Preconditioning crush increases the survival rate of motor neurons after spinal root avulsion
Lin Li, Yizhi Zuo, Jianwen He
Department of Human Anatomy, Nanjing Medical University, Nanjing, Jiangsu Province, China
Preconditioning crush increases the survival rate of motor neurons after spinal root avulsion
Lin Li, Yizhi Zuo, Jianwen He
Department of Human Anatomy, Nanjing Medical University, Nanjing, Jiangsu Province, China
In a previous study, heat shock protein 27 was persistently upregulated in ventral motor neurons following nerve root avulsion or crush. Here, we examined whether the upregulation of heat shock protein 27 would increase the survival rate of motor neurons. Rats were divided into two groups: an avulsion-only group (avulsion of the L4lumbar nerve root only) and a crush-avulsion group (the L4lumbar nerve root was crushed 1 week prior to the avulsion). Immunofluorescent staining revealed that the survival rate of motor neurons was significantly greater in the crush-avulsion group than in the avulsion-only group, and this difference remained for at least 5 weeks after avulsion. The higher neuronal survival rate may be explained by the upregulation of heat shock protein 27 expression in motor neurons in the crush-avulsion group. Furthermore, preconditioning crush greatly attenuated the expression of nitric oxide synthase in the motor neurons. Our fi ndings indicate that the neuroprotective action of preconditioning crush is mediated through the upregulation of heat shock protein 27 expression and the attenuation of neuronal nitric oxide synthase upregulation following avulsion.
nerve regeneration; nerve root avulsion; spinal nerve root; heat shock protein 27; nitric oxide synthase; motor neurons; fluorescent antibody technique; choline acetyltransferase; a grant from Education Ministry of Jiangsu Province; Excellent Discipline of Jiangsu Province; neural regeneration Funding: This study was supported by a grant from Education Ministry of Jiangsu Province, No. 08KJB310002; Excellent Discipline of Jiangsu Province, No. JX10131801096.
Li L, Zuo YZ, He JW. Preconditioning crush increases the survival rate of motor neurons after spinal root avulsion. Neural Regen Res. 2014;9(5):540-548.
Introduction
Spinal nerve root avulsion in adult rodents induces retrograde degeneration of motor neurons, leading to subacute death of motor neurons[1-6]. The molecular mechanisms of this degeneration and cell death are still not fully understood. One consensus is that neural cytotoxic molecules such as superoxide and glutamine accumulate[7-14]and neurotrophic factors, such as glial cell line-derived[15-16]and brain-derived[10,17-19]neurotrophic factors, become depleted. Nitric oxide, mainly produced by neuronal nitric oxide synthase (nNOS) in the central nervous system, is considered a modulator of neurotransmission as well as a protector against neuronal death from several death stimuli[20]. However, besides this protective effect, nitric oxide is also cytotoxic and involved in neuronal degeneration or death after spinal nerve injury[2,7,20-23]. Recent evidence indicates that heat shock proteins are critically involved in protection against the cytotoxicity induced by reactive nitrogen species[23]. Among these proteins, special attention has been paid to a 27 kDa heat shock protein (HSP27) that is constitutively expressed in some populations of neurons. HSP27 plays an important role in cellular defense mechanisms[24-26]. A previous study showed that, 1 week after avulsion of the spinal nerve root, small motor neurons (< 500 μm2) negative for HSP27 immunoreactivity died and only large (> 500 μm2) HSP27-positive motor neurons survived in the spinal cord ventral horn[3]. This was followed by the induction of nNOS in the surviving large motoneurons, which showed a significant upregulation of HSP27. Furthermore, enhancement of HSP27 expression in motor neurons was observed after mild crush of the spinal nerve root, indicating that upregulation of HSP27 might be a protective response in neuronal cell bodies following the injury of peripheral axons[3]. But the effect on the survival rate of motor neurons has not been assessed after crushing before spinal root avulsion.
Here, we investigate whether preconditioning crush can increase the survival rate of motor neurons. We examined the effect of spinal nerve root crush on neuronal survival rate in spinal cord ventral horn at 2, 3 and 5 weeks after spinal nerve root avulsion. We also quantitatively compared the time courses of HSP27 and nNOS expression between the avulsion-only and crush-avulsion groups.
Results
Quantitative analysis of experimental animals
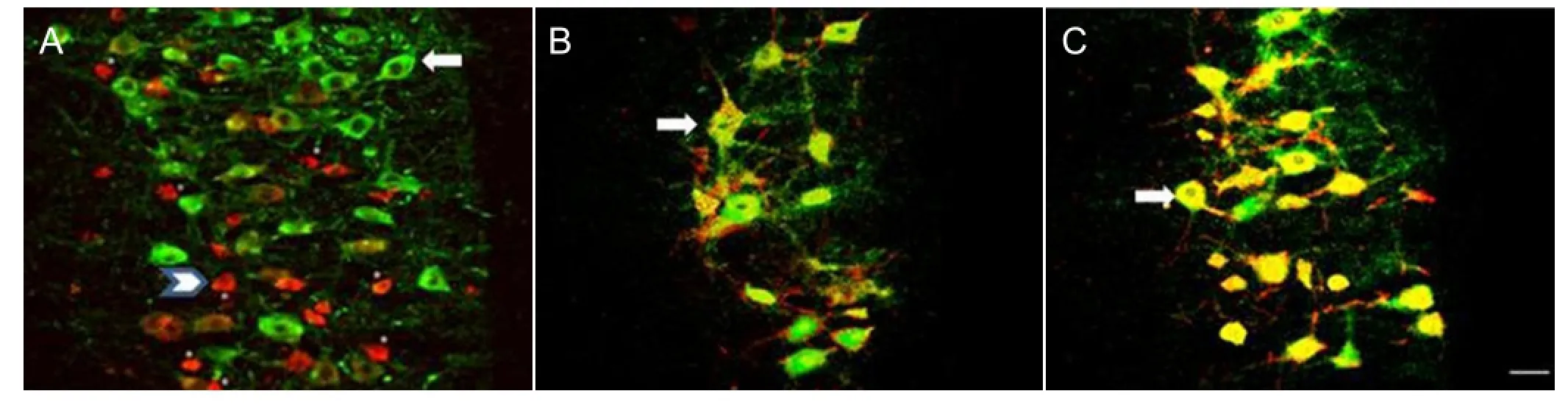
Figure 1 Effect of preconditioning crush on the expression of heat shock protein 27 (HSP27, green) and choline acetyltransferase (ChAT, red) in ventral motor neurons following L4nerve root avulsion.
