Adaptive and regulatory mechanisms in aged rats with postoperative cognitive dysfunction
Yanlin Bi, Shuyun Liu, Xinjuan Yu, Mingshan Wang, Yuelan Wang
1 Department of Anesthesiology, Af fi liated Qianfoshan Hospital, Shandong University, Jinan, Shandong Province, China
2 Department of Anesthesiology, Shaoxing People’s Hospital, Shaoxing, Zhejiang Province, China
3 Department of Anesthesiology, Qingdao Municipal Hospital, Qingdao, Shandong Province, China
Adaptive and regulatory mechanisms in aged rats with postoperative cognitive dysfunction
Yanlin Bi1,2, Shuyun Liu2, Xinjuan Yu3, Mingshan Wang3, Yuelan Wang1
1 Department of Anesthesiology, Af fi liated Qianfoshan Hospital, Shandong University, Jinan, Shandong Province, China
2 Department of Anesthesiology, Shaoxing People’s Hospital, Shaoxing, Zhejiang Province, China
3 Department of Anesthesiology, Qingdao Municipal Hospital, Qingdao, Shandong Province, China
In fl ammation may play a role in postoperative cognitive dysfunction. 5′ Adenosine monophosphate-activated protein kinase, nuclear factor-kappa B, interleukin-1β, and tumor necrosis factor-α are involved in in fl ammation. Therefore, these in fl ammatory mediators may be involved in postoperative cognitive dysfunction. Western immunoblot analysis revealed 5′ adenosine monophosphate-activated protein kinase and nuclear factor-kappa B in the hippocampus of aged rats were increased 1–7 days after splenectomy. Moreover, interleukin-1β and tumor necrosis factor-α were upregulated and gradually decreased. Therefore, these in fl ammatory mediators may participate in the splenectomy model of postoperative cognitive dysfunction in aged rats.
nerve regeneration; postoperative cognitive dysfunction; splenectomy; brain; aging; 5′adenosine monophosphate-activated protein kinase; nuclear factor-kappa B; tumor necrosis factor-α; interleukin-1β; neural regeneration
Bi YL, Liu SY, Yu XJ, Wang MS, Wang YL. Adaptive and regulatory mechanisms in aged rats with postoperative cognitive dysfunction. Neural Regen Res. 2014;9(5):534-539.
Introduction
Postoperative cognitive dysfunction has been widely reported to be a common complication following surgery and injury, particularly in elderly patients[1]. However, no standard, universally accepted diagnostic criteria exists for this disorder[2]. Patients who experience cognitive decline and confusion account for 25–50% of hospitalized patients[1]. The in fl uence of surgical procedures on cognition is mainly reflected in learning, language comprehension, executive functioning, thinking, social integration orientation, and memory[3]. However, the pathogenic mechanisms of postoperative cognitive dysfunction are unclear. Risk factors for this disorder include advanced age, second surgery, postoperative pain, coexisting diseases, and hospitalization[4]. However, advanced age is the most important factor in postoperative cognitive dysfunction. Resisting both injury and repair decreases with increasing age, thus predisposing the elderly to postoperative cognitive dysfunction.
Cognitive dysfunction during and after surgery may be attributed to cerebral ischemia reperfusion, hypoxia, cerebral microemboli and inflammation[5]. Cerebral blood flow is normally independent of perfusion pressure in the physiological range. However, cerebral blood fl ow becomes pressure-dependent when the perfusion pressure is too low or high and consequently increases the susceptibility to ischemia or hyperaemia, respectively owing to the failure in autoregulation of blood flow, which impacts oxygenation, thus leading to cerebral injury[6]. Recent studies have shown that postoperative cognitive dysfunction may result from post-surgery inflammation, particularly in the hippocampus[7-8]. An inflammatory response is common during surgery- and patient-related factors. Tissue damage causes an in fl ammatory reaction through the activation of the immune system, which aims to repair the damage. However, this response can also exacerbate the state of tissue damage[6].
