans play a signi fi cant role in learning and memory, and their structure, number, and regeneration are strongly associated with cognitive function (Eichenbaum, 1999).
In the present study, we performed double immunofluorescence staining with a marker of newly born neurons, doublecortin, and a marker of neuronal maturation, neuronal nuclear antigen, in the dentate gyrus of adult mice with tooth loss. Results from these experiments showed neurogenesis in this brain region of these mice.
Materials and Methods
Animals
A total of 60 healthy adult male CD1 mice aged 3 or 4 months and weighing 20-30 g were provided by the Experimental Animal Center, Lanzhou University, China. All mice were housed in a standard cage placed in a quiet room (away from the sun and noise). Mice were kept at 22-23°C with a relative humidity of 45-50%, and under a 12-hour light/ dark cycle. The protocols were approved by the Animal Ethics Committee, Second Hospital, Lanzhou University, China.
Experimental groups and model establishment
All mice were equally and randomly divided into either the tooth loss group or the normal control group. Mice in the tooth loss group were intraperitoneally injected with 10% chloral hydrate 4 mL/kg and then fi xed in the supine position. The left maxillary and mandibular molars were then extracted 2 days later, thus establishing the tooth loss model. The normal control group did not receive any intervention.
Preparation of tissue sections
All mice were anesthetized with chloral hydrate 4 weeks after model establishment. After the heart was exposed, a puncture was made through the cardiac apex until it reached the ascending aorta. The right auricle was then cut with a pair of eye scissors. Physiological saline (150 mL) was used for washing until the lip and tongue became white. The blood vessels of the heart were fully fi xed with 250 mL 4% paraformaldehyde in phosphate-buffered saline (PBS; 0.01 mol/L, pH 7.4, 150 mL). After craniotomy, brain tissue was fi xed (overnight at 4°C)with 4% paraformaldehyde in PBS. Brain tissues were sliced into coronal sections (thickness of 5 μm) from the superior colliculus to the optic chiasm and from the cephalic side to caudal side. Three serial sections were obtained at intervals of 50 μm and placed on poly-lysine-coated slides for further staining.
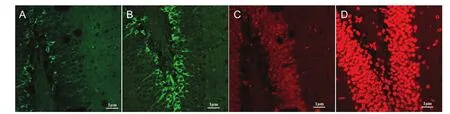
Figure 1 Effects of tooth loss on the distribution and morphology of newborn neurons in the mouse dentate gyrus (immunofuorescence staining).
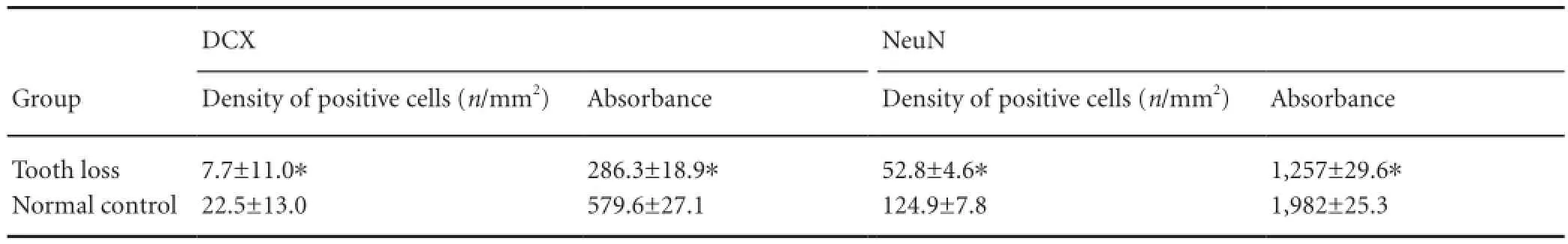
Table 1 Effects of tooth loss on the distribution of newborn neurons in the mouse dentate gyrus
Double immunofuorescence staining for doublecortin and neuronal nuclear antigen
Sections were dehydrated and permeabilized at room temperature (20°C) (Rohr et al., 2001). Antigen was retrieved with citric acid by exposing the sections in the microwave oven for 120 minutes. Sections were then treated with a mixture of hydrogen peroxide and methanol (1:50) at room temperature for 30 minutes to deactivate endogenous peroxidase. Sections were washed (5 minutes × 3) with 0.01 mol/L PBS, These sections were blocked with normal goat serum for 20 minutes, then incubated (overnight at 4°C) with the primary antibodies, donkey anti-doublecortin (1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and mouse anti-neuronal nuclear antigen (1:1,000; Chemicon, Hofheim, Germany). After washing (5 minutes × 3) with 0.01 mol/L PBS, sections were incubated (for 10 minutes at 37°C) with the secondary antibodies, goat anti-mouse IgG, Alexa 555 (1:300; Molecular Probes Gotingen, Germany) and goat anti-donkey IgG, Alexa 488 (1:300; Molecular Probes, Gotingen). All sections were subsequently washed (5 minutes × 3) with PBS 0.01 mol/L and then immersed in water, followed by dehydration using a graded alcohol series. The sections were placed in each alcohol grade for 2 minutes and then fi nally immersed in xylene, and moved in a fume cupboard where they were mounted with neutral resin.
