Negative regulation of miRNA-9 on oligodendrocyte lineage gene 1 during hypoxic-ischemic brain damage
Lijun Yang, Hong Cui, Ting Cao
Department of Pediatrics, Beijing Friendship Hospital, Capital Medical University, Beijing, China
Negative regulation of miRNA-9 on oligodendrocyte lineage gene 1 during hypoxic-ischemic brain damage
Lijun Yang, Hong Cui, Ting Cao
Department of Pediatrics, Beijing Friendship Hospital, Capital Medical University, Beijing, China
Oligodendrocyte lineage gene 1 plays a key role in hypoxic-ischemic brain damage and myelin repair. miRNA-9 is involved in the occurrence of many related neurological disorders. Bioinformatics analysis demonstrated that miRNA-9 complementarily, but incompletely, bound oligodendrocyte lineage gene 1, but whether miRNA-9 regulates oligodendrocyte lineage gene 1 remains poorly understood. Whole brain slices of 3-day-old Sprague-Dawley rats were cultured and divided into four groups: control group; oxygen-glucose deprivation group (treatment with 8% O2+ 92% N2and sugar-free medium for 60 minutes); transfection control group (after oxygen and glucose deprivation for 60 minutes, transfected with control plasmid) and miRNA-9 transfection group (after oxygen and glucose deprivation for 60 minutes, transfected with miRNA-9 plasmid). From the third day of transfection, and with increasing culture days, oligodendrocyte lineage gene 1 expression increased in each group, peaked at 14 days, and then decreased at 21 days. Real-time quantitative PCR results, however, demonstrated that oligodendrocyte lineage gene 1 expression was lower in the miRNA-9 transfection group than that in the transfection control group at 1, 3, 7, 14, 21 and 28 days after transfection. Results suggested that miRNA-9 possibly negatively regulated oligodendrocyte lineage gene 1 in brain tissues during hypoxic-ischemic brain damage.
nerve regeneration; brain injury; miRNA-9; oligodendrocyte lineage gene 1; hypoxic-ischemic; brain damage; premature birth; brain slice culture; NSFC grant; neural regeneration
Funding: This study was supported by the National Natural Science Foundation of China, No. 81241022; the Beijing Municipal Natural Science Foundation in China, No. 7122045, 7072023.
Yang LJ, Cui H, Cao T. Negative regulation of miRNA-9 on oligodendrocyte lineage gene 1 during hypoxic-ischemic brain damage. Neural Regen Res. 2014;9(5):513-518.
Introduction
Repair mechanisms and therapeutic problems of premature infants with hypoxic-ischemic brain damage are becoming increasingly recognized by experts in perinatology[1-5]. Current studies concerning functional rehabilitation after brain injury mainly focus on how to protect neurons, but seldom glial cells (such as oligodendrocytes)[6-11]. Periventricular leukomalacia is a kind of hypoxic-ischemic brain damage. A recent study showed that oligodendrocytes played an important role in the occurrence, development and turnover of periventricular leukomalacia[12]. Cytokines produced toxic effects on white matter by inhibiting the differentiation of oli-godendrocyte precursor cells, inducing glial cell apoptosis, and causing myelin sheath degeneration[13]. Therefore, effective therapy for white matter injury depends on in depth investigation into the mechanisms underlying oligodendrocyte injury and can be considered as a new therapeutic target for promoting the recovery of neural function. These studies further indicated the essential effect of oligodendrocytes on hypoxic-ischemic brain damage.
Oligodendrocyte lineage gene 1 (Olig1) triggers repair of the myelin sheath around injured neurons, indicating a crucial role in myelin sheath repair for this gene. Arnett et al.[14]first reported in Science that Olig1 was expressed in oligodendrocytes after demyelinating injury in the brain and could repair the myelin sheath. A recent study confirmed that Olig1 was necessary for repair of the myelin sheath of transplanted neural progenitor cells in models of virus-induced demyelination[15]. Olig1 is expressed during the maturity and regeneration of human oligodendrocytes[16-17]. Our pilot experiments verified that increased Olig1 gene expression contributed to myelin basic protein expression, indicating that the Olig1 gene played an important role in the repair of the myelin sheath. Previous studies on brain specimens from rodents and multiple sclerosis patients further veri fi ed that Olig1 gene expression in oligodendrocytes was vitally important for repair of the myelin sheath[18-19]. In models of virus-induced demyelination, Olig1 promoted the regeneration of the myelin sheath of transplanted neural progenitor cells[20]. Another study found that oligodendrocyte development was delayed in Olig1 knockout mice[21].