Interleukin-1β and tumor necrosis factor-α respond to numerous activities in the brain[7]. After tibia surgery, mice exhibit hippocampal memory impairment, reactive microglia, and increased levels of interleukin-1β and tumor necrosis factor-α[8-9]. Pro-inflammatory molecules are promptly released in the circulatory system after tissue damage, surgery, and trauma. For example, tumor necrosis factor-α reaches a peak within minutes after surgery[10]. The pro-in fl ammatory transcription factor, nuclear factor-kappa B, plays a crucial role in the pathophysiology of the central nervous system because it regulates neuroimmune responses, such as the production of pro-in fl ammatory cytokines[11]. Injury, infection or stress activates the central nervous system, increasing the expression of cytokines, chemokines, and adhesion molecules[11]. Leukocytes and microglia accumulate and in fi ltrate the central nervous system[12]. Nuclear factor-kappa B can be activated by cytokines, chemokines, and adhesion molecules, and further activates in fl ammatory cytokines, such as interleukin-1 and tumor necrosis factor-α. Nuclear factor-kappa B controls microglial activation by regulating the transcription of in fl ammatory mediators. Nuclear factor-kappa B also reduces the in fl ammation response after brain injury[13].
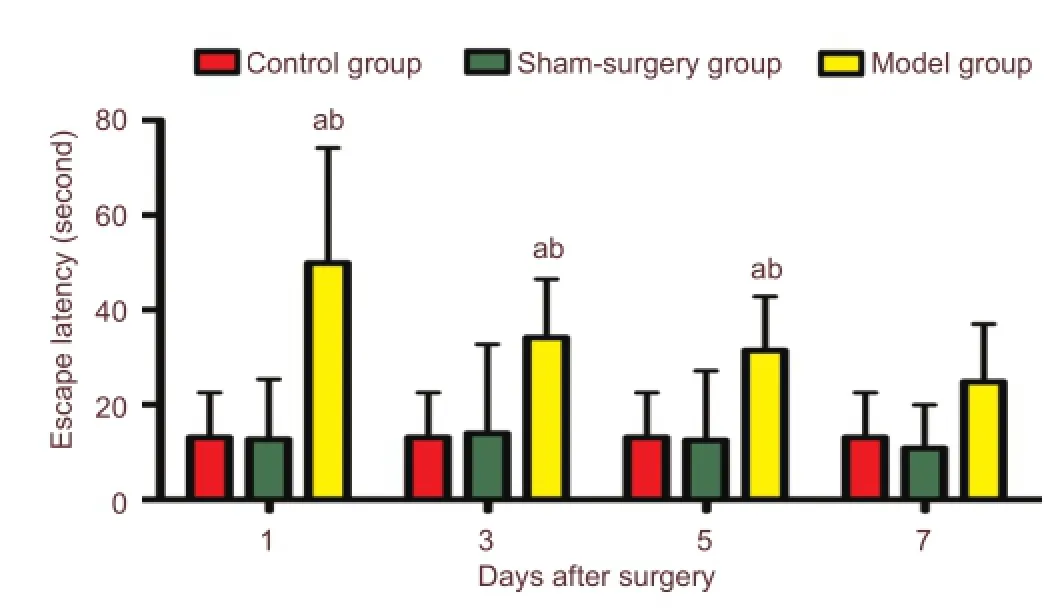
Figure 1 Learning and memory ability is reduced in postoperative cognitive dysfunction rats.
5′ Adenosine monophosphate-activated protein kinase is a protective protein in the central nervous system which is activated by pro- and anti-inflammatory stimuli, such as lipopolysaccharide, ciliary neurotrophic factor, interleukin-10, and transforming growth factor-beta[14-15]. Some common pro-inflammatory cytokines, such as tumor necrosis factor-α, inhibit the activity of 5′ adenosine monophosphate-activated protein kinase by up-regulating the expression of protein phosphatase 2C, which in-turn inhibits 5′ adenosine monophosphate-activated protein kinase signaling[16]. 5′ Adenosine monophosphate-activated protein kinase activation is reduced in interleukin-6 knockout mice[17], indicating that 5′ adenosine monophosphate-activated protein kinase activation is dependent on interleukin-6. Furthermore, 5′adenosine monophosphate-activated protein kinase inhibits in fl ammatory responses in various organs through a wide variety of mechanisms, such as nuclear factor-kappa B[18]In the present study, we investigated the effect of 5′ adenosine monophosphate-activated protein kinase, nuclear factor-kappa B, interleukin-1β, and tumor necrosis factor-α on postoperative cognitive dysfunction via splenectomy in Sprague-Dawley rats by investigating the expression of them in the hippocampus.
Results
Quantitative analysis of experimental animals
A total of 90 aged Sprague-Dawley rats (18–22 months) were randomly divided into 3 groups: control (physiological saline) (n = 10), sham-surgery (anesthesia only) (n = 40), model group (splenectomy model of postoperative cognitive dysfunction) (n = 40). After successfully performing the surgery or sham-operation, rats from both groups were divided into four subgroups: 1, 3, 5 or 7 days after surgery (n = 10 per group). Dead rats were supplemented by new rats. All 90 rats were included in the fi nal analysis.
