Acrylamide exposure impairs blood-cerebrospinal fl uid barrier function
Xue Yao, Licheng Yan, Lin Yao, Weijun Guan, Fanxu Zeng, Fuyuan Cao, Yanshu Zhang
1 College of Public Health, Hebei United University, Tangshan, Hebei Province, China
2 Experimental Animal Center, Hebei United University, Tangshan, Hebei Province, China
3 Key Laboratory of Hebei Health and Safety on Coal Industry, Hebei United University, Tangshan, Hebei Province, China
Acrylamide exposure impairs blood-cerebrospinal fl uid barrier function
Xue Yao1, Licheng Yan1, Lin Yao2, Weijun Guan3, Fanxu Zeng1, Fuyuan Cao2, Yanshu Zhang1
1 College of Public Health, Hebei United University, Tangshan, Hebei Province, China
2 Experimental Animal Center, Hebei United University, Tangshan, Hebei Province, China
3 Key Laboratory of Hebei Health and Safety on Coal Industry, Hebei United University, Tangshan, Hebei Province, China
Previous studies show that chronic acrylamide exposure leads to central and peripheral neuropathy. However, the underlying mechanisms remained unclear. In this study, we examined the permeability of the blood-cerebrospinal fl uid barrier, and its ability to secrete transthyretin and transport leptin of rats exposed to acrylamide for 7, 14, 21 or 28 days. Transthyretin levels in cerebrospinal fl uid began to decline on day 7 after acrylamide exposure. The sodium fl uorescein level in cerebrospinal fl uid was increased on day 14 after exposure. Evans blue concentration in cerebrospinal fl uid was increased and the cerebrospinal fl uid/serum leptin ratio was decreased on days 21 and 28 after exposure. In comparison, the cerebrospinal fl uid/serum albumin ratio was increased on day 28 after exposure. Our fi ndings show that acrylamide exposure damages the blood-cerebrospinal fl uid barrier and impairs secretory and transport functions. These changes may underlie acrylamide-induced neurotoxicity.
nerve regeneration; brain injury; acrylamide; blood-cerebrospinal fluid barrier; tight junction; permeability; thyroid hormone; leptin; cerebrospinal fluid/serum albumin ratio; cerebrospinal fluid; NSFC grant; neural regeneration
Funding: This study was supported by State Key Development Program for Basic Research of China, No. 2012CB525002 and the National Natural Science Foundation of China, No. 30771823.
Yao X, Yan LC, Yao L, Guan WJ, Zeng FX, Cao FY, Zhang YS. Acrylamide exposure impairs blood-cerebrospinal fluid barrier function. Neural Regen Res. 2014;9(5):555-560.
Introduction
Acrylamide is an important chemical and widely used in industrial production of polyacrylamides, which are employed mainly as fl occulating agents in water treatment, and as fl ow control agents in oil well operations[1]. In addition, acrylamide is found in a variety of other sources, such as certain starchy foods cooked at high temperature[2]. Therefore, acrylamide exposure is a serious occupational and public health hazard[3]. Previous studies have shown that acrylamide monomer is a neurotoxin to humans and animals in vivo and in vitro, and can cause central-peripheral neuropathy, which is characterized by ataxia, skeletal muscle weakness, and numbness of the hands and feet[4-6].
Accumulating evidence suggests that acrylamide perturbs neurofilaments and impairs neurotransmission by disrupting presynaptic nitric oxide signaling[7-9]. However, the mechanisms of acrylamide-induced neurotoxicity are still unclear. The blood-brain barrier prevents xenobiotics from entering the central nervous system. Growing evidence indicates that neurotoxins, such as tributyltin, manganese and nanoparticles, may disrupt the function of the blood-brain and blood-cerebrospinal fl uid (CSF) barriers[10-12]. However, very few studies have focused on the effects of acrylamide exposure on these barriers.
The brain barriers include the blood-brain barrier and the blood-CSF barrier, which tightly regulate the entry of xenobiotics into the brain. A large amount of research suggests that neurotoxins have a great impact on the blood-brain barrier and a lower impact on the blood-CSF barrier[13-14]. However, this apparent difference might be due to technical issues and an insuf fi cient understanding of the blood-CSF barrier. The blood-CSF barrier separates blood components from the CSF. Compared with the blood-brain barrier, the blood-CSF barrier has a relatively large surface area, and a large and fast blood supply and tight junctions[15-16]. As technical advancements have enabled the direct insertion of miniature video probes into the ventricles, the role of the blood-CSF barrier in neurotoxin poisoning has attracted increasing attention. Blood-CSF barrier dysfunction is associated with neurodegenerative diseases, such as Alzheimer’s disease, where it may result in reduced Aβ clearance[17]. However, the role of the blood-CSF barrier in acrylamide neurotoxicity is unclear.
The blood-CSF barrier regulates the movement, secretion and transport of substances. The selective permeability of the blood-CSF barrier protects the brain from fl uctuations in plasma composition and circulating agents through the regulation of tight junctions[18]. Some proteins expressed in the blood-CSF barrier regulate permeability. These proteins include integral membrane proteins (occludin, claudins, JAM) and peripheral proteins (ZO-1, ZO-2, ZO-3). Therefore, blood-CSFbarrier integrity is crucial for maintaining homeostasis in the central nervous system. Evans blue is a useful chemical indicator of barrier leakage, and the level of this dye in the CSF can help assess the degree of blood-CSF barrier permeability.