A total of 36 Wistar rats of both sexes, 4–6 weeks old, were randomly divided into an avulsion-only group and a crush-avulsion group (18 rats per group). The avulsion-only group underwent avulsion of the L4nerve root, while the crush-avulsion group was subjected to the same avulsion but 1 week prior to the avulsion also underwent crushing of the same nerve outside the vertebra. All 36 rats survived the surgery and were included in the fi nal analysis.
Survival of HSP27- and choline acetyltransferaseimmunoreactive ventral motor neurons
The number and size of motor neuron cell bodies was determined in the avulsion-only and crush-avulsion groups using double immunofluorescence labeling of HSP27 and choline acetyltransferase. On the intact control side of both groups, at all time points, small motor neurons (< 500 μm2) were positive for choline acetyltransferase, while large motor neurons (> 500 μm2) were double-positive for choline acetyltransferase and HSP27 (Figure 1A). Some ventral motor neurons, mainly of the large type, survived at all time points on both sides in the avulsion-only (Figure 1B) and crush-avulsion (Figure 1C) groups.
Preconditioning crush of the nerve root protected the spinal cord ventral motor neurons from death caused by nerve root avulsion
Nissl fluorescence staining and cell counts showed that there was no significant difference in the number of Nissl-positive ventral horn motor neurons on the intact control side of the L4segment between the two groups at 2, 3 or 5 weeks after avulsion (Figure 2A). However, at each time point, the number of Nissl-positive neurons in the injury side was signi fi cantly lower in the avulsion-only group than in the crush-avulsion group (P < 0.05; Figure 2B). The number of ventral horn motor neurons in the avulsion-only group was 56.5 ± 3.2%, 53.7 ± 3.1% and 49.8 ± 2.8% at 2, 3 and 5 weeks, respectively, while that in the crush-avulsion group was 63.5 ± 3.4%, 61.8 ± 2.9% and 57.9 ± 3.2%. The large motor neurons were particularly abundant in the ventral horn on the injury side, although they seemed to be undergoing light atrophy (Figure 2A).
Preconditioning crush upregulated HSP27 expression and attenuated upregulation of nNOS expression in the spinal cord ventral horn neurons following nerve root avulsionSome motor neurons on the intact control side of the ventral horn expressed HSP27-immuno fl uorescent signals at all time points (Figure 3A), consistent with a previous report[27]. HSP27 expression was upregulated on the injury side in both groups (Figure 3A), but the degree of upregulation was signi fi cantly higher in the crush-avulsion group than in the avulsion-only group (P < 0.01), as shown by a higher number and density of HSP27-positive neurons (Figure 3B, C). The number of HSP27-immunoreactive neurons on the injury side of the crush-avulsion group at 2, 3 and 5 weeks was 63.7 ± 3.4%, 58.9 ± 3.0% and 56.3 ± 3.2% respectively, while that in the avulsion-only group was 56.4 ± 3.2%, 52.3 ± 3.1% and 49.8 ± 2.9% (Figure 3B).
In contrast, at all time points, immunoreactivity of nNOS was attenuated in the crush-avulsion group compared with the avulsion-only group (Figures 4, 5). Immuno fl uorescent staining of nNOS showed that ventral horn neurons in the intact control side of the two groups expressed only faint nNOS (Figures 4, 5), whereas in the injury side, nNOS-immunoreactive neurons were easily detected at the ventral horn in both groups. The preconditioning crush inhibited the upregulation of nNOS expression in the spinal cord ventral horn neurons (Figures 4, 5). Quantitative analysis revealed that the increase in the number of nNOS-immunoreactive neurons in the crush-avulsion group was lower than that in the avulsion-only group at 2, 3 and 5 weeks (P < 0.05).
Finally, among the surviving HSP27-immunoreactive motor neurons in the ventral horn at 2, 3 and 5 weeks, there were a greater number of large (> 500 μm2) than small (<500 μm2) neurons in the two groups (Figure 6). An intrinsic relationship between HSP27 and nNOS expression was demonstrated by a negative correlation between the numberof HSP27-immunoreactive and nNOS-immunoreactive neurons (r = 0.958, P < 0.001; Table 1).
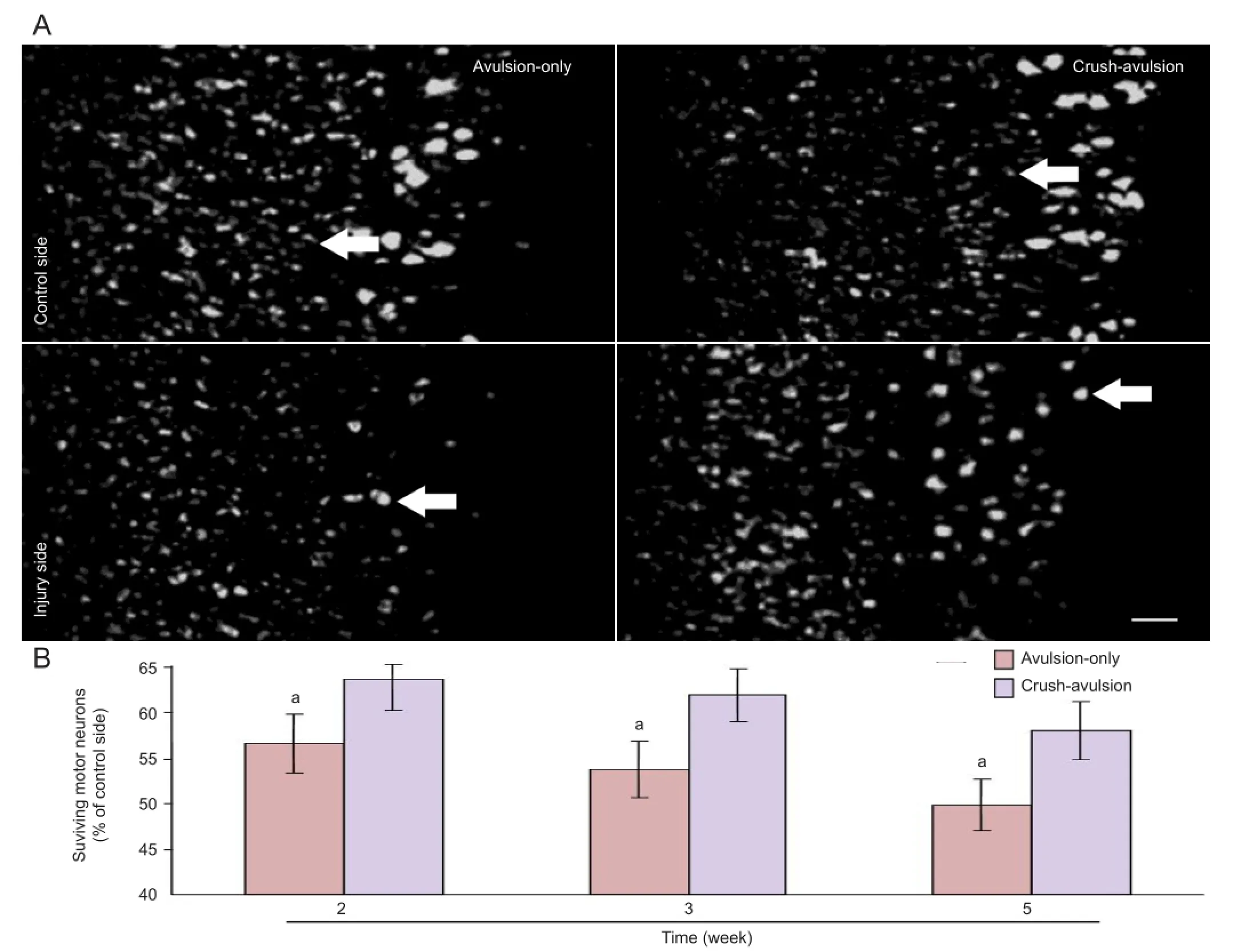
Figure 2 Effect of preconditioning crush on the survival of ventral motor neurons following L4nerve root avulsion.