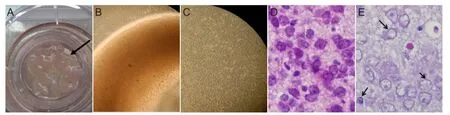
Figure 1 Preparation of whole brain slices from neonatal rats.
miRNA expression probably undergoes dynamic changes after cerebral trauma[22]. After cerebral ischemia, the expression of neurogenesis-related miRNA, such as miRNA-9, is evidently upregulated[23-24]. The expression of miRNA-9 in patients with Alzheimer’s disease or Huntington’s disease is also apparently disordered[25-26]. miRNA-9 has been shown to play a crucial role in repair of the myelin sheath after hypoxic-ischemic brain damage[27-33]. Bioinformatics analysis demonstrated that miRNA-9 complementarily, but incompletely, bound the gene, indicating a direct relationship[31]. A recent study showed that miRNA-9 and Olig1 had a key effect on myelinization and brain injury, and miRNA-9 regulated the differentiation of oligodendrocyte progenitor cells by regulating serum response factor[34]. miRNA-9 also played an important role in maintenance of myelinogenesis[35]. It remains unclear whether there is a regulatory relationship between miRNA-9 and Olig1.
This study established a rat model of oxygen-glucose deprivation in immature tissue to simulate human premature infants with hypoxic-ischemic brain damage. Real-time quantitative PCR was used to explore dynamic expression pattern of Olig1 during hypoxic-ischemic brain damage and after miRNA-9 transfection. This study tried to understand whether the relationship between Olig1 and miRNA-9 was regulatory in nature.
Results
Brain slice culture and establishment of oxygen-glucose deprivation model
Brain tissue from immature neonatal rats was sliced into 450-μm-thick sections using a tissue slicer. Brain slices were incubated in a culture plate insert. Brain slices grew well, were active, shiny, and gradually became translucent over time (Figure 1B, C). Brain slice size became smaller and obviously thinner over time. One week following initial culture, brain slices were less than half their original thickness. Brain slices remained at this thickness for about 30 days (Figure 1A–C).
The experiment contained four groups, with four brain slices in each group. The experiment was performed in triplicate. The control group did not receive any treatment. The oxygen-glucose deprivation group underwent hypoxia (8% O2+ 92% N2) and glucose deprivation (glucose-free medium) for 60 minutes. In the transfection control group, after 60 minutes of oxygen-glucose deprivation, control plasmid (H1-GFP-Hygromycin) was transfected into the tissue. In the miRNA-9 transfection group, miRNA-9 plasmid was transfected (H1-miRNA-9-GFP-Hygromycin) after 60 minutes of oxygen-glucose deprivation. Both miRNA-9 plasmid and control plasmid sequences contained enhanced green fl uorescent protein.
To verify the establishment of the oxygen-glucose deprivation model, 3 days after model induction, hematoxylin-eosin staining was used to compare sections from the control group (Figure 1D). Karyopyknosis and nuclear fragmentation appeared after oxygen-glucose deprivation (Figure 1E).
Effects of miRNA-9 transfection on cell morphology of brain tissues from immature neonatal rats with oxygen-glucose deprivation
Enhanced green fl uorescent protein expression was detected in brain tissue after transfection using fl uorescence microscopy. Results showed that no apparent fl uorescence was detectable in the oxygen-glucose deprivation group (no transfection). Obvious green fl uorescence was visible in the miRNA-9 transfection and transfection control groups (Figure 2).
Effects of miRNA-9 transfection on Olig1 mRNA expression in brain tissue from neonatal rats with oxygen-glucose deprivation
Real-time PCR results exhibited that from the third day of brain slice culture, Olig1 expression gradually increased with prolonged culture time in each group. Olig1 expression peaked at 14 days after transfection, and diminished at 21 days. Olig1 expression reduced at 28 days in the oxygen-glucose deprivation group and transfection control group. Olig1 expression increased in the blank control group and miRNA-9 transfection group at 28 days (Figure 3). Statistical analysis of real-time quantitative PCR results demonstrated that at 1, 3, 7, 14, 21 and 28 days after transfection, Olig1 expression was greater in the oxygen-glucose deprivation group than that in the blank control group (P < 0.05). Olig1 expression was higher in the transfection control group than that in the miRNA-9 transfection group (P < 0.05). At 1 day after transfection, Olig1 expression was higher in the oxygen-glucose deprivation group than that in the transfection control group(P < 0.05). At 3 days, Olig1 expression was lower in the oxygen-glucose deprivation group than that in the transfection control group (P < 0.05).

Figure 2 Cell morphology of brain tissue from neonatal rats undergoing oxygen-glucose deprivation after miRNA-9 and control plasmid transfection (× 100).
