Virtual reality training improves balance function
Yurong Mao, Peiming Chen, Le Li, Dongfeng Huang
Department of Rehabilitation Medicine, First Af fi liated Hospital, Sun Yat-sen University, Guangzhou, Guangdong Province, China
Virtual reality training improves balance function
Yurong Mao, Peiming Chen, Le Li, Dongfeng Huang
Department of Rehabilitation Medicine, First Af fi liated Hospital, Sun Yat-sen University, Guangzhou, Guangdong Province, China
Virtual reality is a new technology that simulates a three-dimensional virtual world on a computer and enables the generation of visual, audio, and haptic feedback for the full immersion of users. Users can interact with and observe objects in three-dimensional visual space without limitation. At present, virtual reality training has been widely used in rehabilitation therapy for balance dysfunction. This paper summarizes related articles and other articles suggesting that virtual reality training can improve balance dysfunction in patients after neurological diseases. When patients perform virtual reality training, the prefrontal, parietal cortical areas and other motor cortical networks are activated. These activations may be involved in the reconstruction of neurons in the cerebral cortex. Growing evidence from clinical studies reveals that virtual reality training improves the neurological function of patients with spinal cord injury, cerebral palsy and other neurological impairments. These fi ndings suggest that virtual reality training can activate the cerebral cortex and improve the spatial orientation capacity of patients, thus facilitating the cortex to control balance and increase motion function.
nerve regeneration; brain injury; spinal cord injury; stroke; virtual reality; balance dysfunction; mechanism; sensorimotor function; neural plasticity; vision; vestibule; proprioception; somatosensory; balance; reviews; rehabilitation; NSFC grant; neural regeneration
Funding:This study was supported by the National Natural Science Foundation of China, No. 30973165 and 81372108; Guangdong Province College Students Innovative Research Projects in 2013.
Mao YR, Chen PM, Li L, Huang DF. Virtual reality training improves balance function. Neural Regen Res. 2014;9(17):1628-1634.
Introduction
Balance dysfunction is one of the main factors leading to impaired mobility and postural control, in fl uences walking after discharge and in the community-dwelling elderly, and also impacts balance control in daily life (Brosseau et al., 1996; Wee et al., 1999; Hsieh et al., 2003). Previous studies have shown that more than 33% of people with chronic balance dysfunction have an increased risk of falling in their daily lives (Colledge et al., 2002), and the cause of more than half of older people’s accidental death was signi fi cantly correlated with falling and balance dysfunction (Agrawal et al., 2009). Some balance ability training methods have demonstrated postural control and mobility improvement (Yavuzer et al., 2006; Lo et al., 2012), but the effectiveness of existing interventions is limited because patients tend not to be fully involved and adhere to the training protocol.
With the development of technology, rehabilitation facilities and technology that can study the simulated real environment are increasingly used to restore motor function and address balance dysfunction (Bower et al., 2014; McEwen et al., 2014). Virtual reality technology is developed on a computer hardware and software environment that enables immersed users to generate visual, audio, and haptic feedback, and obtain an interactive experience in the three-dimensional visual space, which strives to give users the impression of a real environment. The virtual real environment is created by virtual reality technology with a focus on three characteristics: autonomy, interaction and sense of being. Patients who have perceived de fi ciencies and motor dysfunction can use virtual reality technology during their rehabilitation training, to produce the greatest recovery in their impaired motor function. This technique might also partially or fully improve impaired bodily functions. Consequently, following this treatment, patients might be able to achieve full selfcare, and virtual reality training might allow some of them to work independently, improving their quality of life (Berra, 2004). Balance control relies on the central nervous system at multiple levels; some studies have shown that exploring the virtual real environment activates the cortical and sub-cortical regions (Nancy et al., 1980; Bolton et al., 2012). In this current review, the possible central control mechanism of virtual reality technology as well as its clinical application will be introduced.
Neural reorganization mechanism of virtual reality exercise in the balance control of the body
Generally, balancing the body is thought to be achieved by the coordination of three major systems, including visual, vestibular and proprioceptive sensation. Previous studies have shown that the prefrontal cortex is one of the most important brain areas in controlling human balance (Nan-cy, 1980; Virk et al., 2006; Walker et al., 2010; Bolton et al., 2012). They provided information about the position and motion of the head with respect to the surroundings, and based on information in the visual surroundings provided by visual cues. The main role of the proprioceptive and somatosensory system is to sense the distributed tactile input stimuli at the neural level, and to provide a relationship between limb position and the central nervous system (Figure 1). The vestibular system, located in the inner ear, is used to control and perceive the motion and position of the head in space (Virk et al., 2006). The function of these systems reduced after the brain is impaired. When one or two of these sensory input systems are dysfunctional, the allocation of different gains to the sensory inputs can be exploited to compensate for the impaired systems (Nancy, 1980; Walker et al., 2010). Virk et al. (2006) proposed that such an adaptation can take place in everyday life or in a virtual environment. By using a virtual environment to train the eye-head movement, balance in older people can be improved and occupational falls are minimized.