Discussion
We have shown that the time course of ventral motor neuron loss after nerve root avulsion is partially prevented by preconditioning crush. In accordance with previous quantitative analysis results[2-3], our statistical data indicated a progressive decline in the number of Nissl-stained motor neurons following avulsion. Severe loss of motor neurons was observed 2 weeks after avulsion, and neuronal loss progressed steadily and slowly. The extent of motor neuronal loss was signi fi cantly attenuated in the crush-avulsion group at each time point examined. Even 5 weeks after avulsion, 57.9% of the ventral motor neurons had survived. We therefore conclude that preconditioning crush has a persistent effect on the maintenance of surviving motor neurons after nerve root avulsion.
We tentatively propose that the higher motor neuronal sur-vival rate in the crush-avulsion group arises from a long-lasting and strong expression of HSP27. Previous studies have shown that HSP27 plays a role in promoting neuronal survival in the dorsal root ganglion after nerve axotomy[28-29]or nerve ligation[30]. Accumulating evidence[2-3]also demonstrates the persistently protective role of HSP27 in avulsed motor neurons, since all of the surviving large motor neurons continued to express HSP27 intensely over a long period. It is therefore conceivable that this protein might protect motor neurons from cell death. Neuronal protection was further demonstrated by a greater expression of HSP27, associated with a higher survival rate of motor neurons, in the crush-avulsion group compared with the avulsion-only group. Our previous study demonstrated an upregulation of HSP27 expression in ventral neurons by light nerve root crush[3]. Thus, the increase in HSP27 expression after mild nerve damage might protect neurons from degeneration and cell death caused by nerve root avulsion 1 week later. The induction of robust protection against noxious stimuli after a brief stress or toxic insult has been demonstrated in several models in vitro and in vivo[31-33]. For example, increasing endogenous HSP27 by heat shock preconditioning in cultured neonatal dorsal root ganglion neurons inhibits nerve growth factor withdrawal-induced apoptosis[33]. There are two temporally and mechanistically distinct types of protection afforded by preconditioning stimuli: acute and delayed preconditioning[34]. Although they share components of the signaling pathway, they differ in their requirements for new protein synthesis. The protective effect of acute preconditioning is short-lived protein synthesis mediated by post-translational protein modifications. Delayed preconditioning, however, requires new protein synthesis and the effects are sustained for days or weeks[34]. Based on the previously observed persistent upregulation of HSP27 expression following nerve root crush[3]and the long-lasting increase in HSP27 immunoreactivity in the crush-avulsion group in the present study, we hypothesize that the protection against crush lesions induced by preconditioning crush in motor neurons is mediated via the delayed preconditioning pathway. This long-term neuroprotective effect of HSP27 has also been demonstrated in another recent study[35].