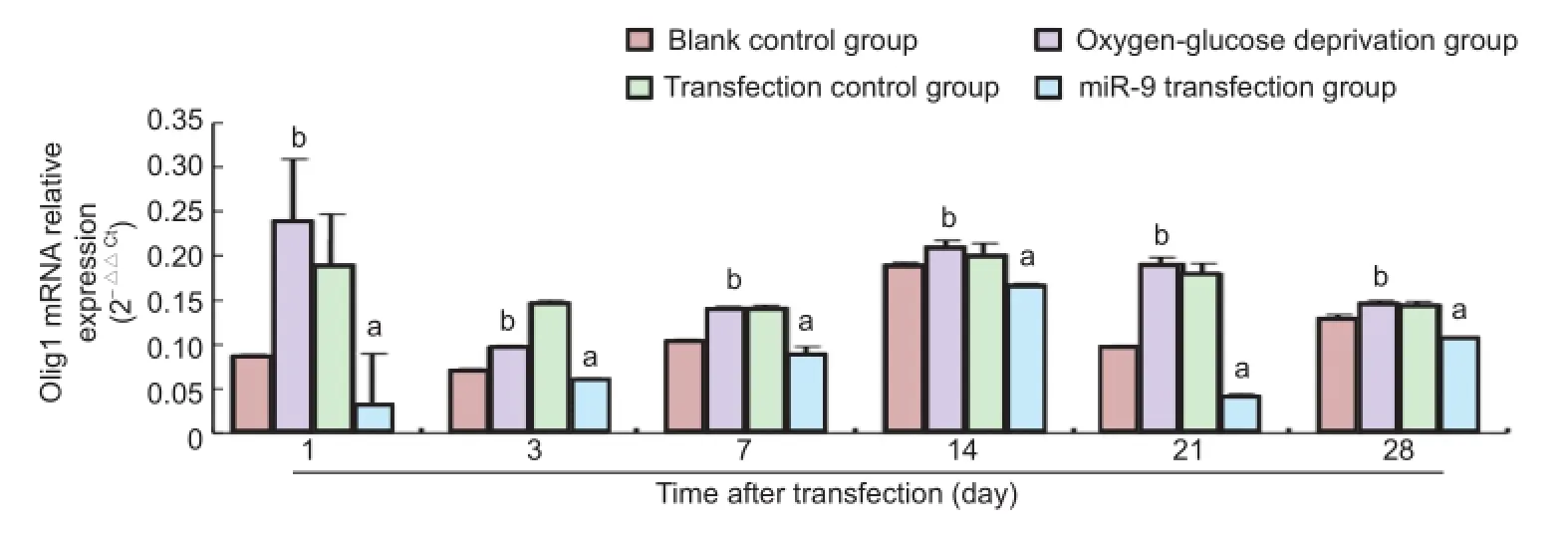
Figure 3 Effects of miRNA-9 (miR-9) transfection on oligodendrocyte lineage gene 1 (Olig1) mRNA expression in brain tissues of neonatal rats undergoing oxygen-glucose deprivation (real-time quantitative PCR).
Discussion
The development of the nervous system in neonatal rats aged 2–4 days is similar to that of the human fetus (28–31 weeks)[36]. Our previous study demonstrated that at 28 days after hypoxia treatment, myelinated axons in the cerebral cortex, hippocampus and lateral ventricle of rats were visible in the normal group, but not obvious in the hypoxia group[37]. Another study showed that expression of various transcription factors, such as Olig1, was upregulated in the subependymal region of adult mammals after cerebral ischemia, and participated in the ischemic pathological process[38]. Hypoxia and ischemia mediated nerve demyelination. In vivo experiments have shown that Olig1 contributed to the differentiation and maturity of oligodendrocytes in rat neocortex, and played an important role in myelinization[39].
miRNA is vital in regulating speci fi c gene expression and determining nerve type[40]. A recent study confirmed that miRNA could regulate self-renewal of neural stem cells and change the fate of nerve cells[41]. Real-time quantitative PCR detected differential expression of nine miRNAs including miRNA-9, and these alterations possibly played a crucial effect in the pathophysiology of hypoxic-ischemic brain damage[42]. miRNA-9 is significant in the differentiation of neural stem cells into neurons[43]. The effects of miRNA-9 in the nervous system are varied. Previous studies confirmed that miRNA-9 suppressed the differentiation of neural progenitor cells, and promoted axon growth, development, and target regulation[44-52].
