Methylmercury chloride damage to the adult rat hippocampus cannot be detected by proton magnetic resonance spectroscopy
Zhiyan Lu, Jinwei Wu,, Guangyuan Cheng, Jianying Tian, Zeqing Lu, Yongyi Bi
1 Department of Radiology, Zhongnan Hospital of Wuhan University, Wuhan, Hubei Province, China
2 Department of Radiology, Hainan Provincial Nongken Hospital, Haikou, Hainan Province, China
3 Department of Anatomy, Ningxia Medical University, Yinchuan, Ningxia Hui Autonomous Region, China
4 School of Public Health, University of Minnesota, Minnesota, MN, USA
5 School of Public Health, Wuhan University, Wuhan, Hubei Province, China
Methylmercury chloride damage to the adult rat hippocampus cannot be detected by proton magnetic resonance spectroscopy
Zhiyan Lu1, Jinwei Wu1,2, Guangyuan Cheng1, Jianying Tian3, Zeqing Lu4, Yongyi Bi5
1 Department of Radiology, Zhongnan Hospital of Wuhan University, Wuhan, Hubei Province, China
2 Department of Radiology, Hainan Provincial Nongken Hospital, Haikou, Hainan Province, China
3 Department of Anatomy, Ningxia Medical University, Yinchuan, Ningxia Hui Autonomous Region, China
4 School of Public Health, University of Minnesota, Minnesota, MN, USA
5 School of Public Health, Wuhan University, Wuhan, Hubei Province, China
Previous studies have found that methylmercury can damage hippocampal neurons and accordingly cause cognitive dysfunction. However, a non-invasive, safe and accurate detection method for detecting hippocampal injury has yet to be developed. This study aimed to detect methylmercury-induced damage on hippocampal tissue using proton magnetic resonance spectroscopy. Rats were given a subcutaneous injection of 4 and 2 mg/kg methylmercury into the neck for 50 consecutive days. Water maze and pathology tests confirmed that cognitive function had been impaired and that the ultrastructure of hippocampal tissue was altered after injection. The results of proton magnetic resonance spectroscopy revealed that the nitrogen-acetyl aspartate/ creatine, choline complex/creatine and myoinositol/creatine ratio in rat hippocampal tissue were unchanged. Therefore, proton magnetic resonance spectroscopy can not be used to determine structural damage in the adult rat hippocampus caused by methylmercury chloride.
nerve regeneration; proton magnetic resonance spectroscopy; methylmercury chloride; cognitive dysfunction; hippocampus; behavior; pathology; NSFC grant; neural regeneration
Funding:This study was supported by the National Natural Science Foundation of China, No. 81060231, 81160338; Hubei Province Natural Science Foundation of China, No. 2013CFB277.
Lu ZY, Wu JW, Cheng GY, Tian JY, Lu ZQ, Bi YY. Methylmercury chloride damage to the adult rat hippocampus cannot be detected by proton magnetic resonance spectroscopy. Neural Regen Res. 2014;9(17):1616-1620.
Introduction
During 1953-1956, a number of Japanese inhabitants suffered from chronic methylmercury poisoning due to high concentrations of edible methylmercury (MeHg), which later presented as Minamata disease 10-20 years after cessation of exposure to MeHg (Yorifuji et al., 2009). Studies have shown that MeHg can directly enter the brain by crossing the blood-brain barrier and accumulate in the hippocampus (Eto et al., 2002; Li et al., 2005; Yin et al., 2011; Roda et al., 2012). Eto et al. (2002) detected severe neurological damage in the cerebral cortex and spongy degeneration in marmosets exposed to MeHg using MRI. Increasing evidence from recent studies has demonstrated that patients have impaired cognitive function following MeHg injury, which is attributed to hippocampal damage (Yin et al., 2011; Roda et al., 2012). In related animal experiments, MeHg chloride (MeHgCl) was shown to affect learning/memory ability and the ultrastructure of hippocampal neurons (Li et al., 2005). At present, there is no non-invasive, safe, and accurate method to detect hippocampal damage (Coccini et al., 2010). Proton magnetic resonance spectroscopy (1H-MRS) is one method that can be used to detect the metabolism ofin vivochemicals in a non-invasive manner, and provide valuable information for the diagnosis and treatment of diseases. In fact, this method has already been applied for the early diagnosis and prevention of cognitive impairment (Christiansen et al., 1993). Therefore, the present study aimed to explore the possibility of detecting methylmercury-induced hippocampal damage in rats using1H-MRS.