The blood-CSF barrier has a secretory function as well, producing and secreting various proteins into the CSF and parenchyma that are involved in brain development, neuronal survival and neurodegeneration. Transthyretin levels in the CSF and brain parenchyma are derived exclusively from the choroid plexus. By contrast, the liver is the primary source of blood transthyretin. The passive diffusion of serum transthyretin into the CSF is blocked by the blood-CSF barrier[19-20]. Therefore, the transthyretin level in the CSF partly re fl ects the secretory function of the blood-CSF barrier, and serves as an indicator of blood-CSF barrier secretory impairment[21].
Furthermore, the blood-CSF barrier has an active transport system, through which nutrients, such as calcium and leptin, are transported into the brain. Under physiology conditions, leptin is secreted by adipose tissue and transported into the central nervous system through the OB-Ra receptor expressed at the brain barriers[22-23]. Thomas et al.[24]demonstrated that the OB-Ra receptor is mainly expressed in the choroid plexus, and to a lesser extent in brain microvessels (the main tissue of the blood-brain barrier). Thus, leptin levels in the CSF are an indicator of transport function. However, it remains unclear whether acrylamide disrupts the transport of leptin through the blood-CSF barrier.
The present study was designed to examine whether acrylamide exposure interferes with blood-CSF barrier function by increasing permeability, decreasing secretion and reducing the transport of leptin. Our fi ndings should contribute to our understanding of acrylamide-induced neurotoxicity and lay the foundation for future studies on the mechanisms of neurotoxicity.
Results
Quantitative analysis of experimental animals
A total of 128 Sprague-Dawley rats were randomly divided into control group (intraperitoneal injection of saline) and acrylamide exposure group (intraperitoneal injection of acrylamide, 20 mg/kg). Of these rats, 64 were used for assessment of Evans blue and sodium fluorescein content, while the remaining 64 were used to evaluate the CSF/serum albumin ratio, and transthyretin and leptin levels. The rats were used for analysis 7, 14, 21 and 28 days after acrylamide or saline control exposure, with 8 rats at each time point.In the end, data from all 128 rats were entered into the fi nal analyses, without dropout.
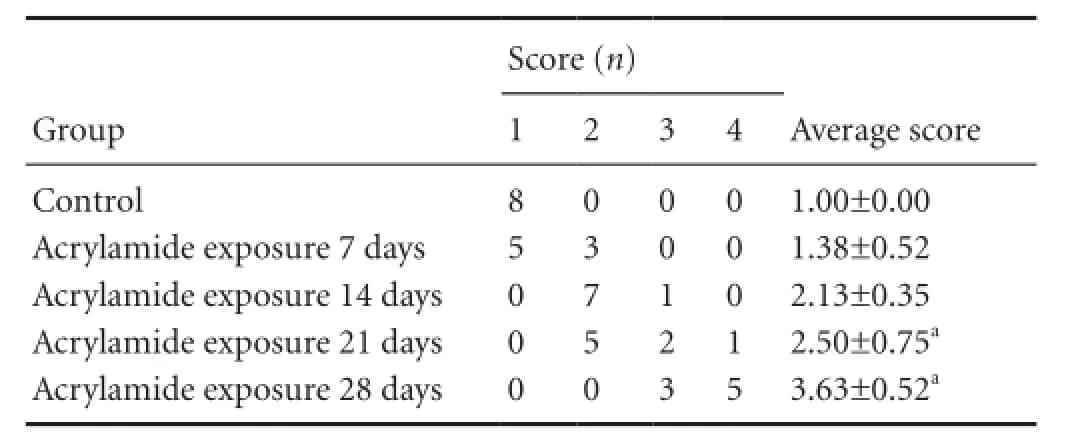
Table 1 Effect of acrylamide exposure on gait scores in rats
Acrylamide exposure increased gait scores
After 7, 14, 21 and 28 days of acrylamide exposure, gait scores were measured and recorded (Table 1). Signi fi cantly higher gait scores were seen in acrylamide-treated rats at 21, 28 days compared with the control group (P < 0.05). No signi fi cant change was seen at other acrylamide exposure time points. Gait scores in rats with acrylamide exposure rose signi fi cantly in a time-dependent manner (P = 0.003).
Acrylamide exposure increased blood-CSF barrier permeability
Evans blue concentration in the CSF was signi fi cantly increased at 21 and 28 days after acrylamide exposure, to 32.88 μg/mL and 54.62 μg/mL, respectively, compared with the control group (21.62 μg/mL; P < 0.05; Table 2). Additionally, the level of sodium fl uorescein in the CSF began to increase at 14 days after acrylamide exposure in comparison with the control group (P < 0.05). Furthermore, the CSF/serum albumin ratio was also significantly increased at 28 days after acrylamide exposure compared with the control group (P < 0.05; Table 2). Collectively, these results indicate that the permeability of the blood-CSF barrier began to increase following 14 days of exposure.