Data analysis
Newborn neurons and the newborn granule cell layer in the dentate gyrus were observed with a confocal laser scanning microscope (LSM 510; Zeiss, Germany). The distribution, density, and absorbance values of newborn neurons were compared. Doublecortin-labeled newborn neurons in the dentate gyrus were quantified with a confocal laser scanning microscopy. The number (n) of newborn neurons in the granular cell layer and subgranular zone in each section was calculated. The area of the granular cell layer and subgranular zone in the dentate gyrus was also calculated, and the number (n/mm2) of doublecortin-positive cells in a unit area of the dentate gyrus was calculated by the grid test method (Zhu et al., 2009). Absorbance values of doublecortin- and neuronal nuclear antigen-labeled cells were obtained from both groups, as previously described (Zhou et al., 1995).
Statistical analysis
Data were expressed as the mean ± SD and were analyzed by the two-samplet-test, which was performed using SPSS 13.0 software (SPSS, Chicago, IL, USA). A value ofP< 0.05 was considered statistically signi fi cant.
Results
Distribution and morphology of newborn neurons in the dentate gyrus of mice with tooth loss
The numbers of both doublecortin and neuronal nuclear antigen-labeled newly born neurons were high in the normal control group, but low in the tooth loss group.
Doublecortin-labeled cells were detected in the granular cell layer and subgranular zone in both groups 4 weeks after model establishment. Dendrites vertically crossed the lamellar structure and were distributed two-cell widths between the granular cell layer and gate region. A few doublecortin-positive cells migrated to the granular cell layer. Doublecortin-positive cells in the gate region were scattered. Neuronal nuclear antigen-labeled cells were visible in the molecular layer, granular layer and subgranular zone, especially in the subgranular zone (Figure 1).
In the normal control group, the number of doublecortin-positive cells was high in the subgranular zone of dentate gyrus. These doublecortin-positive cells were arranged in a cluster, and the synapse was long and continuous (Figure 1B). In the tooth loss group, the number of doublecortin-positive cells was low in the subgranular zone of the dentate gyrus. These doublecortin-positive cells were single (with a few in a cluster) and scattered, and the synapse was short and discontinuous (Figure 1A). In neuronal nuclear antigen-labeled images, neuronal nuclear antigen-positive granule cells in the normal control group were visible in the dentate gyrus, and most of them were mature and densely distributed (about 7-9 layers) with a spherical or elliptical shape (Figure 1D). In the tooth loss group, the number of neuronal nuclear antigen-positive cells was less in the dentate gyrus (Figure 1C).
Effect of tooth loss on neurogenesis in the dentate gyrus
Both the density and absorbance values of doublecortinand neuronal nuclear antigen-positive cells were signi fi cantly (P< 0.05) lower in the tooth loss group compared with the normal control group (Table 1).