Learning and memory ability was reduced in postoperative cognitive dysfunction rats
The Morris water maze test showed that spatial learning and memory were damaged after surgery. Escape latencies were signi fi cantly (P < 0.05) longer at postoperative days 1, 3, and 5 of the model group compared with the control group or the sham-surgery group (Figure 1).
Expression of 5′adenosine monophosphate-activated protein kinase and in fl ammatory mediators increased in the hippocampus of postoperative cognitive dysfunction rats
Western blot analysis of hippocampal tissue revealed that 5′ adenosine monophosphate-activated protein kinase, nuclear factor-kappa B, interleukin-1β, and tumor necrosis factor-α were signi fi cantly (P < 0.05) increased in postoperative cognitive dysfunction rats compared with the control group or the sham-surgery group (Figures 2, 3). Moreover, the expression of interleukin-1β and tumor necrosis factor-α significantly (P < 0.05) decreased with increasing postoperative days (Figures 2, 3).
Discussion
This study indicated that memory and learning declined for a short period in rats after splenectomy. Cognition was impaired 1, 3, and 5 days after splenectomy, and recovery was observed by the 7thday. These results suggested that cognition was transiently reduced after splenectomy.
Activated microglia and interleukin-1β-positive microglia are more abundant in the hippocampus of aged mice compared with young mice[19]. Therefore, in the present study, aged (18–22 months) Sprague-Dawley rats were chosen to investigate postoperative cognitive dysfunction. Splenectomy has been widely used in past studies[20-21]. Splenectomy can be easily performed to establish this model. The recovery time of rats subjected to this surgery is fast, thus results from the Morris water maze task are unaffected[21]. The Morris water maze task is a useful tool to determine learning and memory to re fl ect the severity of injury or the effects of drugs[22].
Furthermore, the expression of 5′ adenosine monophosphate-activated protein kinase peaked 5 days after surgery, which may have been caused by inflammation and stress. The expression of in fl ammatory cytokines and 5′ adenosine monophosphate-activated protein kinase increased 1 day after surgery. These results showed that splenectomy induced neuroin fl ammation and activated 5′ adenosine monophosphate-activated protein kinase. Nuclear factor-kappa B, interleukin-1β, and tumor necrosis factor-α increased earlier than 5′ adenosine monophosphate-activated protein kinase, and moreover, their peak expression occurred earlier. These results suggested that inflammatory cytokines may have activated 5′ adenosine monophosphate-activated protein kinase. Peripheral inflammatory cytokines may induce infl ammatory responses in the central nervous system through direct and/or indirect ways. Peripheral in fl ammatory factors can pass through the blood-brain barrier into the central nervous system more easily under surgical and pathological conditions. During injury and surgeries, peripheral in fl ammatory factors can pass the blood-brain barrier into the central nervous system directly or via specific receptors. This effect results in an in fl ammatory response in the central nervous system[23]. In fl ammatory cytokines, such as interleukin-1β, interleukin-6, and tumor necrosis factor-α, affect neurons and activate astrocytes and microglia[24]. Furthermore, cytokines can induce neurotoxicity, which inturn stimulates astrocytes and microglia in an autocrine and paracrine manner. The subsequent release of in fl ammatory cytokines aggravates ischemia[24]and damages the nervous system. Interleukin-1β is an important mediator in triggering in fl ammation[25].

Figure 2 The expression of AMPK and NF-κB increased in the hippocampus of postoperative cognitive dysfunction rats.

Figure 3 The expression of IL-1β and TNF-α increased in the hippocampus of postoperative cognitive dysfunction rats.
Cytokines can pass through the impaired blood-brain barrier and in fl uence cognitive function by affecting neuronal activity and synaptic connections, and causing neurotoxicity and degeneration. Pro-in fl ammatory cytokines, such as interleukin-1 and tumor necrosis factor-α, have been shownto influence hippocampal-dependent cognition[26-27]. The increase in interleukin-6 and other pro-in fl ammatory cytokines in the hippocampus has been shown to occur in parallel with postoperative cognitive dysfunction in aged rats[28]. Moreover, pro-in fl ammatory cytokines and glial activation may affect postoperative cognitive dysfunction[28]. The blood vessels, neural axis and glial cells form the basic unit for brain function. Pro-inflammatory cytokines are produced from glial and neuronal cells, and thus play a role in neuroin fl ammation. The brain activates in fl ammatory signaling in response to an immune challenge[29].