Acrylamide exposure decreased transthyretin levels in CSFTransthyretin levels in the CSF were signi fi cantly decreasedat 7, 14, 21 and 28 days after acrylamide exposure, and were lower than those in the control group (P < 0.05; Table 3). The 7-day acrylamide treatment regimen resulted in a 61.28% reduction in transthyretin concentration in the CSF (P < 0.05; Table 3), suggesting that acrylamide readily disrupts the secretion of transthyretin by the choroid plexus, even following short-term exposure. However, no signi fi cant change in transthyretin concentration was observed in serum (Table 3).
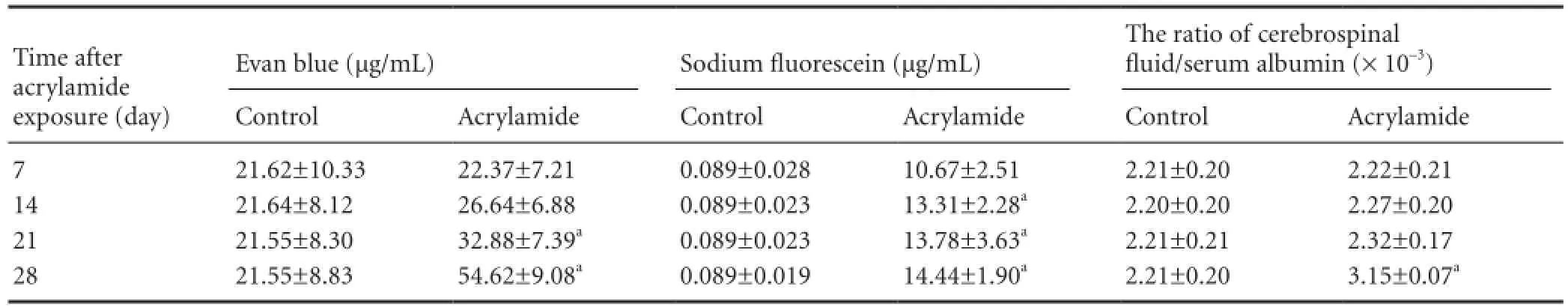
Table 2 Effect of acrylamide exposure on the permeability of the blood-cerebrospinal fl uid barrier
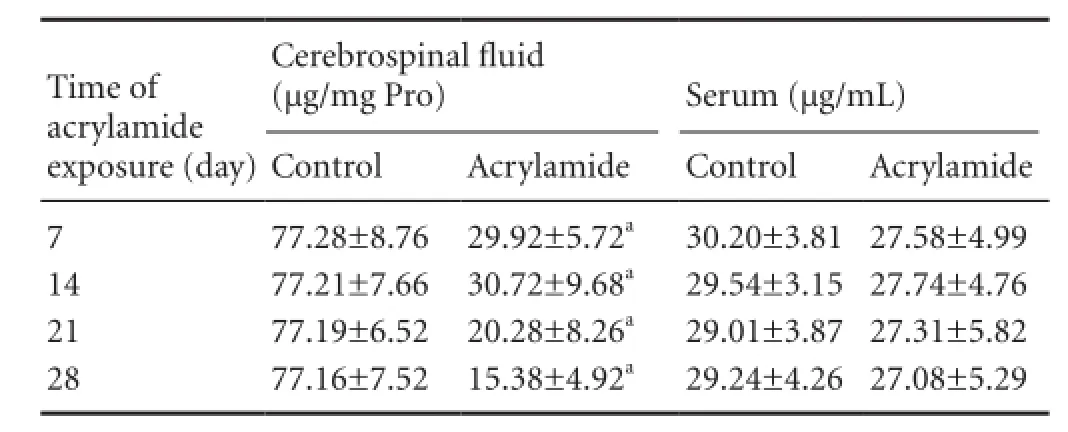
Table 3 Effect of acrylamide exposure on transthyretin levels in cerebrospinal fl uid
Acrylamide exposure decreased leptin levels in CSF
Leptin concentration in serum decreased from 5.17 ng/mL to 4.54 ng/mL following 7-day exposure, and a similar change was found at other exposure time points (P < 0.05; Table 4). However, leptin concentration in CSF was reduced 33.33% after 21-day acrylamide exposure compared with the control group (P < 0.05). The CSF leptin to serum leptin ratios were 0.154 and 0.143, respectively, following 21- and 28-day acrylamide exposure, which were signi fi cantly lower than those in the control group (P < 0.05).
Discussion
Acrylamide exposure leads to cumulative neurotoxicity in workers and laboratory animals[25]. However, the mechanisms of toxicity are not yet clear. Several hypotheses, including interaction with nucleic acids, perturbation of second messenger and neurotransmitter systems, and disruption of oxidative balance, have been proposed[26-27]. LoPachin et al.[28]attempted to explain its neurotoxicity using an organic chemistry approach. However, there is very little research on the effect of acrylamide on blood-CSF barrier function, or on how acrylamide can enter the central nervous system. Our data demonstrates that acrylamide exposure results in the disruption of blood-CSF barrier permeability, and impairs its secretory and transport functions. Our fi ndings offer an alternative mechanism of acrylamide-induced neurotoxicity.