Discussion
Neurogenesis mainly occurs in the subependymal layer and in the dentate gyrus of adult mammals. More specifically, neurogenesis occurs in the subgranular cell zone of the dentate gyrus, and involves neural stem cells/progenitor cells (Altman and Das, 1965; Eriksson et al., 1998). Neural stem cells in the dentate gyrus are located in the subgranule cell layer in hippocampus. Neurogenesis in the infragranular layer consists of three stages: (1) neural stem cell division, (2) gradual migration to the granule cell layer in which newborn cells are semi-mature, and (3) newborn cells integrated in the granule cell layer, with most cells differentiated into mature neural cells (Oyanagi et al., 2001; Leuner et al., 2010). The infragranular layer of the dentate gyrus is considered to be a region for neural stem cell proliferation, with its effects continuing into adulthood. Neural stem cells in the infragranular layer of adult mice constantly proliferate and migrate to the granule cell layer. Moreover, neural stem cells gradually transform into mature cells during migration, and finally differentiated into neurons in the granule cell layer (Corbo et al., 2002). Dentate gyrus is a key region in the brain in which neurogenesis occurs all through life (Nacher et al., 2001). Thus newborn cells may be strongly correlated with learning and memory. A previous study has con fi rmed that newborn cells in the granule cell layer are strongly associated with memory formation, and disruption of neural cell proliferation in the dentate gyrus affects learning and memory (Macklis, 2001).
The present study may indicate that tooth loss plays a role in learning and memory impairment in mice, by observing neuronal regeneration in the dentate gyrus using double immunofluorescence staining for doublecortin and neuronal nuclear antigen. Our results verified that Doublecortin could be used to analyze neuronal regeneration in the dentate gyrus under different conditions such as environmental change, mature, illness or injury (Jin et al., 2002).
Doublecortin is a microtubule-associated protein that is extensively expressed in the developing nervous system of mammas. Furthermore, doublecortin is necessary for neuronal migration and differentiation and can label the fi rst and second stages of neurogenesis in the infragranular layer of the dentate gyrus (Sska et al., 2000). Doublecortin is mainly expressed in neuronal cell bodies and plays a major role in migration and axonal differentiation (Gleeson et al., 1999; Friocourt et al., 2003). Our results from the high-powered confocal laser scanning microscope revealed that doublecortin-positive cells were mainly located in the infragranular layer of dentate gyrus. The number of doublecortin-labeled newly born neurons was high in the normal control group, but low in the tooth loss group. Neuronal nuclear antigen labels the fi rst and second stages of neurogenesis in the infragranular layer of the dentate gyrus, and is mainly expressed in mature neurons. Our staining results demonstrated that the number of neuronal nuclear antigen-positive cells was high in the normal control group, but low in the tooth loss group. This study showed that the number and density of newly born neural cells were lower in the tooth loss group compared with the normal control group. Antigen activity and number of positive cells were higher in the normal control group than in the tooth loss group. These results therefore suggest that the neurogenic capacity in the hippocampus is lower in the tooth loss group than in the normal control group.
Our results from the immuno fl uorescence study demonstrated that tooth loss impaired the distribution, structure, and neurogenic capacity of neurons in the mouse dentate gyrus. These effects may have a negative impact on learning and memory. The alteration in neurotransmitter content, a decrease in cerebral blood fl ow and oxygen levels after tooth loss (Hu et al., 2003), and poor chewing-induced nutritional de fi ciencies may also result in injury to the brain structure and neuronal regeneration at different degrees (Chen et al., 2007). Nevertheless, the impact of tooth loss on learning and memory in mice requires further investigation.
Author contributions:Su SC, Wang JL and Yang L participated in study design and implementation, result analysis, data statistics, manuscript writing, and submission. Wang JL and Yang L participated in theory and practice guidance, result analysis and manuscript submission. Qi T participated in study implementation and result analysis. Su BL and Gu HB participated in experimental statistics. All authors approved the final version of the paper.
Conficts of interest:None declared.
Altman J, Das GD (1965) Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol 124: 319-335.
Bergdahl M, Habib R, Bergdahl J, Nyberg L, Nilsson LG (2007) Natural teeth and cognitive function in humans. Scand J Psychol 48:557-565.
Chen Y, Hong J, Xu J, Liao Y, Wei Z, Huang C (2007) Effects of multi-micronutrients on alleviating physical fatigue and improving learning and memory. Yingyang Xuebao 29:213-216.