This study supposed that a series of miRNAs, including miRNA-9, with altered expression after hypoxia and ischemia, regulated multiple target genes, resulting in nerve repair. This study established brain slice models of hypox-ic-ischemic brain damage using immature rats to simulate human premature infants. Plasmid carrying miRNA-9 was transfected into rat brain slices, and the effects of miRNA-9 on Olig1 expression were observed in the present study. First, blank plasmid and plasmid with miRNA-9 were separately transfected into cells. Green fluorescent protein was expressed in cells transfected with plasmid successfully. Results demonstrated that blank plasmid and plasmid with miRNA-9 were successfully transfected into cells. Subsequently, Olig1 expression was analyzed at six time points in the four groups. Studies found that Olig1 gene expression was higher at six time points after hypoxia and ischemia than that in the normal state. After transfecting plasmid containing miRNA-9, Olig1 mRNA expression was noticeably decreased and reached a normal level. Figure 3 suggested that miRNA-9 possibly directly regulated Olig1. miRNA-9 becomes a key factor for regulation at the post-transcriptional level after Olig1 gene transcription by identifying and combining target gene Olig1 mRNA[53]. Functional miRNA was predicted to probably regulate 20–30% of gene expression in the human genome[53]. Simultaneously, human Olig1 mRNA had a long 3′UTR (about 1,374 bp), suggesting that the reduction in Olig1 expression in the miRNA-9 transfection group was possibly because of negative regulation of miRNA-9 on a post-transcriptional level, which diminished Olig1 transcriptional ef fi ciency or directly inhibited its transcription. The regulatory effects of miRNA-9 on Olig1 require further veri fi cation using a double luciferase reporter gene method.
Simultaneously, results from this study demonstrated that under hypoxia and ischemia, Olig1 expression peaked at 14 days after transfection, and then decreased, which was possibly associated with myelinization. It is assumed that the myelin sheath was in a rapid growth period at 14 days after transfection, and then entered a slow growth period. This theory deserves further investigation.
At 7, 14, 21 and 28 days after transfection, no changes in Olig1 expression were detected between transfection control group and oxygen-glucose deprivation group. This observation indicated that after oxygen-glucose deprivation, transfection of control plasmid did not affect cells. At 1 and 3 days after transfection, differences in Olig1 expression were detected between the transfection control and oxygen-glucose deprivation groups. These findings suggested that transfection of control plasmid could affect experimental results. A possible reason may be that in the fi rst 3 days after hypoxia and ischemia, cells did not recover from this insult, and did not enter a stable period of growth and metabolism. Transfected plasmid entered cells and affected normal Olig1 expression. With increasing culture time, effects of control plasmid on cells gradually disappeared and Olig1 expression recovered to normal.
There are various targets for miRNA-9; therefore its mechanism of action will be rather complicated. Moreover, few investigations have addressed the action of miRNA-9 on the repair of myelin sheath. These possible mechanisms still deserve further veri fi cation.
Materials and Methods
Design
A randomized controlled animal study.
Time and setting
Experiments were performed at the Experimental Animal Center, Capital Medical University and the Laboratory of Department of Pediatrics, Beijing Friendship Hospital, Capital Medical University, China from October 2011 to March 2012.
Materials
Animals
A total of 80 specific-pathogen-free Sprague-Dawley rats aged 3 days and weighing 8–10 g, of both genders, were provided by the Vital River, Beijing, China, animal license No. SCXK(Jing)2007-0001. All animals were housed in a 12-hour light/dark cycle at 24°C and humidity of 40%. The protocols were conducted in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, formulated by the Ministry of Science and Technology of China[54].
Plasmids
H1-GFP-Hygromycin (blank plasmid), H1-miRNA-9-GFPHygromycin (miRNA-9 sequence: 5′-AUA AAG CUA GAU AAC CGA AAG U-3′) was provided by Genechem (Shanghai, China).
Methods
Culture of brain slices from immature neonatal rats
In accordance with the method described by Adamchik et al.[55-56], Sprague-Dawley rats aged 3 days were sterilized with 75% alcohol, anesthetized and decapitated. The brains were rapidly obtained and immersed in precooled buffer, supplemented with 64% Dulbecco’s modified Eagle’s medium (DMEM), 32% Hanks’ balanced salt solution (Gibco, NY, USA), 6.5 g/L D-glucose, 2.98 mg/L hydroxyethyl piperazine ethanesulfonic acid (Sigma, St. Louis, MO, USA), 100 U/mL penicillin, 100 μg/mL streptomycin, and 2.5 μg/mL amphotericin (Gibco), and then cooled for 15 minutes. Meninges were removed under an anatomical microscope (Leica, Wetzlar, Germany). Whole brain tis-sue was sliced (450-μm sections) in a slicer (Ted Pella, Redding, CA, USA) coated with filter paper. Complete culture medium (1 mL), containing 50% DMEM, 25% Hanks’ balanced salt solution, 25% horse serum (Gibco), 2.98 mg/L hydroxyethyl piperazine ethanesulfonic acid, 100 U/mL penicillin, 100 μg/mL streptomycin, 2.5 μg/mL amphotericin, and 6.5 g/L D-glucose, was added in each well of a 6-well culture plate (Costar, Kennebunk, CA, USA). Brain slices were incubated in a culture plate insert (Gibco), placed in 5% CO2incubator (Heraeus, Wetzlar, Germany) at 37°C, 95% O2+ 5% CO2saturated humidity. Four groups contained 16 brain slices, i.e., four neonatal rats. A total of 24 neonatal rats were needed at six time points. Results were repeated in triplicate at each time point, so 4 × 6 × 3 = 72. That is, 72 neonatal rats were needed (80 rats were enrolled to fully ensure experiment needs). Brain slices were incubated in the incubator at 37°C, 95% O2+ 5% CO2and saturated humidity for 36 hours. A semi-mediumchange occurred once every 3 or 4 days. All experiments were performed under aseptic conditions.