Materials and Methods
Establishment of MeHgCl poisoning model
Thirty healthy adult male Sprague-Dawley rats, of specific pathogen free grade, weighing 30-40 g, were provided by the ABSL-III Laboratory, Wuhan University, China (license No. SCXK (E) 2008-0004). Animals were housed in a closed sterile animal room, at 20 ± 2°C under a 12-hour alternating cycle (light 8:00 a.m.; dark 8:00 p.m.). The study conformed tothe Guide for the Care and Use of Laboratory Animals,published by the US National Institutes of Health (NIH publication No. 85-23, revised 1996), and the protocol was approved by the Institutional Animal Care Committee fromWuhan University in China.
Rats were randomly divided into three groups: high-dose MeHgCl, low-dose MeHgCl and control groups. Each group contained 10 rats. After 1 week of feeding, rats were subcutaneously injected with 4 mg/kg of MeHgCl (high-dose; Merck, Darmstadt, Germany), 2 mg/kg of MeHgCl (low-dose), and 4 mL/kg saline (control). The subcutaneous injection was given for 50 consecutive days. Two rats in the high-dose group were excluded owing to skin ulcers, infection and death caused by MeHgCl.
Morris water maze test for cognitive behavior
The water maze tests were performed 1 week after administration of MeHgCl. The Morris water maze apparatus (Beijing Zhongshi Dichuang Science and Technology Development Co., Ltd., Beijing, China) was used to test spatial learning and memory, as well as cognitive behavior, according to a previously described method (Arias et al., 2014). The tests consisted of a navigation test and a spatial probe test.
Navigation test
The navigation test was performed to detect the spatial learning and memory capacity of rats. There were four trials per session and one session per day. For a complete test, a total of fi ve sessions over 5 days were given. In each of the four trials, the animals were placed randomly at four different starting positions (A, B, C, D) at the junction between two adjacent quadrants. The time that an individual rat spent to reach the platform was recorded as the escape latency. The animals were allowed 60 seconds to fi nd the platform. If an animal could not find the platform within 60 seconds, it was guided with a stick to the platform. After mounting the platform, the animals were allowed to stay there for 30 seconds, and the escape latency was recorded as 60 seconds. The training was performed at intervals of 1 hour.
Spatial probe test
The spatial probe test was performed 24 hours after the navigation test was completed, to detect the platform memory capacity of rats. The platform was removed from the pool at 6 days and rats were placed at a unique starting location (E point). The number of times rats crossed the former platform location and the percentage of time spent in the former platform quadrant were recorded over 30 seconds. Each rat performed the task once.
1H-MRS detection of rat hippocampal ultrastructure
At 7 days after the Morris water maze test was completed, the right hippocampus was photographed using1H-MRS as previously described (Coccini et al., 2010). Each group contained eight rats. The scanning room was maintained at 20-22°C and imaging was performed with the Bruker Biospect 7.0 T/20 cm magnetic resonance imager for small animals (Bruker, Bremen, Germany). Sprague-Dawley rats were anesthetized with 10% (v/v) chloral hydrate (0.3 mL/100 g)viaintraperitoneal injection, and placed in a 60 mm dual-channel RF coil. T1-weighted images were captured using spin-echo sequence and T2-weighted images (T2WI) were captured using rapid acquisition with relaxation enhancement sequence. The axial and sagittal T2WI were collected, and the region of interest was defined as the right hippocampus (Figure 1). Avoiding interference from the skull and cerebrospinal fluid, shimming and water suppression were performed using automatic scanning. The free induction signal was acquired for Fourier transformation analysis. The imaging data were phased and corrected using Bruker topspin software. The changes of signal intensity of various metabolites such as nitrogen-acetyl aspartate (NAA) 2.01 ppm, choline compound (Cho) 3.22 ppm, creatine (Cr) 3.03 ppm and myo-inositol (mI) 3.28 ppm were observed. The peak under the curve of the metabolites was calculated and the percentage of each metabolite was also calculated, taking Cr as the reference. Peak area spectrophotometry was applied to calculate the NAA/Cr, Cho/Cr, mI/Cr ratios. Each rat was measured once and the results were averaged.