Corbo JC, Deuel TA, Long JM, LaPorte P, Tsai E, Wynshaw-Boris A, Walsh CA. (2002) Doublecortin is required in mice for lamination of the hippocampus but not the neocortex. J Neurosci 22:7548-7557.
Eichenbaum H (1999) Conscious awareness, memory and the hippocampus. Nat Neurosci 2:775-776.
Eriksson PS, Per fi lieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH (1998) Neurogenesis in the adult human hippocampus. Nat Med 4:1313-1317.
Friocourt G, Koulakoff A, Chafey P, Boucher D, Fauchereau F, Chelly J, Francis F (2003) Doublecortin functions at the extremities of growing neuronal processes. Cereb Cortex 13:620-626.
Gleeson JG, Lin PT, Flanagan LA, Walsh CA (1999) Doublecortin is a microtubule-associated protein and is expressed widely by migrating neurons. Neuron 23:257-271.
Hu H, Huang J, Liu H (2003) Protective effects and mechanisms of serial TCM “Huoxuehuayu” prescriptions on cerebral ischemia-reperfusion injury in rats. Zhejiang Daxue Xuebao: Yixue Ban 32:502-506.
Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA (2002) Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci U S A 99:11946-11950.
Kato T, Usami T, Noda Y, Hasegawa M, Ueda M, Nabeshima T (1997) The effect of the loss of molar teeth on spatial memory and acetylcholine release from the parietal cortex in aged rats. Behav Brain Res 83:239-242.
Leuner B, Gould E (2010) Structural plasticity and hippocampal function. Ann Rev Psychol 61:111-140.
Macklis JD (2001) Neurobiology: newmemories from new neurons. Nature 410:314-315.
Nakata M (1998) Masticatory function and its effects on general health. Int Dent J 48:540-548.
Oyanagi K, Kakita A, Kawasaki K, Hayashi S, Yamada M (2001) Expression of calbindin D-28k and parvalbumin in cerebral cortical dysgenesis induced by administration of ethylnitrosourea to rats at the stage of neurogenesis. Acta Neuropathol 101:375-382.
Sska M, Ono J, Okada S, Nakamura Y, Kurahashi H (2000) Genetic alteration of the DCX gene in Japanese patients with subcortical laminar heterotopia or isolated lissencephaly sequence. J Hum Genet 45:167-170.
Zhou Y, Haugland RP (1995) Use of a new fluorescent probe, seminaphtho fl uorescein-calcein, for determination of intracellular pH by simultaneous dual-emission imaging laser scanning confocal microscopy. J Cell Physiol 164:9-16.
Zhu HL, Bin P, Wu JJ, Xu Q, Zhu WB, Wang BH (2009) A counting method for monoayer cells attached to culture plate in situ. Xibao Shengwuxue Zazhi 164:9-16.
Copyedited by Farso M, de Souza M, Wang J, Qiu Y, Li CH, Song LP, Zhao M
Jianlin Wang, Ph.D., School of Life Sciences, Lanzhou University; Second Hospital, Lanzhou University, Lanzhou 730030, Gansu Province, China, jlwang@lzu.edu.cn. Lan Yang, M.D., School of Life Sciences, Lanzhou University; Second Hospital, Lanzhou University, Lanzhou 730030, Gansu Province, China, ylan2005@163.com.
10.4103/1673-5374.141786
http://www.nrronline.org/
Accepted: 2014-07-30
 中國(guó)神經(jīng)再生研究(英文版)2014年17期
中國(guó)神經(jīng)再生研究(英文版)2014年17期
- 中國(guó)神經(jīng)再生研究(英文版)的其它文章
- Stem cell transplantation for treating stroke: status, trends and development
- Publication trends in studies examining radix notoginseng as a treatment for ischemic brain injury
- Virtual reality training improves balance function
- Neuroprotective effects of Buyang Huanwu decoction on cerebral ischemia-induced neuronal damage
- Methylmercury chloride damage to the adult rat hippocampus cannot be detected by proton magnetic resonance spectroscopy
- Damage of hippocampal neurons in rats with chronic alcoholism