Behavioral experiences including an enriched environment, physical activity and spatial learning substantially improve sensorimotor function outcome after brain injury following ischemia (Johansson et al., 1996; Borlongan, 2000; Johansson, 2000). Virtual reality systems have been shown by many studies to provide similar real-world training effects, such as in the intensity and repetition referred to as constraint-induced movement therapy, body weight support treadmill training and allow patients with stroke to modify their neural organization and promote neuroplastic changes (Viau et al., 2004; Dvorkin et al., 2006). Therefore, we hope to explore the possible mechanism of how virtual reality promotes balance rehabilitation after brain injury in terms of visual, vestibular and proprioceptive feedback.
Cortical mechanisms of balance control
Recent studies have shown that balance control in standing is a complex sensorimotor action based on automatic and reflexive spinal programs under the influence of several distinct and separate supra-spinal centers in the brainstem, cerebellum and cortex (Drew et al., 2004). Different cortical areas were activated, related to these arti fi cial environment tasks. The prefrontal cortex would be activated when participants performed spatial orientation tasks. Multimodal dance training over 4 weeks could activate the region of the prefrontal cortex (Tachibana et al., 2011). Some experiments have allowed patients to play and learn spatial navigation in mazes (Ayaz et al., 2011), and activation could always be found in brain regions in our preliminary study of virtual reality training of stroke patients in SYSU using an Xbox 360 (Figure 2). Although the mechanism is not clear, these results suggest a corresponding relationship between the prefrontal cortex and spatial orientation ability. A further study from Miller suggests that during the incremental swing balance task, when unpredictable and external postural perturbations were applied to healthy standing participants, the prefrontal cortex would bilaterally recruit both visual and proprioceptive information to adaptively modify postural instability in response to changes in the virtual environment to complete the action goal (Miller et al., 2001). Another study by Basso Moro also demonstrated that, when healthy participants performed an incremental swing balance task in a semi-immersive virtual reality environment, the effect of oxygenation increased in the prefrontal cortex of both hemispheres (Basso et al., 2014). In addition, Slobounov et al. (2005) recorded the ankle movement of twelve young healthy participants using force plates, EEG and EMG as they performed oscillatory and discrete postural movements in the anterior and posterior directions. A statistically signi fi cant difference in the amplitude of all three components of movement-related cortical potentials, with respect to the baseline at the front-central electrode sites was found in the EEG data. They also demonstrated that the frontal region may play an important role in postural equilibrium, likes moving continuously in different directions, or only in the forward direction.
During walking, the prefrontal cortex also seems to play a role in controlling balance. Some studies show that when adjusting walking speed on the treadmill (Suzuki et al., 2004) and during the recovery process following ataxic stroke (Mihara et al., 2007), the prefrontal cortex participates in the control of locomotion. Basso used functional near-infrared spectroscopy to study changes in regional activation in the frontal cortices in terms of hemoglobin oxygenation in the brain’s vascular system during walking at 3 and 5 km/h and running at 9 km/h on a treadmill. The density of oxygenated hemoglobin increased, but that of deoxygenated hemoglobin was not found in the frontal cortices. Moreover, other studies found a positive correlation between presence and parietal brain activation, and a negative correlation between presence and frontal brain activation during interactive virtual reality training (Mihara et al., 2008). We can see that alongside the prefrontal cortex, the parietal cortex makes a contribution to the control of balance.
Through investigating the hemodynamics of patients who had cortical activation induced by postural perturbation, Mihara found that there was a strong relationship between continuous activation during ataxic gait and the compensatory mechanisms for ataxic gait after infarct stroke. In conclusion, there are widespread cortical network activations, including the prefrontal, premotor, supplementary motor, and parietal cortical areas in both hemispheres, which have a signi fi cant impact on postural control in post-stroke hemiplegic patients (Kober et al., 2012; Mihara et al., 2012).
Visual input mechanisms of balance control
The simulated real-world scenarios give balance dysfunction patients more and stronger information input than the real world to ensure that they relearn their coordination and sense of balance. The fi delity of virtual reality may play an important role in the effectiveness of recruiting neural circuits and the delivery of desirable outcomes at the functional level.