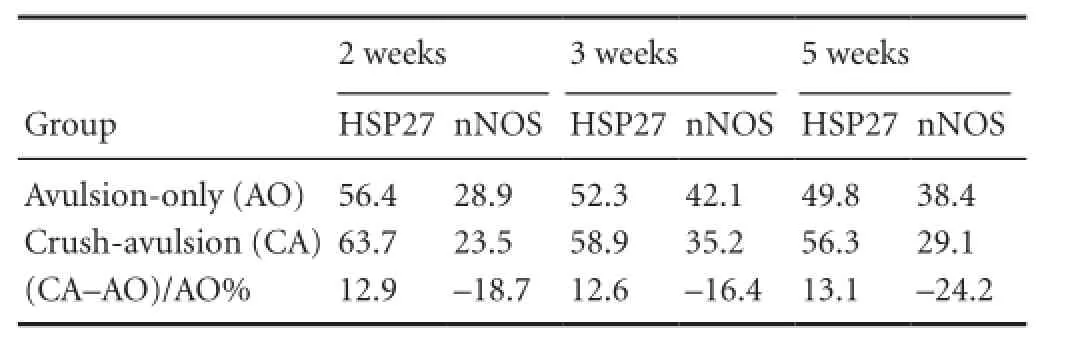
Table 1 Survival rate (%) of heat shock protein 27 (HSP27) and neuronal nitric oxide synthase (nNOS) immunoreactive motor neurons
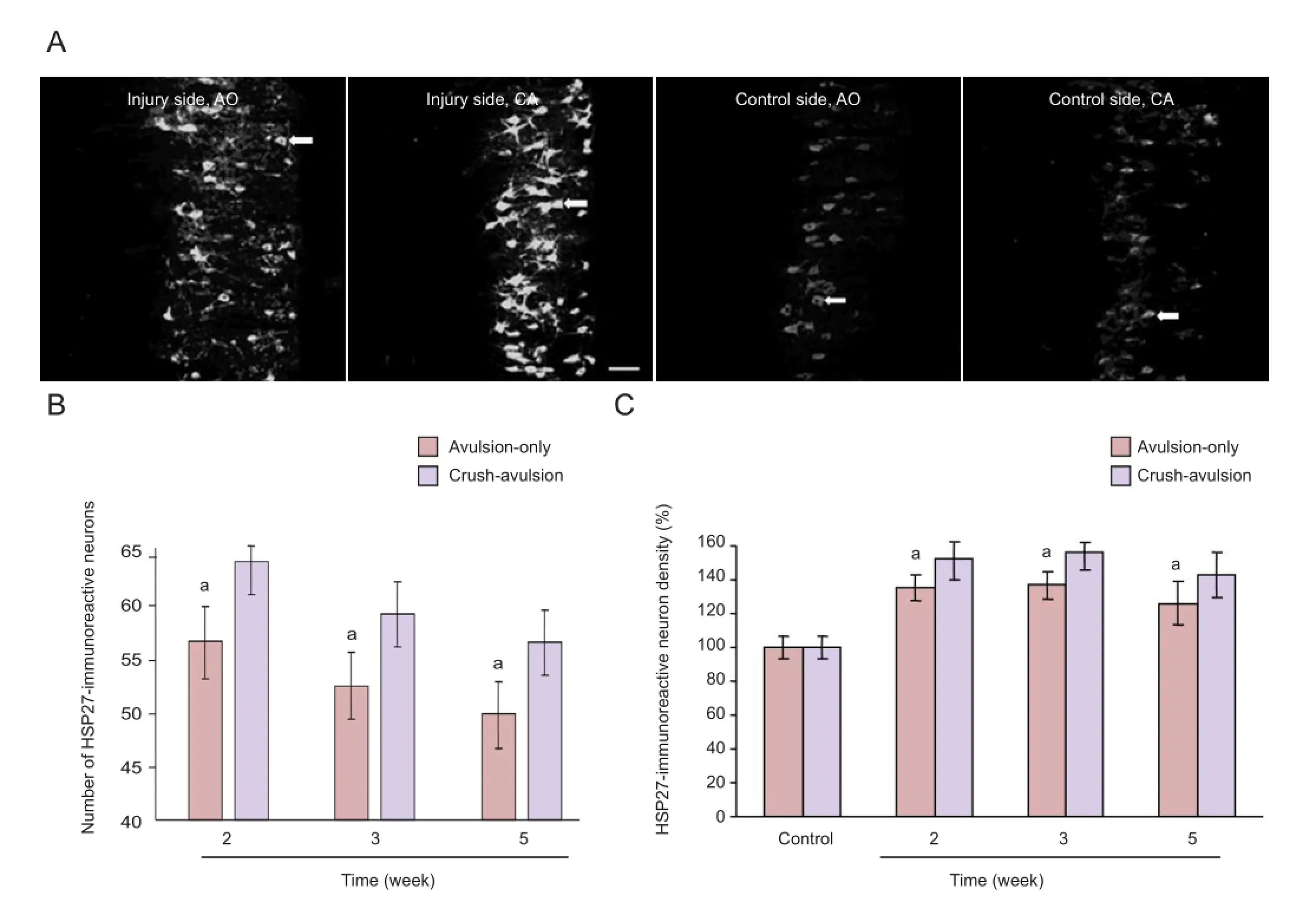
Figure 3 Effect of preconditioning crush on the expression of heat shock protein 27 (HSP27) in ventral motor neurons following L4nerve root avulsion.
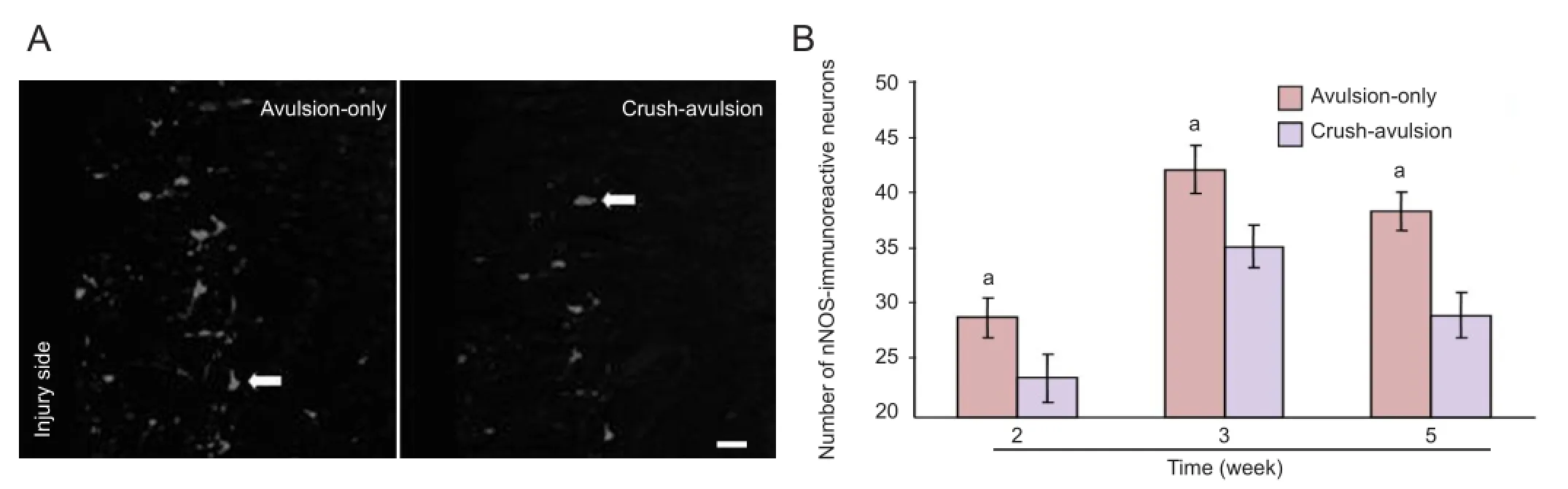
Figure 4 Effect of preconditioning crush on the expression of neuronal nitric oxide synthase (nNOS) in ventral motor neurons following the L4nerve root avulsion.