Establishment and identification of a brain slice model of oxygen-glucose deprivation in immature neonatal rats
Whole brain slices of immature neonatal rats were incubated in glucose-free medium in the oxygen-glucose deprivation group, transfection control group and miRNA-9 transfection group. The above-mentioned brain slices were placed in a sealed container, which was filled with 8% O2+ 92% N2(provided by Beijing Friendship Hospital in China) in a 37°C water bath, 0.5–1.0 L/min. Brain slices were deprived of oxygen and glucose for 60 minutes. Subsequently, brain slices were incubated in a 5% CO2incubator (Heraeus) for further incubation. Brain slices in the blank control group were left intact. Hematoxylin-eosin staining was used to test if the hypoxia-ischemia model was successfully established.
Transfection of miRNA-9 plasmid and corresponding control plasmid into brain slices from immature neonatal rats
Brain slices were transfected with LipofectamineTM2000 (Invitrogen, CA, USA). Plasmid containing target miR-NA-9, blank plasmid (plasmid: OPTI-MEM = 4 μg:125 μL) and Lipofectamine (Lipofectamine: OPTI-MEM = 10 μL:125 μL) were diluted and separately incubated for 5 minutes at room temperature. Plasmid containing target miRNA-9 and blank plasmid were separately mixed with LipofectamineTM2000 for 20 minutes. The mixture was added in the wells containing corresponding brain slices. After gentle shaking, the specimens were placed in a 37°C 5% CO2incubator for 36 hours. At 72 hours after transfection, the transfection was observed under a fl uorescence microscope.
RNA extraction and reverse transcription and fluorescence real-time quantitative PCR for Olig1 gene expression in brain tissue of immature neonatal rats
In accordance with the instructions from the total RNA extraction kit (Tiangen, Beijing, China), total RNA was extracted from animal brain tissue, and was considered as a template. Reverse transcription was performed as follows, with a total volume of 20 μL: 4 μL AMV 5 × reverse transcription buffer, 2 μL dNTP, 0.5 μL RNase inhibitor, 1 μL random primer, 1 μL AMV reverse transcriptase, 11.5 μL RNA sample, at 30°C for 10 minutes, 42°C for 30 minutes, and 99°C for 5 minutes. Synthesized cDNA served as a template. Quantitative PCR was conducted as follows with a total volume of 20 μL: 10 μL 2 × SYBR Green, 1 μL cDNA template, upstream and downstream primers (each 0.5 μL; GAPDH and oligodendrocyte lineage gene 1) and 8 μL distilled water. Reaction system: 95°C for 10 minutes, followed by 40 cycles of 95°C for 15 seconds and 62°C for 1 minute. Product size: Olig1: 182 bp, GAPDH: 75 bp. Primer information is listed as follows:
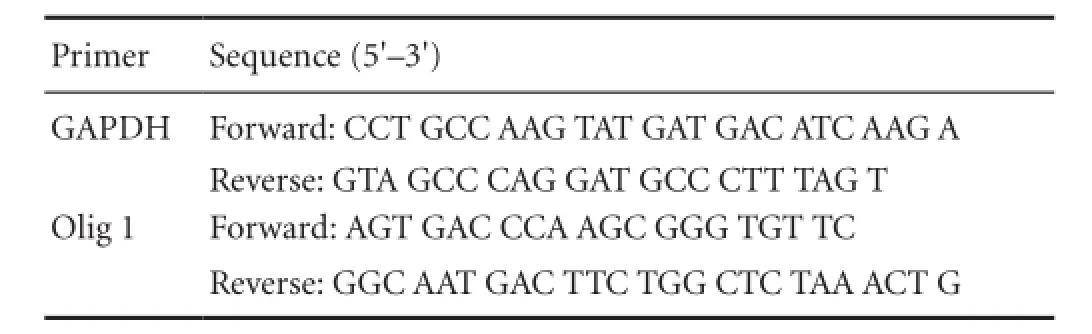
Primer Sequence (5′–3′) GAPDH Forward: CCT GCC AAG TAT GAT GAC ATC AAG A Reverse: GTA GCC CAG GAT GCC CTT TAG T Olig 1 Forward: AGT GAC CCA AGC GGG TGT TC Reverse: GGC AAT GAC TTC TGG CTC TAA ACT G
Target gene and internal gene GAPDH of the same specimen were separately amplified. Relative changes in gene expression were calculated with the following formula: fold change = 2?△△Ct= 2?△Ct(treated)?△ Ct (control), where△Ct = Ct (detected gene)?Ct (housekeeping gene) and Ct is the threshold number.