In the present study, acrylamide exposure increased barrier permeability, as re fl ected in higher sodium fl uorescein and Evans blue levels in the CSF and an elevated CSF/serum albumin ratio. Blood-CSF barrier function is maintained by the lipid bilayer of endothelial cells and the tight junctions between endothelial cells. Under physiological conditions, chemicals with a molecular weight greater than 180 kDa cannot cross the blood-CSF barrier passively. In the present study, sodium fl uorescein (molecular weight: 376 kDa) and Evans blue (molecular weight: 960 kDa) served as markers of blood-CSF barrier leakage[29]. Our study is the fi rst to use CSF samples from animals to assess barrier permeability. Our research group developed a method to directly measure blood-CSF barrier permeability by using a modification of Euser’s method[30]. Evans blue and sodium fluorescein solutions were mixed and infused into the femoral artery, and we used a small needle to directly take CSF samples instead of brain tissue. Moreover, the CSF/serum albumin ratio was also measured in the present study. Dorta-Contreras’s group[31]found that the CSF/serum albumin ratio is an objective index of the impairment of the blood-CSF barrier. However, this ratio might not be very sensitive due to the high molecular weight of albumin (69 kDa). Our results revealed that sodium fluorescein concentration in CSF significantly increased following 14-day acrylamide exposure, and levels continued to rise until the end of the experiment. The concentration of Evans blue in the CSF was signi fi cantly increased to 52.58% at 21 days and 153.46% at 28 days. A higher CSF/serum albumin ratio was observed 28 days after acrylamide exposure compared with control animals. Acrylamide exposure led to an increase in sodium fl uorescein concentration in CSF after 14 days of exposure, an increase in Evans blue concentration in CSF starting at 21 days, and an increase in the CSF/serum albumin ratio starting at 28 days. The molecular weights of sodium fluorescein, Evans blue and albumin are 379 kDa, 960 kDa and 69 kDa, respectively, which demonstrates that acrylamide-induced blood-CSF barrier leakage worsened over time.
Current studies on Alzheimer’s disease, ischemia, traumatic brain injury and heavy metal neurotoxicity have focused on blood-CSF barrier permeability using in vitroor in vivo models[32-33]. Our fi ndings demonstrate that acrylamide exposure disrupts blood-CSF barrier permeability, which might disrupt immune surveillance due to perturbed immune cell trafficking across the choroid plexus into the CSF[34]. Therefore, our data provide insight into the mechanism of acrylamide neurotoxicity.
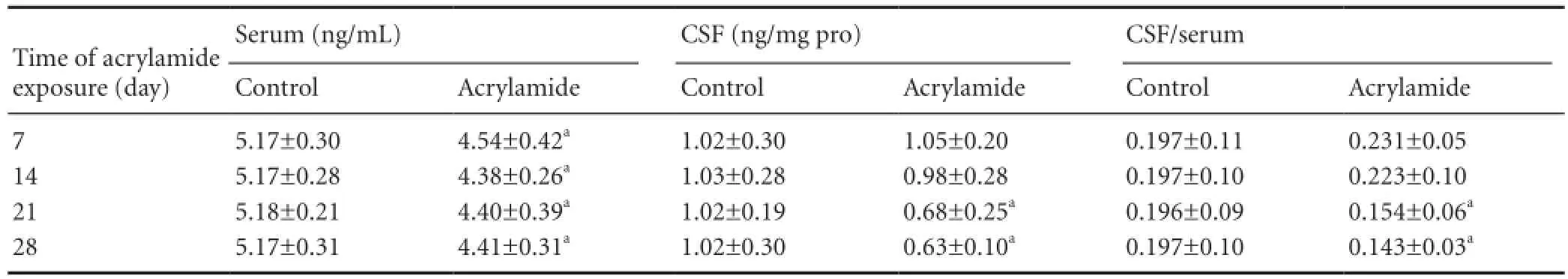
Table 4 Effect of acrylamide exposure on cerebrospinal fl uid (CSF) and serum leptin levels
Apart from permeability, the blood-CSF barrier also possesses the ability to secrete CSF and proteins, such as transthyretin, adrenomedullin, vascular endothelial growth factor and apolipoprotein J. The choroid plexus is the main component of the blood-CSF barrier. Transthyretin is the first protein found to be synthesized solely by the choroid plexus. Approximately 20% of the protein newly synthesized in the choroid plexus and about 50% of the newly synthesized protein secreted into the CSF is transthyretin[35-36]. Therefore, transthyretin level in CSF re fl ects the secretory function of the blood-CSF barrier, and also serves as an indicator for evaluating blood-CSF barrier impairment[21]. We found that transthyretin level in CSF was reduced to 33.6% after shortterm (7-day) acrylamide exposure, and this decline was maintained until 28 days after exposure. This indicates that acrylamide acutely disrupts transthyretin secretion. Transthyretin serves as a thyroxine transporter and is involved in beta-amyloid peptide chelation, attenuating neurotoxicity[37-38]. Sousa et al.[39]showed that absence of transthyretin accelerates cognitive decline associated with aging. In addition, transthyretin is required for normal neural function and enhances neural regeneration[40]. The decrease in transthyretin levels induced by acrylamide might be involved in neurotoxicity by affecting nerve regeneration, although further study is required to investigate this possibility.