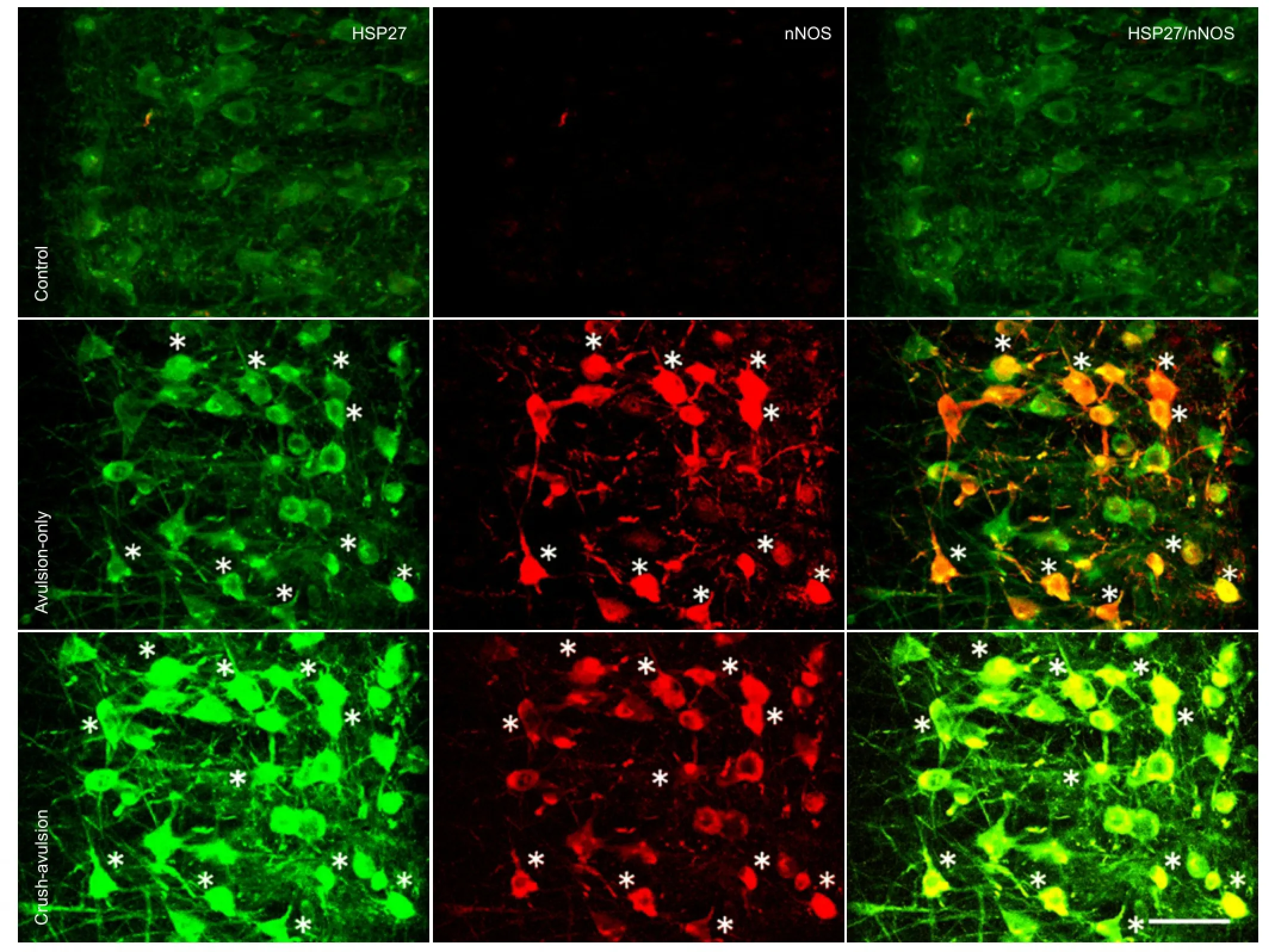
Figure 5 Effect of preconditioning crush on the expression of heat shock protein 27 (HSP27) and neuronal nitric oxide synthase (nNOS) in horizontal sections through the ventral horn of the L4segment following nerve root avulsion.
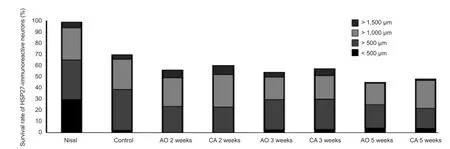
Figure 6 Time changes in cell body size in heat shock protein 27 (HSP27) immunoreactive motor neurons following nerve root crush prior to the avulsion of L4nerve root (CA) or avulsion alone (AO).
We found that, following neonatal nerve crush, HSP27 overexpression in vivo provided substantial rescue of motor neurons 5–6 months after nerve injury. Furthermore, surviving motor neurons were able to regenerate and form motor units to preserve muscle function. These properties of HSP27 have considerable potential for improving long-term muscle function in motor neuron disorders.
How might endogenous HSP27 act to prevent or limit motor neuron degeneration after preconditioning crush? To address the underlying molecular mechanisms, we observed the time course of nNOS expression among the two groups and investigated the correlation between HSP27 and nNOS expression. Although nitric oxide has many functions following neurotrauma, it is generally accepted that the nitric oxide induced in motor neurons mediates motor neuron death, since the induction of nitric oxide occurs in avulsion models but not in other models of axotomy without cell death[3,7,36-37].
The motor neuronal toxicity of nitric oxide has been further demonstrated by the fi nding that nitroarginine, a specific inhibitor of nitric oxide synthase, signi fi cantly reduces the death of motor neurons following avulsion[7]and that nitric oxide mainly contributes to cultured motor neuron apoptosis induced by trophic factor deprivation in vitro[11].
In the present study, the number and staining density of nNOS-immunoreactive neurons was notably lower in the avulsion-only group than in the crush-avulsion group. There was a negative correlation between the number of neurons immunoreactive for HSP27 and for nitric oxide synthase after avulsion. Thus, it is reasonable to presume that the induction of HSP27 by preconditioning crush might attenuate upregulation of nNOS following crush to improve motor neuron survival.