Pathology of the rat hippocampus
After1H-MRS observation, rats were anesthetized with 10% (v/v) chloral hydrate (0.35 mL/100 g) and fi xed in the supine position. The skin on the chest was disinfected with iodine, and an incision was made to fully expose the heart. Rats were rapidly perfused with saline and 4% (w/v) paraformaldehyde. Hippocampal and cortical tissues were harvested, fi xed in paraformaldehyde, stained with hematoxylin-eosin, and observed under a microscope (Olympus, Tokyo, Japan) to observe changes in the morphology of rat hippocampal neurons.
A 1-mm incision was cut along the midline of the right hippocampus and then sectioned at a thickness of 1-2 mm at the sagittal plane. Sections were fi xed and preserved in 2% (v/v) glutaraldehyde and subsequently prepared for observation under S-500A transmission electron microscope (Hitachi, Tokyo, Japan).
Statistical analysis
Morris water maze data are expressed as mean ± SEM.1H-MRS data are expressed as mean ± SD. SPSS 17.0 software (SPSS, Chicago, IL, USA) was used for one-way analysis of variance. Differences between groups were compared using independent samplest-test. AP< 0.05 level was considered statistically signi fi cant.
Results
Effect of different concentrations of MeHgCl on cognitive behavior of adult rats
The results of the navigation test using the Morris water maze showed that the average escape latency of rats in the high-dose MeHgCl group were signi fi cantly longer than that of the control group (P< 0.05), and also longer than that of the low-dose MeHgCl group (P< 0.05). The low-dose MeHgCl group had a similar escape latency to the control group (P>0.05; Table 1).
The spatial probe test using the Morris water maze revealed that the high-dose MeHgCl group crossed the formerplatform location signi fi cantly more times when compared with the low-dose MeHgCl or control groups (P< 0.05). No difference was observed between rats from the low-dose Me-HgCl group and the control group (P> 0.05; Figure 2).
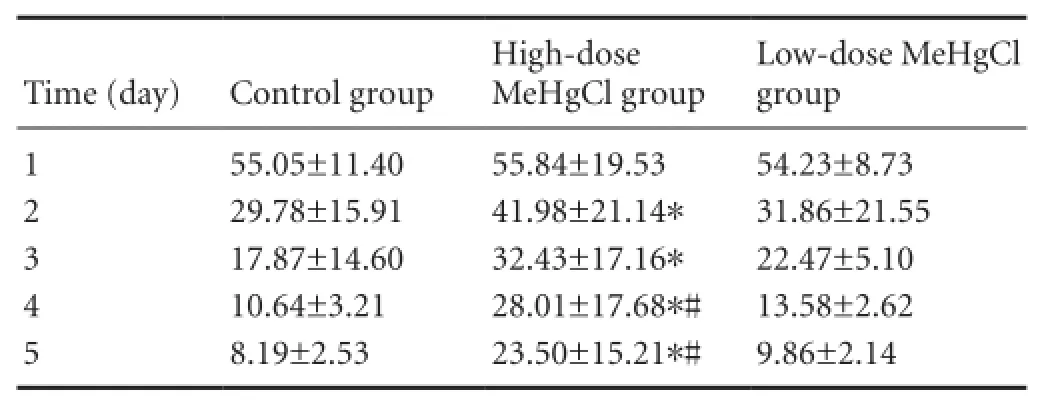