The structure of the brain can be enhanced using visual feedback in virtual reality to augment interconnected,distributed cortical regions. It is suggested that visual information can provide a potent signal for the reorganization of sensorimotor circuits (Lewis et al., 2000; Lewis et al., 2005; Stepniewska et al., 2005). Patients with stroke can use visual feedback to adjust the body’s center of gravity, and to achieve the goal of controlling the body state (Srivastava et al., 2009). In this way, the multi-sensory virtual reality feedback loop can strengthen the control of balance. Fetter et al. (1998) conducted some animal experiments on monkeys, cats and other animals, and showed that visual experience and action seem to be the keys to functional recovery of vestibular function defi ciency (Lacour et al., 1976; Courjon et al., 1977).
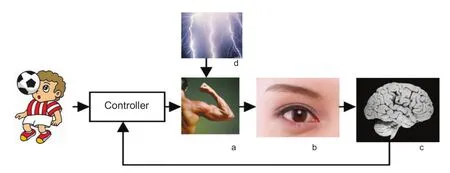
Figure 1 A simplifed representation of the human motion control loop.
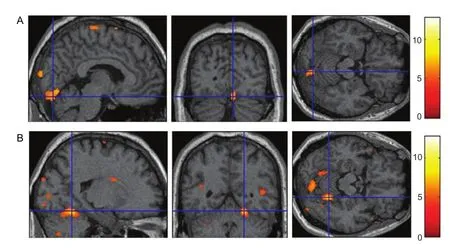
Figure 2 Areas activated during an active foot movement task by a patient with stroke at the First Affliated Hospital, Sun Yat-sen University, China.

Figure 3 Virtual training with bilateral limb movement in the Motor Recovery Laboratory, Department of Rehabilitation, the First Affliated Hospital, Sun Yat-sen University, China.
A large number of clinical trials support this view. In a study by Webster et al. (2010), a virtual environment was created to help people control the mobility of their wheelchairs, and participants had to navigate through a virtual obstacle course. After treatment, compared with participants who did not have training on the virtual course, the experimental group showed a decreased rate of wheelchair accidents and falls and a better performance on obstacle courses (Webster et al., 2001). To study the in fl uence of virtual reality technology on the recovery of balance function following chronic stroke, Belinda and Cho used different kinds of video games to stimulate patients’ balance, coordination, strength and upper limb coordination, and the results showed that all patients had signi fi cantly improvement in balance function (Cho et al., 2012; Michalski et al., 2012). Some other studies have suggested that middle cerebral artery infarcts in the right hemisphere might have a relationship with increased dependence on vision, and non-infarct brain regions are recruited to control postural sway. It is also suggested that the control of lateral posture following a right hemisphere infarct requires more visual input than in a healthy participant (Manor et al., 2010).
Vestibular sensory pathway mechanism of balance control
At present, virtual reality use in vestibular rehabilitation is a relatively new concept, and there are not many studies on the relationship between virtual reality and balance in the vestibular fi eld. Common vestibular function disorders may be caused by abnormalities in the vestibulo-ocular reflex. Viirre suggested that the vestibulo-ocular reflex could be adapted in virtual reality simulations with an increase in vestibulo-ocular re fl ex gain, and virtual reality could be used to increase the rate of adaptation by speci fi cally adapting scenes to a person’s capabilities, thereby facilitating their recovery (Viirre et al., 2000).
Miles’ study suggested that the key to adjusting the vestibular system is the retinal slip, the movement of a visual image across the retina, which is a powerful signal that induces the adaptation of vestibular responses. The primary rationale for using virtual reality for vestibular rehabilitation is that realistic visual environments may enhance adaptation by causing retinal slip (Miles et al., 1980). Pavlou et al. (2001) studied people with uncompensated unilateral peripheral vestibular dysfunction using a customized exercise program or a machine-base optokinetic stimulation exercise program. Preliminary findings suggest that both exercise programs could improve vestibular dysfunction, yet the machine-base group demonstrated that physical therapy involving confl icting visual environments might be more effective than a customized program alone or a program that includes only Cawthorne-Cooksey exercises. Virtual reality scenes may promote rehabilitation more effectively than optokinetic-based therapies, since there is an ability to fi nely control the virtual scene (Pavlou et al., 2001). To discover the potential of virtual reality for vestibular disorders, in a small sample investigation, Wbitney asked two vestibular disorder patients and three healthy people to stand while viewing a sinusoidal waveform on a force plate. The results suggested that virtual reality was a valid way to perform vestibular rehabilitation (Whitney et al., 2002). Nevertheless, because of the small number of participants, the accuracy of the study needs to be validated with further data collection. Another theory to explain the effect of virtual reality on the vestibular system is habituation therapy. Habituation refers to the reduction of patients’ symptoms by provoking the symptoms repetitively. The patients may feel uncomfortable at the beginning, and then a sensory mismatch is sent to the brain and promotes compensation for and adaptation to labyrinthine disorders (Norre et al., 1989).