Although we cannot provide direct evidence to elucidate their intrinsic relationship in the present study, we propose that persistent expression of HSP27 accompanied by nNOS may imply a keen competition in motor neuron survival between cytotoxic and cytoprotective systems. For example, Benn et al.[25]showed that the neuroprotective action of HSP27 is downstream of cytochrome c release from mitochondria and upstream of caspase-3 activation. On the other hand, Figueroa et al.[38]demonstrated that in cortical neurons nitric oxide toxicity is mediated by mitochondrial dysfunction. SNAP, a nitric oxide donor, induces apoptosis in these cells mainly by increasing p53 and inducing cytochrome C release, and activation of caspase-9 and caspase-3. Further study is necessary to elucidate how the molecular mechanism of HSP27 upregulation after preconditioning crush relates to the inhibition of nNOS following avulsion.
In summary, the present study is the fi rst to demonstrate that loss of ventral motor neurons after nerve root avulsion is partially prevented by preconditioning crush. This injury tolerance might act through the induction of HSP27 by preconditioning crush, which inhibits the upregulation of nNOS to attenuate the cytotoxic effects of nitric oxide following crush. The present results open new avenues in the investigation of neuroprotective strategies for the treatment of motor neuron disorders in the future.
Materials and Methods
Design
A randomized, controlled animal experiment.
Time and setting
Experiments were performed from June 2011 to May 2012 at the Human Anatomy Laboratory, Nanjing Medical University, China.
Materials
A total of 36 Wistar rats of both sexes, aged 4–6 weeks, weighing 120–140 g, were purchased from the Experimental Animal Center of Jiangsu Province, China (license No. SYXK (Su) 2002-0013). The rats were housed at 20–26°C and 40–70% humidity in a 12 hour light/dark cycle, with free accessto water and food.
Experimental protocols were in strict accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, issued by the Ministry of Science and Technology of China[39].
Methods
Establishment of an animal model for lumbar nerve root avulsion
L4nerve root crush and avulsion were performed as described previously[2]. Briefly, rats were anesthetized with pentobarbital sodium salt (20 mg/kg). A left paramedial incision (about 2 cm) was made over the iliac crest. The left longissimus muscle was then split at the midline and the left transverse process of the L5vertebra was removed. In the avulsion-only group, the left L4nerve root (consisting of a motor and sensory root and dorsal root ganglion) was raised slightly and then pulled with steady and moderate traction. About 30 mm of the left L4mixed root was excised. The right nerve root was intact and used as the control.
In the crush-avulsion group, the left L4nerve root was crushed for 10 seconds at the exit point from the intervertebral foramen. One week later, the same nerve root was avulsed as described above. Crush and resection models resulted in little motor neuron loss and no expression of nNOS in motor neurons, but a signi fi cant increase of HSP27 was observed.
Perfusion and tissue processing
At 2, 3 and 5 weeks after surgery, six rats from each group were deeply anesthetized with ether and pentobarbital sodium salt (50 mg/kg), then perfused with 0.01 mol/L PBS (pH 7.4) and fixed by perfusion with 4% paraformaldehyde in 0.1 mol/L phosphate buffer (pH 7.4) through the ascending aorta. Under a stereomicroscope, the lumbar spinal cord with dorsal and ventral roots was carefully dissected. The avulsed L4dorsal and ventral nerve roots were confirmed, and the L4spinal cord segment was then dissected out using a pair of microscissors. The specimens were post fi xed for 3 hours and subsequently stored in 30% sucrose solution at 4°C overnight.
The L4segment was cut horizontally from ventral to dorsal at 50 μm on a cryostat (Leica Microsystems, Wetzlar, Germany). Five serial sections from each animal were collected in 0.02 mol/L potassium phosphate-buffered saline (pH 7.4). The fi rst section was used for fl uorescent Nissl staining; the second and third sections were used for double-immuno fl uorescence staining of HSP27 with nNOS and with choline acetyltransferase, respectively. The protocol for each stain is described below.
Fluorescent Nissl staining
NeuroTrace fl uorescent Nissl stains (Molecular Probes Inc., Eugene, Oregon, USA) were used to label the spinal cord ventral horn neurons. Sections were loaded on a slide, permeabilized in 0.1 mol/L PBS (pH 7.2) for at least 40 minutes and washed with PBS plus 0.1% Triton X-100 for 10 minutes followed by PBS twice for 5 minutes each. The NeuroTrace stain, diluted 1:200 in PBS, was applied to the sections and incubated for 20 minutes at room temperature.
After washing once with PBS containing 0.1% Triton X-100, and twice with PBS, the sections were mounted onto glass slides and coverslipped with Gel Mount (Sigma, St. Louis, MO, USA). Staining was visualized with brightfield (cresyl violet) or epifluorescent (fluorescent staining) microscopy under an Olympus LSM-GB200 microscope (Olympus, Tokyo, Japan).
Immunofluorescent histochemical staining
Sections were stained for immunofluorescence of HSP27 or nNOS as described previously[2-3]. Briefly, non-specific binding sites were blocked overnight at 4°C by preincubation with 0.1% bovine serum albumin in potassium phosphate-buffered saline containing 0.5% Triton X-100. Primary antibodies were rabbit polyclonal antibody raised against murine HSP25/27 (1:1,500; Enzo Life Sciences Inc., Farmingdale, NY, USA) or sheep polyclonal antibody raised against murine nNOS (1:2,000; a generous gift from Dr. Emson (Kyushu University, Fukuoka, Japan) and were diluted in potassium phosphate buffered saline. Sections were incubated in primary antibody for 3 days at 4°C. As a negative control, preimmune serum was used in place of primary antibody. After washing, sections were incubated with FITC-conjugated swine anti-rabbit IgG (1:200; Chemicon, Temecula, CA, USA) or Texas red-conjugated donkey anti-sheep IgG (1:200; Chemicon) for 12 hours at 4°C. After washing with PBS, the sections were then mounted onto glass slides and coverslipped with Gel Mount.
For HSP27 and choline acetyltransferase double immuno fl uorescent labeling, a primary antibody mixture of rabbit polyclonal antibodies to HSP25/27 (1:1,500; StressGen, SPA-801) and rat monoclonal antibody to choline acetyltransferase (1:8; Boehringer Mannheim GMBH, Sandhofer Strasse, Mannheim, Germany) was used. Sections were then incubated in a secondary antibody mixture of FITC-conjugated swine anti-rabbit IgG (Dako, Glostrup, Denmark) and biotinylated goat anti-rat IgG (Chemicon). Texas red-conjugated streptavidin was used to visualize the biotin binding site. No difference in the morphology of any of the immunolabeled structures was observed between single and double labeling.