Statistical analysis
Measurement data were expressed as mean ± SD, and analyzed using SPSS 11.5 software (SPSS, Chicago, IL, USA). Measurement data were analyzed with one-way analysis of variance of completely randomized design. Paired comparison of intergroup mean difference was analyzed using least signi fi cant difference test. A value of P < 0.05 was considered statistically signi fi cant.
Author contributions:Yang LJ participated in experiment implementation, data integrity, and manuscript writing. Cui H obtained funding and was in charge of manuscript authorization. Cao T provided study data. All authors approved the final version of the paper.
Con fl icts of interest:None declared.
Peer review:This study established animal models of hypoxic-ischemic brain damage, observed negative regulation of miRNA-9 on Olig1 during hypoxic-ischemic brain damage, and provided experimental and theoretical evidence for simulating hypoxic-ischemic brain damage in premature infants in the clinic.
[1] Een-Ram S, Doyle AD, Conti MA, et al. Myosin IIA regulates cell motility and actomyosin -microtubule crosstalk. Nat Cell Biol. 2007;19(3):299-309.
[2] Gao XJ, Li SJ, Zhu ZL, et al. Advance in relationship between COX-2 and gastric cancer. Zhongguo Meitan Gongye Yixue Zazhi. 2010;13(1):166-167.
[3] Liao SM, Ferradal SL, White BR, et al. High-density diffuse optical tomography of term infant visual cortex in the nursery. J Biomed Opt. 2012;17(8):081414.
[4] Holsti L, Grunau RVE, Whit fi eld MF. Developmental coordination disorder in extremely low birth weight children at nine years. J Dev Behav Pediatr. 2002;23(1):9-15.
[5] Constable RT, Ment LR, Vohr BR, et al. Prematurely born children demonstrate white matter microstructural differences at 12 years of age, relative to term control subjects: an investigation of group and gender effects. Pediatrics. 2008;121(2):306-316.
[6] Alvarez-Díaz A, Hilario E, de Cerio FG, et al. Hypoxic-ischemic injury in the immature brain–key vascular and cellular players. Neonatology. 2007;92(4):227-235.
[7] Volpe JJ. Cerebral white matter injury of the premature infant more common than you think. Pediatrics. 2003;112(1 Pt 1):176-180.
[8] Leviton A, Dammann O, Durum SK. The adaptive immune response in neonatal cerebral white matter damage. Ann Neurol. 2005;58(6):821-828.
[9] Adén U, Favrais G, Plaisant F, et al. Systemic in fl ammation sensitizes the neonatal brain to excitotoxicity through a pro-/anti-in fl ammatory imbalance:key role of TNF-alpha pathway and protection by etanercept. Brain Behav Immun. 2010;24(5):747-758.
[10] Perlman JM. Intervention strategies for neonatal hypoxic-ischemic cerebral injury. Clin Ther. 2006;28(9):1353-1365.
[11] Dammann O, Leviton A. In fl ammatory brain damage in preterm newborns-dry numbers, wet lab, and causal inferences. Early Hum Dev. 2004;79(1):1-15.
[12] Kinney HC, Haynes RL, Xu G, et al. Neuron de fi cit in the white matter and subplate in periventricular leukomalacia. Ann Neurol. 2012;71(3):397-406.
[13] Tekgul H, Yalaz M, Kutukculer N, et al. Value of biochemical markers for outcome in term infants with asphyxia. Pediatr Neurol. 2004;31(5):326-332.
[14] Arnett HA, Fancy SP, Alberta JA, et al. bHLH transcription factor Olig1 is required to repair demyelinated lesions in the CNS. Science. 2004;306(5704):2111-2115.
[15] Whitman LM, Blanc CA, Schaumburg CS, et al. Olig1 function is required for remyelination potential of transplanted neural progenitor cells in a model of viral-induced demyelination. Exp Neurol. 2012;235(1):380-387.