Table 1 Effect of methylmercury chloride (MeHgCl) exposure on the average escape latency (second) of rats in the Morris water maze

Table 2 Effect of different concentrations of methylmercury chloride (MeHgCl) on metabolites in the right hippocampus of adult rats
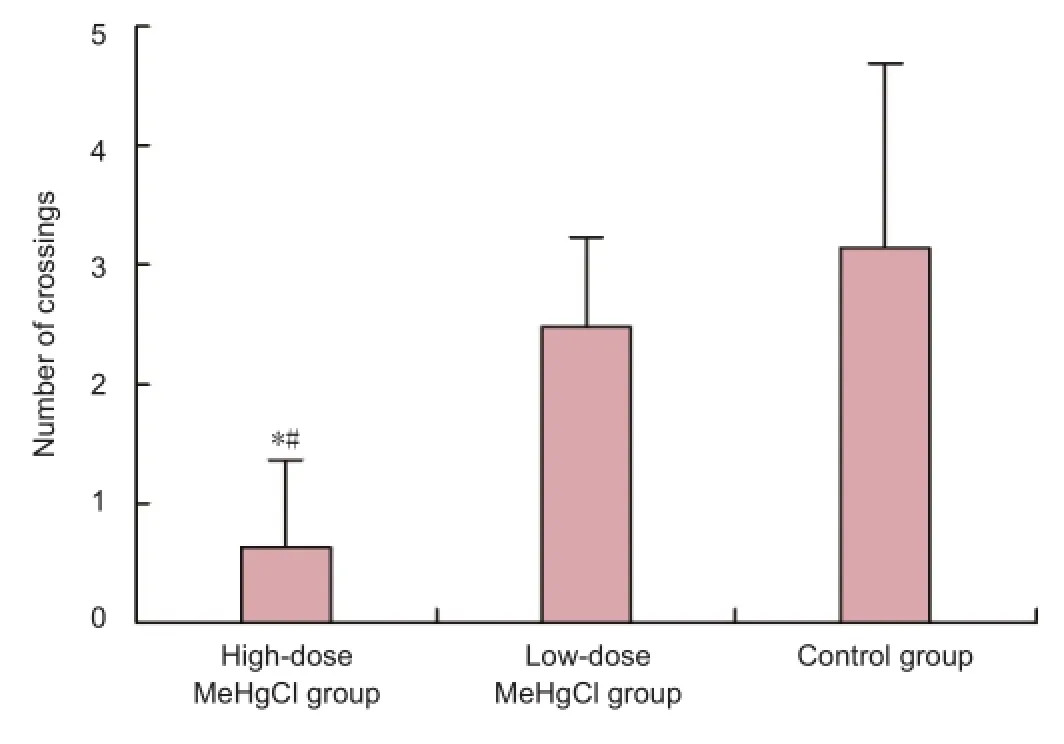
Figure 2 Effect of methylmercury chloride (MeHgCl) exposure on the number of times rats crossed the former platform location during the spatial probe test.

Figure 3 Effect of different concentrations of methylmercury chloride (MeHgCl) on hippocampal injury in adult rats (hematoxylin-eosin staining, × 100).
Effect of different concentrations of MeHgCl on hippocampal injury in adult rats
Hematoxylin-eosin staining showed that there were a small number of hippocampal neurons in the high-dose MeHgCl group that appeared disorderly. In the low-dose MeHgCl group and control group, the hippocampal nerve cells were tightly arranged, had a regular morphology and intact membrane (Figure 3).
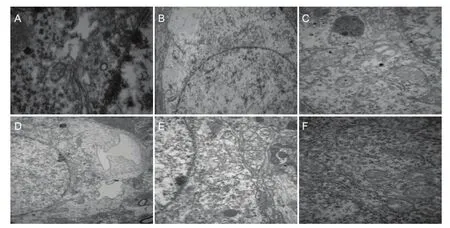
Figure 4 Effect of different concentrations of methylmercury chloride (MeHgCl) on the ultrastructure of hippocampal tissue in adult rats (transmission electron microscopy).
Under transmission electron microscopy, Golgi complex hypertrophy, edema and expansion, and Golgi complex membrane dissolvement were observed in the dentate gyrus of the hippocampus of the high-dose MeHgCl group. Additionally, endoplasmic reticulum expansion, mitochondrial hypertrophy and edema of intercellular substances were visible. In the low-dose MeHgCl group, only the endoplasmic reticulum expanded and edema of intercellular substances was evident in the dentate gyrus. In the control group, hippocampal nerve cells were neatly arranged and had intact cell membranes. There were no obvious changes to mitochondria (Figure 4).
Effect of different concentrations of MeHgCl on the hippocampus of adult rats as detected by1H-MRS
The1H-MRS assay revealed that the NAA/Cr and Cho/Cr ratios were similar among the three groups (P> 0.05; Table 2).