Another major role of the blood-CSF barrier is to transport nutrients in blood into the CSF and to transport waste from the CSF into the blood for clearance. Under physiological conditions, some substances synthesized peripherally are transported into the central nervous system. Leptin is one of those substances and can enter the brain through the OBRa receptor in the choroid plexus[41]. In the present study, leptin concentration in CSF was significantly reduced at 21 days after acrylamide exposure. Leptin level in serum was reduced 7 days after exposure, which needed further research to demonstrate. At present, there is no direct evidence showing that acrylamide exposure disrupts appetite in experimental animals or occupational workers. Our body weight data showed that a little body weight loss was present at 28 days of acrylamide exposure; however, the difference in weight was not signi fi cant (data not shown), similar to the previous study. In order to clarify whether the reduction in leptin level in CSF was mediated by disruption of leptin transport across the blood-CSF barrier or by a reduction in serum leptin level, we calculated the CSF/serum leptin ratio. The CSF/serum leptin ratio was signi fi cantly lower at 21 days in exposed rats compared with the control group, suggesting that acrylamide exposure alters leptin transport across the blood-CSF barrier. Leptin possesses the ability to promote the development and maturation of the brain, and prevents apoptosis of neurons by increasing levels of the anti-apoptotic factor Bcl-2, as well as by decreasing levels of the pro-apoptotic factor caspase-3. Therefore, acrylamide-induced apoptosis of cerebellar granule neurons might partly be due to a decrease in leptin levels[42-43].
In summary, our findings firstly suggest that acrylamide exposure does impair blood-CSF barrier functions, including permeability, secretion and transport, which may be an alternative mechanism of acrylamide-induced neurotoxicity.
Materials and Methods
Design
A randomized controlled animal experiment.
Time and setting
This study was performed at the Central Laboratory, School of Public Health, and the Experimental Animal Center, Hebei United University, China from August 2011 to July 2012.
Materials
Animals
A total of 128 male Sprague-Dawley rats, aged 12 weeks, weighing 180–220 g, of specific pathogen free grade, were purchased from Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China; animal license No. SCXK (Jing) 2006-0009). Upon arrival, the rats were housed in the animal housing facility (a temperature-controlled room, under 12-hour light/dark cycle) of the Experimental Animal Center of Hebei United University, China (license No. SYXK (Ji) 2010-0038). They were acclimated for 1 week prior to experimentation. All animals were allowed free access to standard rat chow and tap water. The experiments described in this study were performed in accordance with the Guidelines for the Care and Use of Laboratory Animals of Hebei United University, China.
Drugs
Acrylamide, C5H5ON, white powder, was purchased from Amresco (Solon, OH, USA), batch No. 20110620. In our experiments, acrylamide was dissolved in saline at 20 mg/mL, and was fresh-made before injection.
Methods
Acrylamide administration
Acrylamide was intraperitoneally administrated to rats at a dose of 20 mg/kg per day, as previously described[44]. The injection schedule was 5 continuous days of injection and 2 days of withdrawal for 7, 14, 21 and 28 days of acrylamide exposure. Control rats were given the same volume of saline. The injection was performed using a syringe (5 mL).
Gait score evaluation
At 7, 14, 21 and 28 days after acrylamide exposure, the exposed and control rats were evaluated for walking ability, and gait score was assessed with a range of 1–4, as described previously[45-46]. A gait score of 1 indicates no degree of impairment, with 4 re fl ecting complete lameness. The evaluators performed gait scoring in a blinded manner and were not involved in animal care after acrylamide exposure.
Sample collection
At different time points after acrylamide exposure, serum and CSF samples were collected from the rats for albumin, transthyretin, and leptin measurements. CSF samples were obtained through a 26-gauge needle inserted between the protuberance and the spine of the atlas, and were centrifuged at 3,000 × g for 5 minutes, immediately after collection, then aliquoted, frozen at ?80°C and stored for further analysis.Blood samples were obtained from the abdominal aorta with a 5 mL needle syringe and placed at 4°C for 4 hours, then centrifuged at 4,000 × g for 10 minutes, aliquoted, and frozen at ?80°C for use.
Determination of blood-CSF barrier permeability using Evans blue and sodium fluorescein
In the present study, Evans blue (molecular weight: 961 kDa; Sigma, St. Louis, MO, USA) and sodium fl uorescein (molecular weight: 376 kDa; Sigma)[47-49]were used to assess the permeability of the blood-CSF barrier. Rats were anesthetized with ketamine HCl (100 mg/kg) via intraperitoneal injection. The skin of a lower limb was cut and the femoral artery was separated. A minimal incision at the femoral artery was made and a plastic PE50 tube was placed inside the artery. The tube was then connected with a syringe containing dye mixture solution (4 mL/kg 2% Evans blue + 0.6 mL/kg 10% sodium fluorescein) with an injection rate of 0.45 mL/min. After 30 minutes of cannulation, heart perfusion was performed to wash off the dye in the vessels using saline for 30 minutes, 3 mL/min. CSF samples were then collected to measure Evans blue and sodium fl uorescein concentrations.
Detection of Evan blue concentration in CSF: CSF sample and Evans blue standard solution were mixed with two volumes of N,N-dimethylformamide, and then incubated in water at 50°C for 48 hours. Samples were centrifuged at 16,000 × g for 10 minutes to remove precipitated protein. The supernatants were put on a microplate and absorbance values were read in an ELISA reader (Bio-rad, Hercules, CA, USA) at 635 nm within 10 minutes. A standard curve was made based on the absorbance value of standard Evans blue solution. The sample concentration was calculated according to a standard curve and expressed as μg/mL.