[16] Othman A, Frim DM, Polak P, et al. Olig1 is expressed in human oligodendrocytes during maturation and regeneration. Glia. 2011;59(6):914-926.
[17] Chakrabarti L, Best TK, Cramer NP, et al. Olig1 and Olig2 triplication causes developmental brain defects in Down syndrome. Nat Neurosci. 2010;13(8):927-934.
[18] Xin M, Yue T, Ma Z, et al. Myelinogenesis and axonal recognition by oligodendrocytes in brain are uncoupled in Olig1-null mice. J Neurosci. 2005;25(6):1354-1365.
[19] Burton A. Olig1 needed for remyelination. Lancet Neurol. 2005;4(2):80.
[20] Whitman LM, Blanc CA, Schaumburg CS, et al. Olig1 function is required for remyelin ation potential of transplanted neural progenitor cells in a model of viral-induced demyelination. Exp Neurol. 2012;235(1):380-387.
[21] Lu QR, Sun T, Zhu Z, et al. Common developmental requirement for Olig function indicates a motor neuron/oligodendrocyte connection. Cell. 2002;109(1):75-86.
[22] Ruan QF, Pan CF, Shen XL, et al. Expression changes of microRNA in hippocampus of mouse after brain injury. Zhonghua Shiyan Waike Zazhi. 2011;28(2):151-152.
[23] Fiore R, Siegel G, Schratt G. MicroRNA function in neuronal development, plasticity and disease. Biochim Biophys Acta. 2008;1779(8):471-478.
[24] Schratt GM, Tuebing F, Nigh EA. et al. A brain-specific microRNA regulates dendritic spine development. Nature. 2006;439(7074):283-289.
[25] Impey S, Davare M, Lasiek A. et al. An activity-induced microRNA controls dendritic spine formation by regulating Racl-PAK signaling. Mol Cell Neurosci. 2010;43(1):146-156.
[26] Otaegui D, Baranzini SE, Arma?anzas R, et al. Differential micro RNA expression in PBMC from Multiple sclerosis patients. PLoS One. 2009;4(7):e6309.
[27] Shin D, Shin JY, McManus MT, et al. Dicer ablation in oligodendrocytes provokes neuronal impairment in mice. Ann Neurol. 2009;66(6):843-857.
[28] Zhao X, He X, Han X, et al. microRNA-mediated control of oligodendrocyte differentiation. Neuron. 2010;65(5):612-626 .
[29] Lehotzky A, Lau P, Tokési N, et al. Tubulin polymerization-promoting protein (TPPP/p25) is critical for oligodendrocyte differentiation. Glia. 2010;58(2):157-168.
[30] Budde H, Schmitt S, Fitzner D, et al. Control of oligodendroglial cell number by the miR-17-92 cluster. Development. 2010;137(13):2127-2132.
[31] Lau P, Verrier JD, Nielsen JA, et al. Identi fi cation of dynamically regulated microRNA and mRNA networks in developing oligodendrocytes. J Neurosci. 2008;28(45):11720-11730.
[32] Deo M, Yu JY, Chung KH, et al. Detection of mammalian microRNA expression by in situ hybridization with RNA oligonucleotides. Dev Dyn. 2006;235(9):2538-2548.
[33] Leucht C, Stigloher C, Wizenmann A, et al. MicroRNA-9 directs late organizer activity of the midbrain-hindbrain boundary. Nat Neurosci. 2008;11(6):641-648.
[34] Buller B, Chopp M, Ueno Y, et al. Regulation of serum response factor by miRNA-200 and miRNA-9 modulates oligodendrocyte progenitor cell differentiation. Glia. 2012;60(12):1906-1914.
[35] Li JS, Yao ZX. MicroRNAs: novel regulators of oligodendrocyte differentiation and potential therapeutic targets in demyelination-related diseases. Mol Neurobiol. 2012;45(1):200-212.
[36] Cui SD. Neural stem cell apoptosis, proliferation and differentiation of subventricular zone in 3-day-old neonatal rats after chronic cerebral ischemia. Shanghai: Fudan University, China. 2006.
[37] Yang LJ, Cui H, Yao YQ, et al. Changes of Myelin in Rat Brain after Hypoxia. Shoudu Yike Daxue Xuebao. 2010;31(6):756-759.
[38] Tonchev AB, Yamashima T, Sawamoto K, et al. Transcription factor protein expression patterns by neural or neuronal progenitor cells of adult monkey subventricular zone. Neuroscience. 2006;139(4):1355-1367.
[39] Meijer DH, Kane MF, Mehta S, et al. Separated at birth? The functional and molecular divergence of OLIG1 and OLIG2. Nat Rev Neurosci. 2012;13(12):819-831.