Surgical trauma can activate the innate immune system, resulting in strong peripheral in fl ammation, as seen by the increased levels of interleukin-1β, interleukin-6, and tumor necrosis factor-α, and impaired cognitive function[30-31]. Nuclear factor-kappa B plays an important role in this process. Nuclear factor-kappa B regulates the expression of a number of genes, many of which regulate inflammation. After surgery, nuclear factor-kappa B is activated by inflammatory factors, cytokines, and calcium overload factors[32-33]. Nuclear factor-kappa B activation promotes the expression of infl ammatory molecules (such as interleukin-1β, interleukin-6 and tumor necrosis factor-α) and calcium. An in fl ammatory cycle response is formed, thus injuring the blood brain barrier and neurons. Overall, nuclear factor-kappa B regulates the expression of tumor necrosis factor-α and interleukin-1β. The balance between these two cytokines is reduced during nervous system injury[34].
Ser/Thr kinase 5′ adenosine monophosphate-activated protein kinase reduces energy use and increases energy production via glucose and lipid metabolism[35-36]. The phosphorylation of tau protein and the accumulation of amyloid in aged people triggers neuronal damage, and may be associated with cognitive dysfunction[37-39]. The activation of 5′ adenosine monophosphate-activated protein kinase protects the central nervous system by promoting autophagic degradation, regulating tau protein phosphorylation, and reducing amyloidogenesis in neurons[40-41]. In various model systems, 5′ adenosine monophosphate-activated protein kinase activators, such as 5-aminoimidazole-4-carboxamide 1-β-D-ribofuranoside, inhibit inflammatory responses[42-43]. 5-Aminoimidazole-4-carboxamide 1-β-D-ribofuranoside inhibits in fl ammation in primary rat microglial cells, and this effect is 5′ adenosine monophosphate-activated protein kinase-independent[44]. Knockdown of the gene encoding 5′ adenosine monophosphate-activated protein kinase or expression of a dominant negative mutant in macrophages promotes a pro-in fl ammatory state[45]. In addition, numerous studies have indicated that activation of 5′ adenosine monophosphate-activated protein kinase signaling suppresses nuclear factor-kappa B[46-48]. 5′ Adenosine monophosphate-activated protein kinase activates silent mating type information regulation 2 homolog-1 and forkhead box O (FOXO) proteins (FOXO3a and FOXO4), which inhibit nuclear factor-kappa B signaling, thereby preventing in fl ammation[46-48]. Therefore, 5′ adenosine monophosphate-activated protein kinase can inhibit inflammation through different pathways, which may be one of the mechanisms reducing hippocampal damage.
These downstream proteins also regulate the rate of neuronal survival. Silent mating type information regulation 2 homolog-1 inhibits amyloidogenesis, triggers autophagic degradation, and affects oxidative stress[48]. The 5′ adenosine monophosphate-activated protein kinase activators, metformin and cilostazol, inhibit tumor necrosis factor-α-induced activation of nuclear factor-kappa B, inhibitor κ-gene binding molecule kinase activity, and tumor necrosis factor-α-induced inhibitor κ-gene binding molecule degradation in humans[49-50]. Nuclear factor-kappa B activation is inhibited more in 5′ adenosine monophosphate-activated protein kinase-over-expressed cells than cells with kinase-dead 5′adenosine monophosphate-activated protein kinase[51]. Overall, 5-aminoimidazole-4-carboxamide 1-β-D-ribofuranoside regulates nuclear factor-kappa B by activating 5′ adenosine monophosphate-activated protein kinase and phosphorylation of a protein substrate that forms a part of the nuclear factor-kappa B signing pathway.
Overall, 5′ adenosine monophosphate-activated protein kinase and nuclear factor-kappa B may play a role in postoperative cognitive dysfunction in aged rats. Our fi ndings indicated that 5′ adenosine monophosphate-activated protein kinase may have been activated by nuclear factor-kappa B, which in-turn inhibited nuclear factor-kappa B expression. The increased expression of 5′ adenosine monophosphate-activated protein kinase in rats with postoperative cognitive dysfunction may have indicated that reduced 5′ adenosine monophosphate-activated protein kinase activation enhanced the expression. The increased expression of hippocampal interleukin-1β, interleukin-6, and tumor necrosis factor-α in post-surgery rats may have indicated that these cytokines played a role in postoperative cognitive dysfunction. In conclusion, splenectomy stimulated the expression of 5′ adenosine monophosphate-activated protein kinase, nuclear factor-kappa B, interleukin-1β, and tumor necrosis factor-α, and caused postoperative cognitive dysfunction. However, the precise mechanisms of these relationships require further investigation.