Detection of sodium fluorescein in CSF: CSF sample and sodium fl uorescein standard solution were mixed with three volumes of PBS and centrifuged at 15,000 × g for 10 minutes. The supernatant was isolated, diluted with 20% trichloroacetic acid (1:2), and centrifuged again at 100,000 × g for 15 minutes to precipitate protein. The supernatant was neutralized with 5 mol/L NaOH and transferred to a microplate, and its fl uorescence intensity value was read by a spectro fl uorophotometer (RF-5301PC, Shimadzu Corporation, Japan) at an excitation wavelength of 480 nm and an emission wavelength of 525 nm within 5 minutes. A standard curve was plotted based on the fl uorescence intensity value of standard sodium fl uorescein solution. The sample concentration was calculated according to a standard curve and expressed as μg/mL.
Quantification of albumin, transthyretin and leptin using ELISA Albumin concentrations in CSF and serum were quanti fi ed using an albumin ELISA kit (R & D Systems, Minneapolis, MN, USA; lower detection limit of 1.0 ng/mL). The concentrations of transthyretin and leptin in CSF and serum were determined using a transthyretin ELISA kit (R & D Systems; detection limit of 1.5 μg/mL) and a leptin ELISA kit (R & D Systems; detection limit of 0.25 μg/L), respectively. Albumin, transthyretin and leptin rabbit anti-rat polyclonal antibodies were used. Briefly, a 96-well polystyrene microplate coated with a rabbit anti-rat polyclonal antibody against rat albumin, transthyretin or leptin were used at room temperature. 50-μL aliquots of standards at different concentrations and 5-fold diluted serum and 10-fold diluted CSF samples were added into wells. After 1 hour of incubation, samples were rinsed and incubated with 50 μL of chromogen solution A and B for 15 minutes. When optimal blue color was seen, the termination solution was added to terminate the reaction. The absorbance values of the samples were immediately read on a microplate reader (Bio-Rad, Hercules, CA, USA) at 450 nm. The total protein concentration of the sample was measured using a Bradford protein assay kit and read on a microplate reader at 595 nm. A standard curve was plotted based on the absorbance value of standard albumin, transthyretin and leptin solutions. The sample concentration was calculated according to standard curves.
Statistical analysis
All data are represented as mean ± SD. SPSS 13.0 software (SPSS, Chicago, IL, USA) was used for data analysis. Oneway analysis of variance was used to examine the differences among groups for albumin, transthyretin and leptin concentrations in CSF and serum. Intergroup paired comparison was performed using least signi fi cant difference test. All P values were two-tailed, and P < 0.05 was considered signi fi cant.
Acknowledgments:We thank Fu X from the School of Health Science, Purdue University, USA, for her help in reviewing the manuscript.
Author contributions:Zhang YS, Yao X, Yan LC and Yao L designed the study. Yao X, Yan LC, Yao L, Zeng FX and Cao FY participated in experiments, prepared the animal model and performed measurements. Yao X, Yan LC, Guan WJ and Zhang YS analyzed experimental data and wrote the manuscript. Zhang YS was in charge of funding and was responsible for experimental concept and design, validation and guidance of the study. All authors approved the final version of the manuscript.
Con fl icts of interest:None declared.
Peer review:This study examined changes in the permeability, secretory function and transport function of the blood-CSF barrier in acrylamide-exposed rats. The results are reliable. Further studies focusing on morphology and ex vivo experiments are required to provide additional insight into the mechanisms of acrylamide-induced blood-CSF barrier damage.
[1] Pennisi M, Malaguarnera G, Puglisi V, et al. Neurotoxicity of acrylamide in exposed workers. Int J Environ Res Public Health. 2013;10(9):3843-3854.
[2] Obón-Santacana M, Slimani N, Lujan-Barroso L, et al. Dietary intake of acrylamide and pancreatic cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort. Ann Oncol. 2013;24(10):2645-2651.
[3] Powers SJ, Mottram DS, Curtis A, et al. Acrylamide concentrations in potato crisps in Europe from 2002 to 2011. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2013;30(9):1493-1500.
[4] LoPachin RM, Gavin T. Molecular mechanism of acrylamide neurotoxicity: lessons learned from organic chemistry. Environ Health Perspect. 2012;120(12):1650-1657.
[5] Prasad SN, Muralidhara. Evidence of acrylamide induced oxidative stress and neurotoxicity in Drosophila melanogaster - its amelioration with spice active enrichment: relevance to neuropathy. Neurotoxicology. 2012;33(5):1254-1264.
[6] LoPachin RM, Gavin T. Acrylamide-induced nerve terminal damage: relevance to neurotoxic and neurodegenerative mechanisms. J Agric Food Chem. 2008;56(15):5994-6003.
[7] Seale SM, Feng Q, Agarwal AK, et al. Neurobehavioral and transcriptional effects of acrylamide in juvenile rats. Pharmacol Biochem Behav. 2012;101(1):77-84.
[8] Barber DS, Stevens S, LoPachin RM. Proteomic analysis of rat striatal synaptosomes during acrylamide intoxication at a low dose rate. Toxicol Sci. 2007;100(1):156-167.