Discussion
In this study, adult Sprague-Dawley rats were exposed to 4 and 2 mg/kg MeHgClviasubcutaneous injection for 50 days, to damage hippocampal neurons and cause cognitive impairment. Subsequently, cognitive function was determined using the Morris water maze test. The escape latency of rats in the high-dose MeHgCl group was significantly longer, and the number of times rats crossed the former platform quadrant decreased when compared with the low-dose and control groups. These results indicate that MeHgCl leads to impairment of cognitive behavior in rats.
Liu et al. (2009) found that Sprague-Dawley rats at 7-14 days after birth had a signi fi cantly reduced capability of performing the Morris water maze task after continuous exposure to 5 mg/kg MeHgCl for 7 days. This was consistent with the findings of Nishioku et al. (2000). Additionally, Kakita et al. (2000) and Goulet et al. (2003) showed that the motor, spatial and learning ability of neonatal rats in the Morris water maze was impaired after pregnant rats were exposed to MeHgCl during lactation. These results were supported by the fi ndings of Gao et al. (2006). The results of the Morris water maze test in this study showed that neurotoxicity of certain doses. MeHgCl leads to damage of hippocampal neurons, which may affect the cognitive function of rats (Zhang et al., 2007).
After neonatal rats were exposed to low-dose poisons (1 mg/kg) (Ang and Lee, 2007), the number of cells in the hippocampus reduced, cell membrane rupture and nuclear condensation occurred and necrosis was visible. However, we did not detect pyknosis, membrane dissolvement or cytoplasmic over fl ow; hematoxylin-eosin staining revealed that the number of neurons was reduced and nerve cells were arranged in a disorderly manner after injection of highdose MeHgCl, while these changes were not observed in the low-dose group. This indicates that adult rats have a strong capacity for repairing the damage caused by MeHgCl. Under electron microscopy, Golgi complex hypertrophy, edema and expansion were observed in the hippocampal dentate gyrus, and the Golgi complex membrane was dissolved. Additionally, endoplasmic reticula expanded, and mitochondrial hypertrophy and intercellular edema were apparent after injection of high-dose MeHgCl. In contrast, in the low-dose MeHgCl group, only endoplasmic reticulum expansion andintercellular edema were found in the hippocampal dentate gyrus. Gao et al. (2006) found that hippocampal ultrastructure was slightly changed, but that previously described characteristic features of mercury exposure, such as apoptosis and mitochondrial disintegration, were not evident. However, mercury exposure may affect normal development of the cerebellum and hippocampus.
At present, few efforts have been focused directly on studying proton spectroscopy of rats exposed to MeHgCl. Previous studies suggest that spectral characteristics in Alzheimer’s patients include a decline of NAA and the elevation of mI (Baddeley, 2003; McHugh et al., 2007). In this study, we determined the concentration of the main metabolites in the hippocampus of rats exposed to MeHgCl using1H-MRS. The results showed that the NRR/Cr ratio in the right hippocampus of rats was not changed after exposure to MeHgCl. This result could be due to brain volume and size, which were obviously increased during poisoning, resulting in dilution of the mercury concentration in the brain, and the initiation of self-repair. Therefore, the1H-MRS assay revealed that the NAA/Cr, CHO/Cr, mI/Cr ratios in each group remained unchanged indicating that1H-MRS cannot detect MeHgCl-induced hippocampal damage in adult rats.
Author contributions:Lu ZY and Tian JY were responsible for the study design. Wu JW and Cheng GY implemented the experiments. Lu ZQ performed statistical analysis. Lu ZY wrote the manuscript. Bi YY instructed the experiments and supervised the manuscript. All authors approved the final version of the manuscript.
Conficts of interest:None declared.
Ang HH, Lee KL (2007) Evaluation of mercury contamination in Smilax myosoti fl ora herbal preparations. Int J Toxicol 26:433-439.
Baddeley A (2003) Working memory: looking back and looking forward. Nat Rev Neurosci 4:829-839.