Role of proprioceptive rehabilitation
Ninety consecutive patients with hemiplegic involvement following a single cerebrovascular accident were recruited to assess the relative importance of factors affecting balance, and it was found that balance function and mobility poststroke were both strongly influenced by proprioception (Keenan et al., 1984). Proprioceptive training affects mobility and balance function by altering how proprioceptive receptors input information, and improves the control of the musculoskeletal motion system and balance function in stroke patients. Some studies have demonstrated that proprioceptive neuromuscular facilitation promotes neuromuscular control and motor function recovery by stimulating proprioceptive stimuli, such as stretching the limbs, joint compression, traction and so on. In addition, the use of spiral diagonal movement patterns can stimulate the joint and muscle proprioceptors. By training the proprioceptive system, the responsiveness of muscles can be enhanced and the recovery of neuromuscular and motor function can be stimulated (Pan et al., 2011). Furthermore, virtual reality training could improve standing balance in stroke patients, including normal and abnormal proprioceptive function (Song et al., 2014).
But compared with proprioceptive neuromuscular facilitation technology, virtual reality training exercises can not only train the limbs, but also train the body by enriching proprioceptive information input. Pan et al. (2011) trained 31 patients using the Pro-Kin training system and found that the score on the Berg Balance Scale in these patients was significantly increased after training, which indicates that proprioception training played an active role in improving balance function in stroke patients.
The feasibility and effect of virtual reality technology applied to balance dysfunction recovery
Compared with conventional therapies (physiotherapy, occupational therapy, or other exercises), virtual reality technology has unique advantages in the fi eld of rehabilitation. First, it makes treatment more interesting and brings patients more enthusiasm, and the sports favored by the patients in daily life can be used as a training program in a virtual reality system (Figure 3). Second, patients can undergo familiar training in a wheelchair or sitting in a chair as well as standing or walking, so there is no need for stabilized posture control, rehabilitation dif fi culty is reduced, and the safety of the rehabilitation exercise is increased. Third, the game system is cheap and easy to carry, and can be easily operated in the hospital, at home or in the community.
Ding et al. (2013) used a simple computer game involving virtual reality-based constraint-induced movement therapy training for balance dysfunction in three chronic stroke survivors, which improved dynamic stability and weight symmetry by encouraging them to use their paretic leg with more effort. The patients received motion signals from virtual avatars in virtual reality as well as augmenting the control gain. This study also demonstrated that this training pattern could be used for balance improvement. Measured by functional gait assessment, the Berg Balance Scale and a walking speed test, Horlings and coworkers con fi rmed that virtual reality balance training could provide more realistic proprioceptive and visual input, and improved the patient’s reaction time, postural stability, balance and walking function effectively (Bisson, 2007; Horlings et al., 2009; Singh et al., 2012). Therefore, virtual reality balance games can be used as a potential and useful tool to train adults with balance dysfunction (Schwesig et al., 2011).
Virtual environments are used in many areas, such as in entertainment, training situations and medicine, including the rehabilitation of behavioral and neurological dysfunction. The use of virtual reality technology in the functional rehabilitation of various debilitating diseases not only provides patients with a very authentic virtual environment and an immersive experience, but also greatly improves their participation enthusiasm and initiation in rehabilitation. Compared with conventional rehabilitation therapy, there is a more significant improvement in motor function and activities of daily living when treated using virtual reality (Bryanton et al., 2006; Deutsch et al., 2009).
Virtual reality therapy is an effective therapy in stroke, spinal cord injury, cerebral palsy and other motor function de fi cient diseases. Wii games combined with virtual reality technology have been used to treat balance dysfunction. In clinical trials, Deutsch and colleagues randomly allocated stroke patients into a wii exercise training group and a conventional training group, and the experiment demonstrated that the wii group exhibited a higher degree of functional recovery of balance and the patients had higher enthusiasm when they performed the virtual reality exercises (Deutsch et al., 2009). In another study, children with spastic CP completed ankle selective motor control exercises using a virtual reality exercise system and conventional exercises. Ankle movements were recorded by an electrogoniometer. The results showed that greater fun and enjoyment were expressed during the virtual reality exercises, and children completed more repetitions of conventional exercises. Furthermore, the range of motion and hold time in the stretched position were greater during virtual reality exercises. These data suggest that using virtual reality to train or guide exercises may improve exercise compliance and raise exercise effectiveness (Bryanton et al., 2006). However, a randomized controlled trial by Eser et al. (2008) did not fi nd a statistically signi ficant difference between virtual reality therapy and conventional therapy in terms of lower extremity motor recovery, mobility or activity level.