Materials and Methods
Design
A randomized controlled animal experiment.
Time and setting
This experiment was performed in the Animal Laboratory of Qingdao Municipal Hospital in China from July to December 2012.
Materials
A total of 90 clean aged (18–22 months) male Sprague-Dawley rats (480–560 g) were used in this study. Animals were provided by the Dongchuang Animal Science and Technology Service Company (license No. SCXK (Xiang) 2009-0012) (Changsha, Hunan province, China). Animals were housed under a 12-hour light/dark cycle with free access to water and food. All rats acclimatized to their new environment (24–26°C) for 7 days before the commencement of experiments. The transportation and experimental procedures were performed in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, issued by the Ministry of Science and Technology of China[52]. The cages and procedures of transportation were all in accordance with the Association of Business Ethics. This study was approvedby Animal Ethics Committee, Qingdao Municipal Hospital, China.
Methods
Splenectomy
Chloral hydrate (10%) was injected in the abdominal cavity. The abdominal wall was shaved and disinfected, then a 2–4 cm incision was made in the upper left abdomen through the skin and muscle wall. The spleen was isolated from the surrounding tissue. The blood vessels were ligated by a 3-silk suture, and the spleen was removed. The wound was infiltrated with 1% lidocaine, and then closed with sterile 0-silk sutures. The sham-surgery group only received 10% chloral hydrate. The control group received 0.3 mL/100 g physiological saline.
Morris water maze task
Spatial learning and memory were evaluated in the Morris water maze using a computerized video track system (Xinruan Information Technology Co., Ltd., Shanghai, China) before model establishment. A round pool (120 cm in diameter and 40 cm in height) was divided into four quadrants. A round platform (10 cm in diameter and 30 cm in height) was placed 1 cm below the water surface, located in the center of the southeast quadrant. Both room and water temperatures were maintained between 24 and 26°C. Rats were trained for 5 days with three trials per day (90 seconds at most for a trial and 20 seconds for a rest on the platform before treatment). If the platform was not located within 90 seconds, the automatic recording system was stopped and the rat was guided to the platform arti fi cially and allowed to stay there for approximately 20 seconds. On the 6thday, rats were subjected to the model surgery, sham operation or saline injection. The rats were then tested in the Morris water maze 1, 3, 5, and 7 days after treatment, as described above. The time to reach the platform was recorded by video tracking system.
Following the test on 1, 3, 5, and 7 days, rats from the three groups were injected with chloral hydrate and then sacri fi ced by decapitation. The cerebral cortex was then removed, and the hippocampus immediately dissected[53]. The hippocampi were stored in liquid nitrogen for further use.
Western blot assay
Hippocampal homogenates were rapidly solubilized in lysate buffer containing the protease inhibitor (phenylmethylsulfonyl fl uoride, and then incubated on ice for 2 hours, followed by centrifugation (12,000 r/min at 4°C for 10 minutes). The supernatant was then extracted, mixed with 1 × sodium dodecyl sulphate buffer, and denaturated at 95°C for 5 minutes. Protein samples were separated by 12% sodium dodecylsulfate polyacrylamide gel electrophoresis (80 V followed by 100 V). The samples were transferred to polyvinylidene fl uoride membranes (60 V for 90 minutes). The membranes were blocked with 5% nonfat milk containing phosphate buffered saline at room temperature for 1 hour. Membranes were incubated with the primary antibodies: rabbit anti-rat 5′ adenosine monophosphate-activated protein kinase monoclonal antibody (1:300; Cell Signaling, Boston, MA, USA), rabbit anti-rat nuclear factor-kappa B monoclonal antibody (1:1,000; Abcam, London, UK), rabbit anti-rat interleukin-1β monoclonal antibody (1:500; Cell Signaling), or rabbit anti-rat tumor necrosis factor-α monoclonal antibody (1:500; Cell Signaling) for 2 hours at room temperature on a shaking table. Anti-rat glyceraldehyde 3-phosphate dehydrogenase (GAPDH) monoclonal antibody (1:500; Zhongshan Golden Bridge Biotechnology, Beijing, China) as the house-keeping protein. After membranes were washed (three times, 10 minutes each, with PBS containing Tween-20), they were incubated with the secondary antibodies, goat anti-mouse or -rabbit IgG (1:6,000; Zhongshan Golden Bridge Biotechnology) at room temperature for 1 hour. Horseradish-conjugated secondary antibody labeling was detected by enhanced chemiluminescence and blots were exposed with VILBER FUSION FX7 (Beijing Wuzhou Oriental Science and Technology Development Co., Ltd., Beijing, China). The absorbance was normalized to that of GAPDH. Quantitative analysis was performed by Quantity One Software (Bio Rad, Hercules, CA, USA).