Counting of motor neurons
For each animal, motor neurons containing a clearly visible nucleus were counted in every fi fth Nissl-stained, HSP27 and nNOS immuno fl uorescence-labeled section. The ratio of the number of motor neurons in the lesion side to that in the control side was expressed as a percentage of all surviving motor neurons. In immunofluorescence-labeled sections, the number of choline acetyltransferase- and HSP27-immunoreactive motor neurons on the ipsilateral side was counted separately in every fi fth serial section.
The ratio of choline acetyltransferase- and HSP27-positive neurons to Nissl-stained motor neurons in the control side was expressed as a percentage of the surviving immunoreactive motor neurons.
Quantification of the size of HSP27-immunoreactive motor neurons
HSP27 was constitutively expressed in some motor neurons in the intact spinal cord, as indicated previously[27]. This labeling appeared to represent the complete figure of a motor neuron, so the size of HSP27-immunoreactive motor neurons on the lesion side was determined at 2, 3 and 5 weeks after nerve root avulsion using ImageJ (NIH software, Bethesda, Maryland, USA).
One of the criteria for including cells for measurement was that the nucleus be discernible. However, the fluorescence was bright and nuclei were difficult to visualize in some cases. In these cases, motor neurons were checked by scanning through different planes, and were included if they contained a major part of the cell body. Fifty to 100 spinal motor neurons were measured at each time point.
The upregulation of HSP27 expression on the lesion side and the density of HSP27 immunoreactivity was quanti fi ed by measuring the number of pixels per μm2in the area of the motor neuron displayed on the monitor of a computer running NIH Image version 1.62 software. The data from both groups were compared, and the density of HSP27 immunoreactivity in the control side was taken as 100%.
Data analysis
Immuno fl uorescent micrographs in each section were captured by confocal laser scanning microscopy (LSM-GB200 Olympus, Tokyo, Japan) as described previously[40]. Briefly, the optical section 2 μm from the upper surface was used as the lookup section, and sections 4–24 μm from the surface were reference sections after taking the lost caps bias into consideration[40].
The numbers of Nissl-positive, HSP27-immunoreactive and nNOS-immunoreactive motor neurons containing a clearly visible nucleus were counted in the spinal cord ventral horn of the L4segment.
The percentage of surviving motor neurons on the injured side was calculated by dividing the number of Nissl-positive neurons on the lesion side by that on the control side and multiplying by 100.
Statistical analysis
Excel 2007 (Microsoft Corporation, Redmond, Washington, USA) was used for statistical analysis. Data were presented as mean ± SEM. Statistical comparisons were made using Student’s t-test, with P < 0.01 considered significant. The Pearson correlation test was used to investigate the relationship between HSP27 and nNOS expression, with P < 0.05 considered signi fi cant.
Author contributions:Li L participated in the study concept and design, experimental implementation, data integrity and analysis, and manuscript writing. Zuo YZ participated in the study implementation. He JW was in charge of the study concept and design, served as principle investigator, and was responsible for manuscript authorization. All authors approved the final version of the manuscript.
Con fl icts of interest:None declared.
Peer review:In this study, we avulsed spinal lumbar nerve roots in the rats, which caused a large number of neuronal degeneration and death in anterior horn, in an effort to compare the effect of avulsion-only versus crush-avulsion on the survival of motor neurons and the expression of heat shock protein 27. Experimental findings indicate that crush + avulsion of spinal nerve roots led to increased survival of motor neurons, and the underlying mechanism is mediated by the upregulation of heat shock protein 27 expression and the down-regulation of nitric oxide synthase expression.
[1] Koliatsos VE, Price WL, Pardo CA, et al. Ventral root avulsion: an experimental model of death of adult motor neurons. J Comp Neurol. 1994;342(1):35-44.
[2] He JW, Hirata K, Kuraoka A, et al. An improved method for avulsion of lumbar nerve roots as an experimental model of nitric oxide-mediated neuronal degeneration. Brain Res Proto. 2000;5(3):223-230.
[3] He JW, Hirata K, Wang S, et al. Expression of nitric oxide synthase and 27-kD heat shock protein in motor neurons of ventral rootavulsed rats. Arch Histol Cytol. 2003;66(1):83-93.
[4] Bergerot A, Shortland PJ, Anand P, et al. Co-treatment with riluzole and GDNF is necessary for functional recovery after ventral root avulsion injury. Exp Neurol. 2004;187(2):359-366.
[5] Chew DJ, Leinster VHL, Sakthithasan M, et al. Cell death after dorsal root injury. Neurosci Lett. 2008;433(3):231-234.
[6] Penas C, Casas C, Robert I, et al. Cytoskeletal and activity-related changes in spinal motoneurons after root avulsion. J Neurotrauma. 2009;26(5):763-779.
[7] Wu W, Li L. Inhibition of nitric oxide synthase reduces motoneuron death due to spinal root avulsion. Neurosci Lett. 1993;153(2):121-124.
[8] Wu W, Han K, Li L, et al. Implantation of PNS graft inhibits the induction of neuronal nitric oxide synthase and enhances the survival of spinal motoneurons following root avulsion. Exp Neurol. 1994;129(2):335-339.
[9] Wu Y, Li Y, Liu H, et al. Induction of nitric oxide synthase and motoneuron death in newborn and early postnatal rats following spinal root avulsion. Neurosci Lett. 1995;194(1-2):109-112.
[10] Novikov L, Novikova L, Kellerth JO. Brain-derived neurotrophic factor promotes survival and blocks nitric oxide synthase expression in adult rat spinal motoneurons after ventral root avulsion. Neurosci Lett. 1995;200(1):45-48.
[11] Estevez AG, Spear N, Manuel SM, et al. Nitric oxide and superoxide contribute to motor neuron apoptosis induced by trophic factor deprivation. J Neurosci. 1998;18(3):923-931.
[12] Zhou L, Wu W. Antisense oligos to neuronal nitric oxide synthase aggravate motoneuron death induced by spinal root avulsion in adult rat. Exp Neurol. 2006;197(1):84-92.
[13] Yang JY, Kim HS, Lee JK. Changes in nitric oxide synthase expression in young and adult rats after spinal cord injury. Spinal Cord. 2007;45(11):731-738.
[14] Conti A, Miscusi M, Cardali S, et al. Nitric oxide in the injured spinal cord: synthases cross-talk, oxidative stress and in fl ammation. Brain Res Rev. 2007;54(1):205-218.