Discussion
There is no doubt that the intervention time for equilibrium dysfunction rehabilitation is one of the most critical factors in functional recovery after stroke, which has important research value. At present, many studies about virtual reality training mainly focus on the clinical effects; the mechanisms, especially of neural reorganization and neuroplasticity, and which regions are mainly activated at cortical and sub-cortical levels, are still not clear. In addition, how virtual reality training influences the individual involves the central nervous system at multiple levels. Virtual reality therapy is mostly used in the chronic phase of stroke rehabilitation (Lacour et al., 1976; Kizony et al., 2005; Kim et al., 2009), instead of the early stages. However, the early stages are the prime time of exercise for balance recovery in patients with balance dysfunction. According to the literature, sufficient and early rehabilitation training can greatly improve the prognosis of patients with stroke, and reduce complications (Langhorne et al., 2010; Dennis et al., 2011; Guo et al., 2012), improve motor function of the hemiplegic limb (Li et al., 2011), promote their skills (Craig et al., 2010) and improve their quality of life (Tyedin et al., 2010). Therefore, the early phase of brain injury rehabilitation is the most important time in the recovery of patients with stroke. However, during this phase the patients are often too paretic to do training, an important reason for which is that they have a lack of cortical stimulation. Therefore, using virtual reality in the early phase of brain injury could be feasible. In addition, to explore the safety and therapeutic effects and search for the most suitable intervention time point for balance recovery,virtual reality exercise training should be used for acute and subacute patients, which is a necessary and valuable research direction.
Meanwhile, the study should also explore virtual reality exercise training methods and intensity. Many studies (Flynn et al., 2007; Cikajlo et al., 2012; Pluchino et al., 2012) have shown that virtual reality exercise training is likely to be an effective therapy for patients with balance dysfunction at home in the future. But there are still many problems to solve, such as the evaluation criteria for the treatment and the interactive performance of the virtual reality system. In addition, what kind of model of virtual reality should be chosen, how long the use should be and what precautions should be taken need to be carefully considered when performing virtual reality training. There is still no clear reference index at present.
The next stage of the study of virtual reality technology applied to the rehabilitation of balance function needs to explore the mechanism of the integrated central and peripheral nervous system, visual, vestibular and proprioceptive sensation, and more detailed clinical techniques. Establishing a quantitative standard of virtual reality therapy is highly warranted. Furthermore, the most appropriate way of applying virtual reality intervention and suitable intervention intensity according to different patients’ brain function mechanisms should be determined. In these ways, functional recovery of stroke patients will be promoted and the operational feasibility of virtual reality will be improved by formulating better plans, and the technology can be widely used in the family in the future. In conclusion, virtual reality technology based on the study of the mechanisms of functional rehabilitation and its application following stroke has wide space to develop.
Author contributions:Mao YR, Chen PM and Li L helped in reviewing the studies and experimental data. Huang DF critically revised the manuscript. All authors approved the final version of the paper.
Conficts of interest:None declared.
Agrawal Y, Carey JP, Della Santina CC, Schubert MC, Minor LB (2009) Disorders of balance and vestibular function in US adults: data from the National Health and Nutrition Examination Survey, 2001-2004. Arch Intern Med 169:938-944.
Ayaz H, Shewokis PA, Curtin A, Izzetoglu M, Izzetoglu K, Onaral B (2011) Using MazeSuite and functional near infrared spectroscopy to study learning in spatial navigation. Vis Exp 3443.
Basso Moro S, Bisconti S, Muthalib M, Spezialetti M, Cutini S, Ferrari M, Placidi G, Quaresima V (2014) A semi-immersive virtual reality incremental swing balance task activates prefrontal cortex: a functional near-infrared spectroscopy study. Neuroimage 85:451-460.
Berra K (2004) Virtual rehabilitation: dream or reality? Clin Invest Med 29:187-192.
Bisson E, Contant B, Sveistrup H, Lajoie Y (2007) Functional balance and dual-task reaction times in older adults are improved by virtual reality and biofeedback training. Cyberpsychol Behav 10:16-23.
Blennerhassett JM, Dite W, Ramage ER, Richmond ME (2012) Changes in balance and walking from stroke rehabilitation to the community: a follow-up observational study. Arch Phys Med Rehabil 93:1782-1787.