Statistical analysis
All data were expressed as mean ± SD and were analyzed by one-way analysis of variance followed by the least signi fi cant difference test to compare between two groups. All statistical data were analyzed using SPSS 17.0 (SPSS, Chicago, IL, USA). Signi fi cance was reached at values of P < 0.05.
Acknowledgments:We thank the staffs in the Laboratory of Qingdao Eastern Municipal Hospital in China.
Author contributions:Bi YL was study designer and writer. Liu SY was an experiment and data worker. Yu XJ was statistical data worker. Wang YL and Wang MS conducted the experiment. All authors approved the final version of the manuscript.
Con fl icts of interest:None declared.
Peer review:Postoperative cognitive dysfunction is very common in the clinic. This study used classical models and found that inflammatory factors and AMP-activated protein kinase expression obviously altered in the hippocampus of rats with postoperative cognitive dysfunction and provided an experimental basis for the occurrence of postoperative cognitive dysfunction.
[1] Terrando N, Brzezinski M, Degos V, et al. Perioperative cognitive decline in the aging population. Mayo Clin Proc. 2011;86(9):885-893.
[2] van Dijk D, Kalkman CJ. Why are cerebral microemboli not associated with cognitive decline? Anesth Analg. 2009;109(4):1006-1008.
[3] Monk TG, Weldon BC, Garvan CW, et al. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008; 108(1):18-30.
[4] Krenk L, Rasmussen LS, Kehlet H. New insights into the pathophysiology of postoperative cognitive dysfunction. Acta Anaesthesiol Scand. 2010;54(8):951-956.
[5] van Harten AE, Scheeren TW, Absalom AR. A review of postoperative cognitive dysfunction and neuroin fl ammation associated with cardiac surgery and anaesthesia. Anaesthesia. 2012;67(3):280-293.
[6] Wan Y, Xu J, Ma D, et al. Postoperative impairment of cognitive function in rats: a possible role for cytokine-mediated in fl ammation in the hippocampus. Anesthesiology. 2007;106(3):436-443.
[7] Cao XZ, Ma H, Wang JK, et al. Postoperative cognitive deficits and neuroin fl ammation in the hippocampus triggered by surgical trauma are exacerbated in aged rats. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(8):1426-1432.
[8] Orellana DI, Quintanilla RA, Maccioni RB. Neuroprotective effect of TNFalpha against the beta-amyloid neurotoxicity mediated by CDK5 kinase. Biochim Biophys Acta. 2007;1773(2):254-263.
[9] Cibelli M, Fidalgo AR, Terrando N, et al. Role of interleukin-1beta in postoperative cognitive dysfunction. Ann Neurol. 2010; 68(3):360-368.
[10] Terrando N, Monaco C, Ma D, et al. Tumor necrosis factor-alpha triggers a cytokine cascade yielding postoperative cognitive decline. Proc Natl Acad Sci U S A. 2010;107(47):20518-20522.
[11] Ben-Neriah Y, Schmitz ML. Of mice and men. EMBO Rep. 2004; 5(7):668-673.
[12] Lucas SM, Rothwell NJ, Gibson RM. The role of in fl ammation in CNS injury and disease. Br J Pharmacol. 2006;147 Suppl 1:S232-240. [13] Liu Q, Liu FL, Huang BY. Expression of nuclear factor kappa B in rats after cerebral ischemia-reperfusion. Zuzhong yu Shenjing Jibing. 2002;9(2):104-106.
[14] Sag D, Carling D, Stout RD, et al. Adenosine 5′-monophosphateactivated protein kinase promotes macrophage polarization to an anti-inflammatory functional phenotype. J Immunol. 2008; 181(12):8633-8641.
[15] Watt MJ, Dzamko N, Thomas WG, et al. CNTF reverses obesityinduced insulin resistance by activating skeletal muscle AMPK. Nat Med. 2006;12(5):541-548.
[16] Steinberg GR, Michell BJ, van Denderen BJ, et al. Tumor necrosis factor alpha-induced skeletal muscle insulin resistance involves suppression of AMP-kinase signaling. Cell Metab. 2006;4(6):465-474.