Christiansen P, Henriksen O, Stubgaard M, Gideon P, Larsson HB (1993) In vivo quanti fi cation of brain metabolites by1H-MRS using water as an internal standard. Magn Reson Imaging 11:107-118.
Coccini T, Roda E, Sarigiannis DA, Manzo L (2010) Assessing health effects of environmental contaminants by molecular markers. Studies on methylmercury and polychlorinated biphenyls as examples of translational research in environmental toxicology. G Ital Med Lav Ergon 32:5-12.
Eto K, Yasutake A, Korogi Y, Akima M, Shimozeki T, Tokunaga H, Kuwana T, Kaneko Y (2002) Methylmercury poisoning in common marmosets--MRI findings and peripheral nerve lesions. Toxicol Pathol 30:723-734.
Gao Y, Yan CH, Yu XD, Wu SH (2006) Effects of perinatal exposure to methylmercury on the structure of hippocampus and cerebellum in young rats. Wei Sheng Yan Jiu 35:402-405.
Goulet S, Doré FY, Mirault ME (2003) Neurobehavioral changes in mice chronically exposed to methylmercury during fetal and early postnatal development. Neurotoxicol Teratol 25:335-347.
Kakita A, Wakabayashi K, Su M, Yoneoka Y, Sakamoto M, Ikuta F, Takahashi H (2000) Intrauterine methylmercury intoxication: consequence of the inherent brain lesions and cognitive dysfunction in maturity. Brain Res 877:322-330.
Li YJ, Fang Q, Zhang CX, Bi XY, Li Z (2005) Effects of prenatal methylmercury exposure on learning and memory ability of mice and ultrastructure of hippocampus neurons in mice. Wei Sheng Yan Jiu 34:284-286.
Liu W, Wang X, Zhang R, Zhou Y (2009) Effects of postnatal exposure to methylmercury on spatial learning and memory and brain NMDA receptor mRNA expression in rats. Toxicol Lett 188:230-235.
McHugh TL, Saykin AJ, Wishart HA, Flashman LA, Cleavinger HB, Rabin LA, Mamourian AC, Shen L (2007) Hippocampal volume and shape analysis in an older adult population. Clin Neuropsychol 21:130-145.
Nishioku T, Takai N, Miyamoto KI, Murao K, Hara C, Yamamoto K, Nakanishi H (2000) Involvement of caspase 3-like protease in methylmercury-induced apoptosis of primary cultured rat cerebral microglia. Brain Res 871:160-164.
Roda E, Manzo L, Coccini T (2012) Application of Neurochemical Markers for Assessing Health Effects after Developmental Methylmercury and PCB Coexposure. J Toxicol 2012:216032.
Yin Z, Lee E, Ni M, Jiang H, Milatovic D, Rongzhu L, Farina M, Rocha JB, Aschner M (2011) Methylmercury-induced alterations in astrocyte functions are attenuated by ebselen. Neurotoxicology 32:291-299.
Yorifuji T, Kashima S, Tsuda T, Harada M (2009) What has methylmercury in umbilical cords told us? - Minamata disease. Sci Total Environ 408:272-276.
Zhang H, Yan CH, Wu SH, Xu J, Tang FG, Zou XY (2007) Effects of perinatal exposure to methylmercury on changes of hippocampal ultrastructure and expression of metabotropic glutamate receptor 2, 3, 5mRNAs in offspring’s mice. Linchuang Erke Zazhi 25:788-791.
Copyedited by Diwakarla S, Norman C, Yu J, Yang Y, Li CH, Song LP, Zhao M
Yongyi Bi, Ph.D., School of Public Health, Wuhan University, Wuhan 430071, Hubei Province, China, yongyib@aliyun.com.
10.4103/1673-5374.141789
http://www.nrronline.org/
Accepted: 2014-06-18
- 中國神經(jīng)再生研究(英文版)的其它文章
- Stem cell transplantation for treating stroke: status, trends and development
- Publication trends in studies examining radix notoginseng as a treatment for ischemic brain injury
- Virtual reality training improves balance function
- Neuroprotective effects of Buyang Huanwu decoction on cerebral ischemia-induced neuronal damage
- Damage of hippocampal neurons in rats with chronic alcoholism
- Tooth loss inhibits neurogenesis in the dentate gyrus of adult mice