Bolton DA, Brown KE, McIlroy WE, Staines WR (2012) Transient inhibition of the dorsolateral prefrontal cortex disrupts somatosensory modulation during standing balance as measured by electroencephalography. Neuroreport 23:369-372.
Borlongan CV (2000) Motor activity-mediated partial recovery in ischemic rats. Neuroreport 11:4063-4067.
Bos EJ, Bles W, Hosman R (2002) The cause of spatial disorientation. RTO HFM symposium on “Spatial Disorientation in Military Vehicles: Causes, Consequences and Cures. La Coruna, Spain.
Bower KJ, Clark RA, McGinley JL, Martin CL, Miller KJ (2014) Clinical feasibility of the Nintendo Wii? for balance training post-stroke: a phase II randomized controlled trial in an inpatient setting. Clin Rehabil 28:912-923.
Bryanton C, Bossé J, Brien M, McLean J, McCormick A, Sveistrup H (2006) Feasibility, motivation, and selective motor control virtual reality compared to conventional home exercise in children with cerebral palsy. Cyberpsychol Behav 9:123-128.
Cho KH, Lee KJ, Song CH (2012) Virtual-reality balance training with a video-game system improves dynamic balance in chronic stroke patients. J Exp Med 228:69-74.
Cikajlo I, Rudolf M, Goljar N, Burger H, Matja?i? Z (2012) Telerehabilitation using virtual reality task can improve balance in patients with stroke. Disabil Rehabil 34:13-18.
Colledge N, Lewis S, Mead G, Sellar R, Wardlaw J, Wilson J (2002) Magnetic resonance brain imaging in people with dizziness: a comparison with non-dizzy people. J Neurol Neurosurg Psychiatry 72:587-589.
Courjon JH, Jeannerod M, Ossuzio I, Schmid R (1977) The role of vision in compensation of vestibule ocular re fl ex hemilabyrinthectomy in the cat. Exp Brain Res 28:235-248.
Craig LE, Bernhardt J, Langhorne P, Wu O (2010) Early mobilization after stroke: an example of an individual patient data meta-analysis of a complex intervention. Stroke 41:2632-2636.
Dennis M, Mordi N, Graham C, Sandercock P, CLOTS trials collaboration (2011) The timing, extent, progression and regression of deep vein thrombosis in immobile stroke patients: observational data from the CLOTS multicenter randomized trials. J Thromb Haemost 9:2193-2200.
Deutsch JE, Robbins D, Morrison J (2009) Wii-based compared to standard of care balance and mobility rehabilitation for two individuals post-stroke. IEEE 117-120.
Ding Q, Stevenson IH, Wang N (2013) Motion games improve balance control in stroke survivors: A preliminary study based on the principle of constraint induced movement therapy. Displays 34:125-131.
Drew T, Prentice S, Schepens B (2004) Cortical and brainstem control of locomotion. Prog Brain Res 143:251-261.
Dvorkin AY, Shahar M, Weiss PL (2006) Reaching within video-capture virtual reality: using virtual reality as a motor control paradigm. Cyberpsychol Behav 9:133-136.
Eser F, Yavuzer G, Karakus D, Karaoglan B (2008) The effect of balance training on motor recovery and ambulation after stroke: a randomized controlled trial. Eur J Phys Rehabil Med 44:19-25.
Fetter M, Zee DS (1988) Recovery from unilateral labyrinrthectomy in Rhesus monkeys. J Neurophysiol 59:370-393.
Flynn S, Palma P, Bender A (2007) Feasibility of using the Sony PlayStation 2 gaming platform for an individual post-stroke: a case report. J Neurol Phys Ther 31:180-189.
Guo HR, Li L (2012) Effect of Early Rehabilitation Intervention on the Prognosis of Patients with Stroke. Jinlin Yixue 33:933-934.
Horlings CG, Carpenter MG, Küng UM, Honegger F, Wiederhold B, Allum JH (2009) Influence of virtual reality on postural stability during movements of quiet stance. Neurosci Lett 451:227-231.
Hsieh CL, Sheu CF, Hsueh IP, Wang CH (2002) Trunk control as an early predictor of comprehensive activities of daily living function in stroke patients. Stroke 33:2626-2630.
Johansson BB (2000) Brain plasticity and stroke rehabilitation: the Willis lecture. Stroke 31:322-327.
Johansson BB, Ohlsson AL (1996) Environment, social interaction, and physical activity as determinants of functional outcome after cerebral infarction in the rat. Exp Neurol 139:322-327.
Keenan MA, Perry J, Jordan C (1984) Factors affecting balance and ambulation following stroke. Clin Orthop Relat Res 182:165-171.