[17] Kelly M, Keller C, Avilucea PR, et al. AMPK activity is diminished in tissues of IL-6 knockout mice: the effect of exercise. Biochem Biophys Res Commun. 2004;320(2):449-454.
[18] Salminen A, Hyttinen JM, Kaarniranta K. AMP-activated protein kinase inhibits NF-κB signaling and inflammation: impact on healthspan and lifespan. J Mol Med (Berl). 2011;89(7):667-676.
[19] Chen J, Buchanan JB, Sparkman NL, et al. Neuroinflammation and disruption in working memory in aged mice after acute stimulation of the peripheral innate immune system. Brain Behav Immun. 2008;22(3):301-311.
[20] Newman S, Stygall J, Hirani S, et al. Postoperative cognitive dysfunction after noncardiac surgery: a systematic review. Anesthesiology. 2007;106(3):572-590.
[21] Wan Y, Xu J, Ma D, et al. Postoperative impairment of cognitive function in rats: a possible role for cytokine-mediated in fl ammation in the hippocampus. Anesthesiology. 2007;106(3):436-443.
[22] Rasmussen LS, Larsen K, Houx P, et al. The assessment of postoperative cognitive function. Acta Anaesthesiol Scand. 2001; 45(3):275-289.
[23] Quan N, Banks WA. Brain-immune communication pathways. Brain Behav Immun. 2007;21(6):727-735.
[24] Allan SM, Tyrrell PJ, Rothwell NJ. Interleukin-1 and neuronal injury. Nat Rev Immunol. 2005;5(8):629-640.
[25] Yin QS, Zhou SM. The role of IL-1β and IL-1β receptor antagonist in diabetes. Zhonghua Baojian Yixue Zazhi. 2011;13(5):431-433.
[26] Perry VH, Cunningham C, Holmes C. Systemic infections and infl ammation affect chronic neurodegeneration. Nat Rev Immunol. 2007;7(2):161-167.
[27] Pickering M, Cumiskey D, O’Connor JJ. Actions of TNF-alpha on glutamatergic synaptic transmission in the central nervous system. Exp Physiol. 2005;90(5):663-670.
[28] Cao XZ, Ma H, Wang JK, et al. Postoperative cognitive deficits and neuroin fl ammation in the hippocampus triggered by surgical trauma are exacerbated in aged rats. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(8):1426-1432.
[29] Tonelli LH, Postolache TT. Tumor necrosis factor alpha, interleukin-1 beta, interleukin-6 and major histocompatibility complex molecules in the normal brain and after peripheral immune challenge. Neurol Res. 2005;27(7):679-684.
[30] Katerelos M, Mudge SJ, Stapleton D, et al. 5-aminoimidazole-4-carboxamide ribonucleoside and AMP-activated protein kinase inhibit signalling through NF-κB. Immunol Cell Biol. 2010; 88(7):754-760.
[31] Jawa RS, Anillo S, Huntoon K, et al. Interleukin-6 in surgery, trauma, and critical care part II: clinical implications. J Intensive Care Med. 2011;26(2):73-87.
[32] Sun T, Wang X, Liu Z, et al. Plasma concentrations of pro- and anti-in fl ammatory cytokines and outcome prediction in elderly hip fracture patients. Injury. 2011;42(7):707-713.
[33] Lenardo MJ, Baltimore D. NF-kappa B: a pleiotropic mediator of inducible and tissue-speci fi c gene control. Cell. 1989;58(2):227-229.
[34] Pradillo JM, Romera C, Hurtado O, et al. TNFR1 upregulation mediates tolerance after brain ischemic preconditioning. J Cereb Blood Flow Metab. 2005;25(2):193-203.
[35] Cantó C, Auwerx J. AMP-activated protein kinase and its downstream transcriptional pathways. Cell Mol Life Sci. 2010;67(20): 3407-3423.
[36] Hardie DG, Carling D. The AMP-activated protein kinase--fuel gauge of the mammalian cell? Eur J Biochem. 1997;246(2):259-273.
[37] Boekhoorn K, Terwel D, Biemans B, et al. Improved long-term potentiation and memory in young tau-P301L transgenic mice before onset of hyperphosphorylation and tauopathy. J Neurosci. 2006;26(13):3514-3523.
[38] Nordberg A, Hellstr?m-Lindahl E, Lee M, et al. Chronic nicotine treatment reduces beta-amyloidosis in the brain of a mouse model of Alzheimer’s disease (APPsw). J Neurochem. 2002;81(3):655-658.