[15] Li L, Wu W, Lin LF, et al. Rescue of adult mouse motoneurons from injury-induced cell death by glial cell line-derived neurotrophic factor. Proc Natl Acad Sci U S A. 1995;92(21):9771-9775.
[16] Eggers, R, Hendriks WT, Tannemaat MR, et al. Neuroregenerative effects of lentiviral vector-mediated GDNF expression in reimplanted ventral roots. Mol Cell Neurosci. 2008;39(1):105-117.
[17] Novikova L, Novikov L, Kellerth JO. Effects of neurotransplants and BDNF on the survival and regeneration of injured adult spinal motoneurons. Eur J Neurosci. 1997;9(12):2774-2777.
[18] Kishino A, Ishige Y, Tatsuno C, et al. BDNF prevents and reverses adult rat motor neuron degeneration and induces axonal outgrowth. Exp Neurol. 1997;144(2):273-286.
[19] Eggers R, Tannemaat MR, Ehlert EM, et al. A spatio-temporal analysis of motoneuron survival, axonal regeneration and neurotrophic factor expression after lumbar ventral root avulsion and implantation. Exp Neurol. 2010;223(1):207-220.
[20] Iadecola C. Bright and dark sides of nitric oxide in ischemic brain injury. Trends Neurosci. 1997;20(3):132-139.
[21] Dawson VL, Dawson TM, Bartley DA, et al. Mechanisms of nitric oxide-mediated neurotoxicity in primary brain cultures. J Neurosci. 1993;13(6):2651-2661.
[22] He BP, Tay SS, Leong SK. Motoneuronal death without expression of NADPH-diaphorase activity after sciatic nerve cut in 5-day-old mice. Brain Res. 1996;733(1):125-128.
[23] Calabrese V, Bates TE, Stella AM. NO synthase and NO-dependent signal pathways in brain aging and neurodegenerative disorders: the role of oxidant/antioxidant balance. Neurochem Res. 2000;25(9-10):1315-1341.
[24] Costigan M, Mannion RJ, Kendall G, et al. Heat shock protein 27: developmental regulation and expression after peripheral nerve injury. J Neurosci. 1998;18(15):5891-5900.
[25] Benn SC, Perrelet D, Kato AC, et al. Hsp27 upregulation and phosphorylation is required for injured sensory and motor neuron survival. Neuron. 2002;36(1):45-56.
[26] Stetler RA, Signore AP, Gao Y, et al. HSP27: mechanisms of cellular protection against neuronal injury. Curr Mol Med. 2009;9(7):863-872.
[27] Plumier JC, Armstrong JN, Landry J, et al. Expression of the 27,000 mol. wt heat shock protein following kainic acid-induced status epilepticus in the rat. Neuroscience. 1996;75(3):849-856.
[28] Lewis SE, Mannion RJ, White FA, et al. A role for HSP27 in sensory neuron survival. J Neurosci. 1999;19(20):8945-8953.
[29] Wagstaff MJ, Collaco-Moraes Y, Smith J, et al. Protection of neuronal cells from apoptosis by Hsp27 delivered with a herpes simplex virus-based vector. J Biol Chem. 1999;274(8):5061-5069.
[30] Liu W, Hirata K, Kawabuchi M. The occurrence of nitric oxide synthase-containing axonal baskets surrounding large neurons in rat dorsal root ganglia after sciatic nerve ligation. Arch Histol Cytol. 2005;68(1):29-40.
[31] Kabakov AE, Budagova KR, Bryantsev AL, et al. Heat shock protein 70 or heat shock protein 27 overexpressed in human endothelial cells during posthypoxic reoxygenation can protect from delayed apoptosis. Cell Stress Chaperones. 2003;8(4):335-347.
[32] Whitlock NA, Agarwal N, Ma JX, et al. Hsp27 upregulation by HIF-1 signaling offers protection against retinal ischemia in rats. Invest Ophthalmol Vis Sci. 2005;46(3):1092-1098.
[33] Dodge ME, Wang J, Guy C, et al. Stress-induced heat shock protein 27 expression and its role in dorsal root ganglion neuronal survival. Brain Res. 2006;1068(1):34-48.
[34] Nandagopal K, Dawson TM, Dawson VL. Critical role for nitric oxide signaling in cardiac and neuronal ischemic preconditioning and tolerance. J Pharmacol Exp Ther. 2001;297(2):474-478.
[35] Sharp P, Krishnan M, Pullar O, et al. Heat shock protein 27 rescues motor neurons following nerve injury and preserves muscle function. Exp Neurol. 2006;198(2):511-518.
[36] Snider WD, Elliott JL, Yan Q. Axotomy-induced neuronal death during development. J Neurobiol. 1992;23(9):1231-1246.
[37] Clowry GJ. Axotomy induces NADPH diaphorase activity in neonatal but not adult motoneurones. NeuroReport. 1993;5(3):361-364.
[38] Figueroa S, Oset-Gasque MJ, Arce C, et al. Mitochondrial involvement in nitric oxide-induced cellular death in cortical neurons in culture. J Neurosci Res. 2006;83(3):441-449.
[39] The Ministry of Science and Technology of the People’s Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006-09-30.
[40] Jinno S, Kosaka T. Patterns of expression of neuropeptides in gabaergic nonprincipal neurons in the mouse hippocampus: quantitative analysis with optical disector. J Comp Neurol. 2003; 461(3):333-349.
Copyedited by Murphy S, Raye W, Xu WS, Bai WZ, Wang J, Yang Y, Li CH, Song LP, Zhao M
10.4103/1673-5374.130096
Jianwen He, M.D., Department of Human Anatomy, Nanjing Medical University, Nanjing 210029, Jiangsu Province, China, jianghe6562@163.com.
http://www.nrronline.org/
Accepted: 2014-01-08
- 中國神經(jīng)再生研究(英文版)的其它文章
- Acrylamide exposure impairs blood-cerebrospinal fl uid barrier function
- Analysis of the effect of repeated-pulse transcranial magnetic stimulation at the Guangming point on electroencephalograms
- Adaptive and regulatory mechanisms in aged rats with postoperative cognitive dysfunction
- Increased expression of Notch1 in temporal lobe epilepsy: animal models and clinical evidence
- Transient axonal glycoprotein-1 induces apoptosisrelated gene expression without triggering apoptosis in U251 glioma cells
- Negative regulation of miRNA-9 on oligodendrocyte lineage gene 1 during hypoxic-ischemic brain damage