Kim JH, Jang SH, Kim CS, Jung JH, You JH (2009) Use of virtual reality to enhance balance and ambulation in chronic stroke: a double-blind, randomized controlled study. Am J Phys Med Rehabil 88:693-701.
Kizony R, Raz L, Katz N, Weingarden H, Weiss PL (2005) Video-capture virtual reality system for patients with paraplegic spinal cord injury. J Rehabil Res Dev 42:595-608.
Kober SE, Kurzmann J, Neuper C (2012) Cortical correlate of spatial presence in 2D and 3D interactive virtual reality: an EEG study. Int J Psychophysiol 83:365-374.
Lacour M, Roll JP, Appaix M (1976) Modifications and development of spinal re fl ex in the alert baboon following a unilateral vestibular neurotomy. Brain Res 113:255-269.
Langhorne P, Stott D, Knight A (2010) Very early rehabilitation or intensive telemetry after stroke: a pilot randomised trial. Cerebrovasc Dis 29:352-360.
Lewis JW, Van Essen DC (2000) Corticocortical connections of visual, sensorimotor, and multimodal processing areas in the parietal lobe of the macaque monkey. J Comp Neurol 428:112-137.
Lewis SJ, Slabosz A, Robbins TW, Barker RA, Owen AM (2005) Dopaminergic basis for deficits in working memory but not attentional set-shifting in Parkinson’s disease. Neuropsychologia 43:823-832.
Li M (2011) Application of early rehabilitation intervention on hemiplegic. Zhongguo Shiyong Shenjing Jibing Zazhi 14:71-72.
Lo HC, Hsu YC, Hsueh YH, Yeh CY (2012) Cycling exercise with functional electrical stimulation improves postural control in stroke patients. Gait Posture 35:506-510.
Manor B, Hu K, Zhao P, Selim M, Alsop D, Novak P, Lipsitz L, Novak V (2010) Altered control of postural sway following cerebral Infarction. Neurology 74:458-464.
McEwen D, Taillon-Hobson A, Bilodeau M, Sveistrup H, Finestone H (2014) Virtual reality exercise improves mobility after stroke: an inpatient randomized controlled trial. Stroke 45:1853-1855.
Michalski A, Glazebrook CM, Martin AJ, Wong WW, Kim AJ, Moody KD, Salbach NM, Steinnagel B, Andrysek J, Torres-Moreno R, Zabjek KF (2012) Assessment of the postural control strategies used to play two Wii Fit video games. Gait Posture 36:449-453.
Mihara M, Miyai I, Hatakenaka M, Kubota K, Sakoda S (2007) Sustained prefrontal activation during ataxic gait: a compensatory mechanism for ataxic stroke. Neuroimage 37:1338-1345.
Mihara M, Miyai I, Hatakenaka M, Kubota K, Sakoda S (2008) Role of the prefrontal cortex in human balance control. Neuroimage 43:329-336.
Mihara M, Miyai I, Hattori N, Hatakenaka M, Yagura H, Kawano T, Kubota K (2012) Cortical control of postural balance in patients with hemiplegic stroke. Neuroreport 23:314-319.
Miles FA, Eighmy BB (1980) Long-term adaptive changes in primate vestibuloocular reflex: behavioral observations. J Neurophysiol 43:1406-1425.
Miller EK, Cohen JD (2001) An integrative theory of prefrontal cortex function. Annu Rev Neurosci 24:167-202.
Nancy LC (1980) The vestibular and proprioceptive systems to dysfunction in verticality perception, posture and movement afterr stroke. J Physiother 26:5-16.
Norre M, Beckers A (1989) Vestibular habituation training: exercise treatment for vertigo based upon the habituation effect. Otolaryngnol Head Neck Surg 1989:14-19.
Pan HP, Feng H, Li YJ, Jin HZ (2011) Effects of load-controlled proprioceptive training on lower extremity motor and balance function of stroke patients. Zhongguo Kangfu Yixue Zazhi 26:1025-1028.
Pavlou M, Lingerwaran A, Davies RA (2001) Machine-based vs customized rehabilitation for the treatment of chronic vestibular disorders. ISPG Symposium.
Pluchino A, Lee SY, Asfour S, Roos BA, Signorile JF (2012) Pilot study comparing changes in postural control after training using a video game balance board program and 2 standard activity-based balance intervention programs. Arch Phys Med Rehabil 93:1138-1146.
Schwesig R, Goldich Y, Hahn A (2011) Postural control in subjects with visual impairment. Eur J Ophthalmol 21:303-309.