[40] Liu C, Zhao X. MicroRNAs in adult and embryonic neurogenesis. Neuromolecular Med. 2009;11(3):141-152.
[41] Cheng LC, Pastrana E, Tavazoie M, et al. MiR-124 regulates adult neurogenesis in the subventricular zone stem cell niche. Nat Neurosci. 2009;12(4):399-408.
[42] Peng T, Jia YJ, Wen QQ, et al. Expression of microRNA in neonatal rats with hypoxic-ischemic brain damage. Zhongguo Dangdai Erke Zazhi. 2010;12(5):373-376.
[43] Conaco C, Otto S, Han JJ. Reciprocal actions of REST and a mircoRNA promote neuronal identify. Proc Natl Acad Sci U S A. 2006;103(7):2422-2427.
[44] Packer A, Xing Y, Harper S, et al. The bifunctional microRNA miR-9/miR-9? regulates REST and CoREST and is downregulated in Huntington’s disease. J Neurosci. 2008;28(53):14341-14346.
[45] Saunders L, Sharma A, Tawney J, et al. miRNAs regulate SIRT1 expression during mouse embryonic stem cell differentiation and in adult mouse tissues. Aging. 2010;2(7):415-431.
[46] Shibata M, Kurokawa D, Nakao H, et al. MicroRNA-9 modulates Cajal–Retzius cell differentiation by suppressing Foxg1 expression in mouse medial pallium. J Neurosci. 2008;28(41):10415-10421.
[47] Shibata M, Nakao H, Kiyonari H, et al. MicroRNA-9 regulates neurogenesis in mouse telencephalon by targeting multiple transcription factors. J Neurosci. 2011;31(9):3407-3422.
[48] Laneve P, Gioia U, Andriotto A, et al. A minicircuitry involving REST and CREB controls miR-9-2 expression during human neuronal differentiation. Nucleic Acids Res. 2010;38(20):6895-6905.
[49] Otaegi G, Pollock A, Hong J, et al. MicroRNA miR-9 modifies motor neuron columns by a tuning regulation of FoxP1 levels in developing spinal cords. J Neurosci. 2011;31(3):809-818.
[50] Bonev B, Stanley P, Papalopulu N. MicroRNA-9 modulates hes1 ultradian oscillations by forming a double-negative feedback loop. Cell Rep. 2012;2(1):10-18.
[51] Dajas-Bailador F, Bonev B, Garcez P, et al. microRNA-9 regulates axon extension and branching by targeting Map1b in mouse cortical neurons. Nat Neurosci. 2012;15:697-699.
[52] Zhao C, Sun G, Li S, et al. A feedback regulatory loop involving microRNA-9 and nuclear receptor TLX in neural stem cell fate determination. Nat Struct Mol Biol. 2009;16(4):365-371.
[53] Xie X, Lu J, KulbokasE J, et al. System atic discovery of regulatory motifs in hum an promoters and 3 ‘UTRs by com parison of severalm ammals. Nature. 2005;434(7031):338-345.
[54] The Ministry of Science and Technology of the People’s Republic of China Guidance Suggestions for the Care and Use of Laboratory Animals. 2006-09-30.
[55] Adamchik Y, Frantseva MV, Weisspapir M, et al. Methods to induce primary and secondary traumatic damage in organotypic hippocampal slice cultures. J Brain Res Brain Res Protoc. 2000;5(2):153-158.
[56] Yang H, Shew WL, Roy R, et al. Maximal variability of phase synchrony in cortical networks with neuronal avalanches. J Neurosci. 2012;32(3):1061-1072.
Copyedited by Apricò K, Han F, Chen XH, Wang J, Qiu Y, Li CH, Song LP, Zhao M
10.4103/1673-5374.130077
Hong Cui, Department of Pediatrics, Beijing Friendship Hospital, Capital Medical University, Beijing 100050, China, cuihong100@sina.com.
http://www.nrronline.org/
Accepted: 2013-11-02
- 中國神經(jīng)再生研究(英文版)的其它文章
- Acrylamide exposure impairs blood-cerebrospinal fl uid barrier function
- Analysis of the effect of repeated-pulse transcranial magnetic stimulation at the Guangming point on electroencephalograms
- Preconditioning crush increases the survival rate of motor neurons after spinal root avulsion
- Adaptive and regulatory mechanisms in aged rats with postoperative cognitive dysfunction
- Increased expression of Notch1 in temporal lobe epilepsy: animal models and clinical evidence
- Transient axonal glycoprotein-1 induces apoptosisrelated gene expression without triggering apoptosis in U251 glioma cells