[39] Wan Y, Xu J, Meng F, et al. Cognitive decline following major surgery is associated with gliosis, β-amyloid accumulation, and τ phosphorylation in old mice. Crit Care Med. 2010;38(11):2190-2198.
[40] Vingtdeux V, Giliberto L, Zhao H, et al. AMP-activated protein kinase signaling activation by resveratrol modulates amyloid-beta peptide metabolism. J Biol Chem. 2010;285(12):9100-9113.
[41] Won JS, Im YB, Kim J, et al. Involvement of AMP-activated-protein-kinase (AMPK) in neuronal amyloidogenesis. Biochem Biophys Res Commun. 2010;399(4):487-491.
[42] Bai A, Ma AG, Yong M, et al. AMPK agonist downregulates innate and adaptive immune responses in TNBS-induced murine acute and relapsing colitis. Biochem Pharmacol. 2010;80(11):1708-1717.
[43] Pilon G, Dallaire P, Marette A. Inhibition of inducible nitric-oxide synthase by activators of AMP-activated protein kinase: a new mechanism of action of insulin-sensitizing drugs. J Biol Chem. 2004;279(20):20767-20774.
[44] ?abuzek K, Liber S, Gabryel B, et al. AICAR (5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside) increases the production of toxic molecules and affects the profile of cytokines release in LPS-stimulated rat primary microglial cultures. Neurotoxicology. 2010;31(1):134-146.
[45] Sag D, Carling D, Stout RD, et al. Adenosine 5’-monophosphateactivated protein kinase promotes macrophage polarization to an anti-inflammatory functional phenotype. J Immunol. 2008; 181(12):8633-8641.
[46] Cantó C, Gerhart-Hines Z, Feige JN, et al. AMPK regulates energy expenditure by modulating NAD+metabolism and SIRT1 activity. Nature. 2009;458(7241):1056-1060.
[47] Greer EL, Oskoui PR, Banko MR, et al. The energy sensor AMP-activated protein kinase directly regulates the mammalian FOXO3 transcription factor. J Biol Chem. 2007;282(41):30107-30119.
[48] Zhou W, Cao Q, Peng Y, et al. FoxO4 inhibits NF-kappaB and protects mice against colonic injury and in fl ammation. Gastroenterology. 2009;137(4):1403-1414.
[49] Hattori Y, Suzuki K, Hattori S, et al. Metformin inhibits cytokineinduced nuclear factor kappaB activation via AMP-activated protein kinase activation in vascular endothelial cells. Hypertension. 2006;47(6):1183-1188.
[50] Hattori Y, Suzuki K, Tomizawa A, et al. Cilostazol inhibits cytokine-induced nuclear factor-kappaB activation via AMP-activated protein kinase activation in vascular endothelial cells. Cardiovasc Res. 2009;81(1):133-139.
[51] Katerelos M, Mudge SJ, Stapleton D, et al. 5-aminoimidazole-4-carboxamide ribonucleoside and AMP-activated protein kinase inhibit signalling through NF-κB. Immunol Cell Biol. 2010; 88(7):754-760.
[52] The Ministry of Science and Technology of the People’s Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006-09-30.
[53] Xia CB, Qin XY, Jiang CW, et al. Hippocampal anatomy and stereo positioning in rats. Zhongguo Yiliao Qianyan. 2009;4(21):27.
Copyedited by Mark F, Sui RB, Sun DJ, Yu J, Qiu Y, Li CH, Song LP, Zhao M
10.4103/1673-5374.130084
Yuelan Wang, M.D., Department of Anesthesiology, Affiliated Qianfoshan Hospital, Shandong University, Jinan 250014, Shandong Province, China, wyldgf@163. com.
http://www.nrronline.org/
Accepted: 2014-01-28
- 中國神經(jīng)再生研究(英文版)的其它文章
- Acrylamide exposure impairs blood-cerebrospinal fl uid barrier function
- Analysis of the effect of repeated-pulse transcranial magnetic stimulation at the Guangming point on electroencephalograms
- Preconditioning crush increases the survival rate of motor neurons after spinal root avulsion
- Increased expression of Notch1 in temporal lobe epilepsy: animal models and clinical evidence
- Transient axonal glycoprotein-1 induces apoptosisrelated gene expression without triggering apoptosis in U251 glioma cells
- Negative regulation of miRNA-9 on oligodendrocyte lineage gene 1 during hypoxic-ischemic brain damage