Singh DK, Rajaratnam BS, Palaniswamy V, Pearson H, Raman VP, Bong PS (2012) Participating in a virtual reality balance exercise program can reduce risk and fear of falls. Maturitas 73:239-243.
Slobounov S, Hallett M, Stanhope S, Shibasaki H (2005) Role of cerebral cortex in human postural control: an EEG study. Clin Neurophysiol 116:315-323.
Song YB, Chun MH, Kim W, Lee SJ, Yi JH, Park DH (2014) The effect of virtual reality and tetra-ataxiometric posturography programs on stroke patients with impaired standing balance. Ann Rehabil Med 38:160-166.
Srivastava A, Taly AB, Gupta A, Kumar S, Murali T (2009) Post-stroke balance training: role of force platform with visual feedback technique. J Neurol Sci 287:89-93.
Stepniewska I, Fang PC, Kaas JH (2005) Microstimulation reveals specialized subregions for different complex movements in posterior parietal cortex of prosimian galagos. Proc Natl Acad Sci U S A 102:4878-4883.
Suttanon P, Hill KD, Said CM, Logiudice D, Lautenschlager NT, Dodd KJ (2012) Balance and mobility dysfunction and falls risk in older people with mild to moderate Alzheimer disease. Am J Phys Med Rehabil 91:12-23.
Suzuki M, Miyai I, Ono T, Oda I, Konishi I, Kochiyama T, Kubota K (2004) Prefrontal and premotor cortices are involved in adapting walking and running speed on the treadmill: an optical imaging study. Neuroimage 23:1020-1026.
Tachibana A, Noah JA, Bronner S, Ono Y, Onozuka M (2011) Parietal and temporal activity during amultimodal dance video game: an fNIRS study. Neurosci Lett 503:125-130.
Tyedin K, Cumming TB, Bernhardt J (2010) Quality of life: an important outcome measure in a trial of very early mobilization after stroke. Disabil Rehabil 32:875-884.
Viau A, Feldman AG, McFadyen BJ, Levin MF (2004) Reaching in reality and virtual reality: a comparison of movement kinematics in healthy subjects and in adults with hemiparesis. J Neuroeng Rehabil 1:11.
Viirre E, Buskirk J (2000) Utilization of virtual reality technology in the rehabilitation of balance disorder patients. Micromedical Technologies Vestibular Update 24:1-4.
Virk S, McConville KM (2006) Virtual reality applications in improving postural control and minimizing falls. Conf Proc IEEE Eng Med Biol Soc 1:2694-2697.
Walker ML, Ringleb SI, Maihafer GC, Walker R, Crouch JR, Van Lunen B, Morrison S (2010) Virtual reality-enhanced partial body weight-supported treadmill training post-stroke: feasibility and effectiveness in 6 subjects. Arch PhysMed Rehabil 91:115-122.
Webster JS, McFarland PT, Rapport LJ, Morrill B, Roades LA, Abadee PS (2001) Computer-assisted training for improving wheelchair mobility in unilateral neglect patients. Arch Phys Med Rehabil 82:769-775.
Whitney S, Sparto PJ, Brown KE (2002) The potential use of virtual reality in vestibular rehabilitation: preliminary findings with the BNAVE. J Neurol PhysTher Report 26:72-78.
Yavuzer G, Eser F, Karakus D, Karaoglan B, Stam HJ (2006) The effects of balance training on gait late after stroke: a randomized controlled trial. Clin Rehabil 20:960-969.
Copyedited by Jackson C, Norman C, Wang J, Yang Y, Li CH, Song LP, Zhao M
Dongfeng Huang, M.D., Department of Rehabilitation Medicine, First Affiliated Hospital, Sun Yat-sen University, Guangzhou 510080, Guangdong Province, China, huangdf@mail.sysu.edu.cn.
10.4103/1673-5374.141795
http://www.nrronline.org/
Accepted: 2014-07-09
 中國(guó)神經(jīng)再生研究(英文版)2014年17期
中國(guó)神經(jīng)再生研究(英文版)2014年17期
- 中國(guó)神經(jīng)再生研究(英文版)的其它文章
- Stem cell transplantation for treating stroke: status, trends and development
- Publication trends in studies examining radix notoginseng as a treatment for ischemic brain injury
- Neuroprotective effects of Buyang Huanwu decoction on cerebral ischemia-induced neuronal damage
- Methylmercury chloride damage to the adult rat hippocampus cannot be detected by proton magnetic resonance spectroscopy
- Damage of hippocampal neurons in rats with chronic alcoholism
- Tooth loss inhibits neurogenesis in the dentate gyrus of adult mice