[9] Zhang MA, Chen FH, Huang ZY, et al. Elaidic acid enhanced the simultaneous neurotoxicity attributable to the cerebral pathological lesion resulted from oxidative damages induced by acrylamide and benzo(a)pyrene. Toxicol Ind Health. 2011;27(7):661-672.
[10] Mitra S, Gera R, Siddiqui WA, et al. Tributyltin induces oxidative damage, inflammation and apoptosis via disturbance in bloodbrain barrier and metal homeostasis in cerebral cortex of rat brain: an in vivo and in vitro study. Toxicology. 2013;310:39-52.
[11] Kof fi e RM, Farrar CT, Saidi LJ, et al. Nanoparticles enhance brain delivery of blood-brain barrier-impermeable probes for in vivo optical and magnetic resonance imaging. Proc Natl Acad Sci U S A. 2011;108(46):18837-18842.
[12] Bornhorst J, Wehe CA, Hüwel S, et al. Impact of manganese on and transfer across blood-brain and blood-cerebrospinal fluid barrier in vitro. J Biol Chem. 2012;287(21):17140-17151.
[13] Yorulmaz H, Seker FB, Demir G, et al. The effects of zinc treatment on the blood-brain barrier permeability and brain element levels during convulsions. Biol Trace Elem Res. 2013;151(2):256-262.
[14] Monnot AD, Zheng G, Zheng W. Mechanism of copper transport at the blood-cerebrospinal fl uid barrier: in fl uence of iron de fi ciency in an in vitro model. Exp Biol Med (Maywood). 2012;237(3): 327-333.
[15] Segal MB. Fluid compartments of the central nervous system. Boca Raton: the blood-cerebrospinal fl uid barrier, USA. 2005.
[16] Szmydynger-Chodobska J, Strazielle N, Gandy JR, et al. Posttraumatic invasion of monocytes across the blood-cerebrospinal fl uid barrier. J Cereb Blood Flow Metab. 2012;32(1):93-104.
[17] Bolos M, Spuch C, Ordo?ez-Gutierrez L, et al. Neurogenic effects of β-amyloid in the choroid plexus epithelial cells in Alzheimer’s disease. Cell Mol Life Sci. 2013;70(15):2787-2797.
[18] Niehof M, Borlak J. Expression of HNF4alpha in the human and rat choroid plexus: implications for drug transport across the blood-cerebrospinal-fluid (CSF) barrier. BMC Mol Biol. 2009; 10:68.
[19] Soprano DR, Herbert J, Soprano KJ, et al. Demonstration of transthyretin mRNA in the brain and other extrahepatic tissues in the rat. J Biol Chem. 1985;260(21):11793-11798.
[20] Choi SH, Leight SN, Lee VM, et al. Accelerated Abeta deposition in APPswe/PS1deltaE9 mice with hemizygous deletions of TTR (transthyretin). J Neurosci. 2007;27(26):7006-7010.
[21] Kassem NA, Deane R, Segal MB, et al. Role of transthyretin in thyroxine transfer from cerebrospinal fluid to brain and choroid plexus. Am J Physiol Regul Integr Comp Physiol. 2006;291(5):R1310-1315.
[22] Veyrat-Durebex C, Poher AL, Caillon A, et al. Rohner-Jeanrenaud F. Improved leptin sensitivity as a potential candidate responsible for the spontaneous food restriction of the lou/c rat. PLoS One. 2013;8(9):e73452.
[23] Egleton RD, Davis TP. Bioavailability and transport of peptides and peptide drugs into the brain. Peptides. 1997;18(9):1431-1439.
[24] Thomas SA, Preston JE, Wilson MR, et al. Leptin transport at the blood--cerebrospinal fl uid barrier using the perfused sheep choroid plexus model. Brain Res. 2001;895(1-2):283-290.
[25] LoPachin RM, Gavin T. Molecular mechanism of acrylamide neurotoxicity: lessons learned from organic chemistry. Environ Health Perspect. 2012;120(12):1650-1657.
[26] Zhang L, Gavin T, Barber D, et al. Role of the Nrf2-ARE pathway in acrylamide neurotoxicity. Toxicol Lett. 2011;205(1):1-7.
[27] Bowyer JF, Latendresse JR, Delongchamp RR, et al. The mRNA expression and histological integrity in rat forebrain motor and sensory regions are minimally affected by acrylamide exposure through drinking water. Toxicol Appl Pharmacol. 2009;240(3):401-411.
[28] LoPachin RM, Gavin T, DeCaprio AP, et al. Application of the Hard and Soft, Acids and Bases theory to toxicant-target interactions. Chem Res Toxicol. 2012;25(2):239-251.
[29] Wang LK, Hong Z, Wu GF, et al. Perihematomal endothelin-1 level is associated with an increase in blood-brain barrier permeability in a rabbit model of intracerebral hematoma. Chin Med J (Engl). 2013;126(18):3433-3438.
[30] Euser AG, Bullinger L, Cipolla MJ. Magnesium sulphate treatment decreases blood-brain barrier permeability during acute hypertension in pregnant rats. Exp Physiol. 2008;93(2):254-261.
[31] Vries J, Thijssen WA, Snels SE, et al. Intraoperative values of S-100 protein, myelin basic protein, lactate, and albumin in the CSF and serum of neurosurgical patients. J Neurol Neurosurg Psychiatry. 2001;71(5):671-674.
[32] Sharma HS, Zimmermann-Meinzingen S, Johanson CE. Cerebrolysin reduces blood-cerebrospinal fl uid barrier permeability change, brain pathology, and functional de fi cits following traumatic brain injury in the rat. Ann N Y Acad Sci. 2010;1199:125-137.
[33] Shi LZ, Zheng W. Early lead exposure increases the leakage of the blood-cerebrospinal fl uid barrier, in vitro. Hum Exp Toxicol. 2007;26(3):159-167.
[34] Baruch K, Ron-Harel N, Gal H, et al. CNS-speci fi c immunity at the choroid plexus shifts toward destructive Th2 in fl ammation in brain aging. Proc Natl Acad Sci U S A. 2013;110(6):2264-2269.
[35] Martinho A, Gon?alves I, Costa M, et al. Stress and glucocorticoids increase transthyretin expression in rat choroid plexus via mineralocorticoid and glucocorticoid receptors. J Mol Neurosci. 2012;48(1):1-13.
[36] Dickson PW, Aldred AR, Marley PD, et al. Rat choroid plexus specializes in the synthesis and the secretion of transthyretin (prealbumin). Regulation of transthyretin synthesis in choroid plexus is independent from that in liver. J Biol Chem. 1986;261(8):3475-3478.
[37] Behl M, Zhang Y, Monnot AD, et al. Increased beta-amyloid levels in the choroid plexus following lead exposure and the involvement of low-density lipoprotein receptor protein-1. Toxicol Appl Pharmacol. 2009;240(2):245-254.
[38] Cuenco KT, Friedland R, Baldwin CT, et al. Association of TTR polymorphisms with hippocampal atrophy in Alzheimer disease families. Neurobiol Aging. 2011;32(2):249-256.
[39] Sousa JC, Marques F, Dias-Ferreira E, et al. Transthyretin in fl uences spatial reference memory. Neurobiol Learn Mem. 2007;88(3):381-385.
[40] Fleming CE, Saraiva MJ, Sousa MM. Transthyretin enhances nerve regeneration. J Neurochem. 2007;103(2):831-839.
[41] Zlokovic BV, Jovanovic S, Miao W, et al. Differential regulation of leptin transport by the choroid plexus and blood-brain barrier and high af fi nity transport systems for entry into hypothalamus and across the blood-cerebrospinal fl uid barrier. Endocrinology. 2000;141(4):1434-1441.
[42] Liu C, Jiang C, Zhou L. Protective effect of epigallocatechin-3-gallate on apoptosis of rat cerebellar granule neurons induced by acrylamide. Zhong Nan Da Xue Xue Bao: Yi Xue Ban. 2012;37(9):944-950.
[43] Lakshmi D, Gopinath K, Jayanthy G, et al. Ameliorating effect of fi sh oil on acrylamide induced oxidative stress and neuronal apoptosis in cerebral cortex. Neurochem Res. 2012;37(9):1859-1867.
[44] Rahangadale S, Kurkure N, Prajapati B, et al. Neuroprotective effect of vitamin e supplementation in wistar rat treated with acrylamide. Toxicol Int. 2012;19(1):1-8.
[45] Lehning EJ, Jortner BS, Fox JH, et al. Gamma-diketone peripheral neuropathy. I. Quality morphometric analyses of axonal atrophy and swelling. Toxicol Appl Pharmacol. 2000;165(2):127-140.
[46] Bizeray D, Leterrier C, Constantin P, et al. Sequential feeding can increase activity and improve gait score in meat-type chickens. Poult Sci. 2002;81(12):1798-1806.
[47] Kozler P, Pokorny J. Altered blood-brain barrier permeability and its effect on the distribution of evans blue and sodium fl uorescein in the rat brain applied by intracarotid injection. Physiol Res. 2003;52(5):607-614.
[48] Euser AG, Bullinger L, Cipolla MJ. Magnesium sulphate treatment decreases blood-brain barrier permeability during acute hypertension in pregnant rats. Exp Physiol. 2008;93(2):254-261.
[49] Olsen AL, Morrey JD, Smee DF, et al. Correlation between breakdown of the blood-brain barrier and disease outcome of viral encephalitis in mice. Antiviral Res. 2007;75(2):104-112.
Copyedited by Patel B, Norman C, Wang X, Lv J, Wang J, Yang Y, Li CH, Song LP, Zhao M
10.4103/1673-5374.130080
Yanshu Zhang, Ph.D., College of Public Health, Hebei United University, Tangshan 063000, Hebei Province, China, Yanshu_zhang@163.com.
http://www.nrronline.org/
Accepted: 2014-01-27
- 中國神經(jīng)再生研究(英文版)的其它文章
- Analysis of the effect of repeated-pulse transcranial magnetic stimulation at the Guangming point on electroencephalograms
- Preconditioning crush increases the survival rate of motor neurons after spinal root avulsion
- Adaptive and regulatory mechanisms in aged rats with postoperative cognitive dysfunction
- Increased expression of Notch1 in temporal lobe epilepsy: animal models and clinical evidence
- Transient axonal glycoprotein-1 induces apoptosisrelated gene expression without triggering apoptosis in U251 glioma cells
- Negative regulation of miRNA-9 on oligodendrocyte lineage gene 1 during hypoxic-ischemic brain damage

